Abstract
We describe lead compound MIDD0301 for the oral treatment of asthma based on previously developed positive allosteric α5β3γ2 selective GABAA receptor (GABAAR) ligands. MIDD0301 relaxed airway smooth muscle at single micromolar concentrations as demonstrated with ex vivo guinea pig tracheal rings. MIDD0301 also attenuated airway hyperresponsiveness (AHR) in an ovalbumin murine model of asthma by oral administration. Reduced numbers of eosinophils and macrophages were observed in mouse broncho-alveolar lavage fluid without changing mucous metaplasia. Importantly, lung cytokine expression of IL-17A, IL-4, and TNF-α were reduced for MIDD0301 treated mice without changing anti-inflammatory cytokine IL-10 levels. Automated patch clamp confirmed amplification of GABA induced current mediated by α1-3,5β3γ2 GABAARs in the presence of MIDD0301. Pharmacodynamically, transmembrane currents of ex vivo CD4+ T cells from asthmatic mice were potentiated by MIDD0301 in the presence of GABA. The number of CD4+ T cell observed in the lung of MIDD0301 treated mice were reduced by an oral treatment of 20 mg/kg b.i.d. for 5 days. A half-life of almost 14 hours was demonstrated by pharmacokinetic studies (PK) with no adverse CNS effects when treated mice were subjected to sensorimotor studies using the rotarod. PK studies also confirmed very low brain distribution. In conclusion, MIDD0301 represents a safe and improved oral asthma drug candidate that relaxes airway smooth muscle and attenuates inflammation in the lung leading to a reduction of AHR at a dosage lower than earlier reported GABAAR ligands.
Keywords: Asthma, GABAA receptor, airway hyperresponsiveness, airway inflammation, MIDD0301, imidazobenzodiazepine
Graphical abstract
INTRODUCTION
One hallmark of asthma are chronically inflamed airways resulting in hyperactivity to external stimuli and airway obstruction.1 Common clinical features include recurrence or episodes of cough, chest tightness, shortness of breath, and wheezing. Asthma is a major global health concern, estimated at 300 million people affected worldwide, with 21 million in the United States alone. Global asthma prevalence is estimated to reach 400 million in year 2025.2, 3 Despite the growing challenge of asthma, currently approved therapeutic options are limited.
The preferred therapy for chronic persistent asthma includes inhaled corticosteroids and inhaled long acting β2 adrenergic receptor agonists (LABAs).4 Aerosol administration allows for targeted lung drug delivery, thus avoiding systemic side effects associated with these drugs. Nevertheless, asthma morbidity and mortality continues to rise. One important factor is the failure of patients to properly use inhaled medications leading to non-compliance and loss of asthma control.5, 6 An alternative for patients whose symptoms are poorly controlled with inhaled steroids is oral leukotriene receptor antagonists.2 However, genetic variations in leukotriene signaling genes may preclude their widespread efficacy.7 It has been reported that 35–78% of patients are nonresponsive to such medications.8, 9 Finally, injectable biologics such as Omalizumab and Meolizumab were developed to reduce asthma lung inflammation, but patient costs for these drugs of up to $30,000 a year limits their use to only severe disease.10, 11 These biologics are also unable to protect against acute bronchospasm and present anaphylaxis risks.
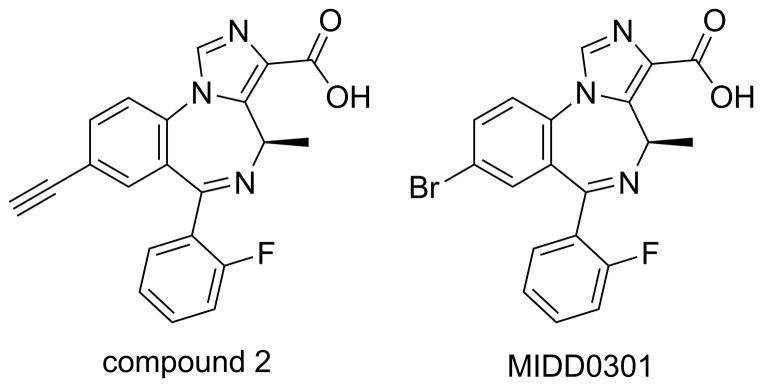
An improved drug for asthma would alleviate the multiple acute and chronic disease symptoms such as airway smooth muscle constriction and airway inflammation. Importantly, it should be orally active to eliminate poor aerosol compliance and have limited cardiovascular and central nervous system (CNS) adverse effects. To address these needs, our laboratory has advanced the development of a new asthma medication based on positive allosteric subtype selective GABAA receptor (GABAAR) ligands. These compounds do not cross the blood brain barrier and target asthma pathophysiology without systemic side effects.12–14 The ligand-gated chloride ion channels, GABAARs, are well-known for their CNS inhibitory neurotransmission. GABAARs are heteropentameric membrane receptors mainly consisting of combinations of 19 different subunits (α1–6, β1–3, γ1–3, δ, ε, π, θ, ρ1-3). Classical GABAARs consist of 2 alpha, 2 beta, and one “tertiary” subunit (γ, δ, ε, θ, or π).15, 16 Importantly, discrete GABAAR subtypes have been identified in airway smooth muscle, airway epithelium, and inflammatory cells.12, 13, 17–21 Our group and others have shown that the activation by positive allosteric modulators relaxed isolated pre-constricted airway smooth muscles and reduced inflammation and airway hyperresponsiveness (AHR) in murine asthma models.12, 13, 18, 19, 21–25 Notably, we described an α5β3γ2 GABAAR selective compound 2 (Figure 1) introduced by us that attenuated asthma disease measures in ovalbumin sensitized and challenged (ova s/c) BALB/c mice at a dose of 100 mg/kg for 5 days.13
Figure 1.
Structures of α5β3γ2 GABAAR selective positive allosteric modulators
In addition, the number of leukocytes, especially eosinophils, in ova s/c mouse lungs were reduced with compound 2 treatment. However, the number of lung CD4+ T cells was not reduced statistically compared to vehicle-treated mice, and electrophysiological analysis showed that compound 2 potentiation of GABA-induced current responses of isolated CD4+ T cells from ova s/c mice was negligible. To improve the performance of compound 2, further development produced lead compound MIDD0301 (Figure 1) that reduced AHR at lower dosage, significantly limited the number of CD4+ T cells, and reduced the expression of pro-inflammatory cytokines.
EXPERIMENTAL SECTION
Chemicals
MIDD0301 was synthesized using a published procedure.26 The purity was of >98% was confirmed by HPLC. Identity was determined by 1H-NMR, 13C-NMR, and high resolution mass spectroscopy. (Supporting Information for spectra)
Experimental animals
For the in vivo experiments, we used male Balb/c and female Swiss Webster mice (Charles River Laboratory). For ex vivo muscle relaxation studies, adult male Hartley Guinea pigs (Charles River Laboratory, 435–450g) were used. The housing of animals was pathogen-free and a twelve hour light and dark cycle was followed. Animals had free access to food and water. UWM and Columbia University confirmed that all in vivo experiments were in compliance with their IACUCs.
Ovalbumin sensitization and challenge
Randomized 35–38 day old male Balb/c mice in groups of ten were sensitized with intraperitoneal (i.p.) injections of 2 mg/kg/d (total volume of 100 μL) of ovalbumin (ova) (Sigma-Aldrich, St. Louis, MO) mixed in 2 mg of alum (Imject Alum; Thermo Scientific, Pierce, Rockford, IL) on days 0, 7, and 14. Mice were anesthetized with isoflurane and challenged intranasally (i.n.) with 1 mg/kg/d ova for 5 consecutive days.27 The control group of mice was sensitized with ova and challenged with i.n. saline. The effects of MIDD0301 at 20, 50 and 100 mg/kg administered 5 days during the ova challenge period were tested in separate groups of ten ova s/c Balb/c mice. As a positive control, separate groups of ova s/c BALB/c mice received salmeterol at 1 mg/kg b.i.d. for 5 days. Airway hyper-responsiveness parameters were assessed on day 28, and mice were sacrificed using an overdose of ketamine/xylazine i.p. on day 29 for assessment of inflammatory cells and mucus metaplasia.
Drug treatment protocol
Suspensions of MIDD0301 and salmeterol were prepared in 2% hydroxypropyl methylcellulose solution (Sigma-Aldrich, St. Louis, MO) and 2.5% polyethylene glycol (Sigma-Aldrich, St. Louis, MO) in a biological safety cabinet. First, a fine suspension was obtained by grinding MIDD0301 and polyethylene glycol with a mortar and pestle. A 2% hydroxypropyl methylcellulose solution was added in small potions followed by 1–2 minute grinding. MIDD0301 or salmeterol were administered by oral gavage at different concentrations with 20G gavage needles (Kent Scientific Corporation, Torrington CT) to groups of ova s/c Balb/c twice daily for 5 days during the ova challenge period. Mice received a single p.o. dose of compound just before airway parameter measurements. Mice were monitored daily after drug administration.
Relaxation of airway smooth muscle
This in vivo experiment was approved by Columbia University. Pentobarbital (100 mg/kg) was injected intraperitoneal to euthanize adult Guinea pigs. After the removal of the tracheas by transection, two cartilaginous rings were isolated. The rings were washed for one hour with at least five buffer exchanges to remove any pentobarbital. After the removal of the epithelium with a cotton swab, 2 silk threads were used to suspense the rings in an organ bath with a 4 ml jacket (Radnoti Glass Technology). A Grass FT03 force transducer was attached and connected to a computer that controlled and recorded the muscle tension using an Acknowledge 7.3.3. software. The bath solution consisted of 118 mM NaCl, 5.6 mM KCl, 0.5 mM CaCl2, 0.2 mM MgSO4, 25 mM NaHCO3, 1.3 mM NaH2PO4, 5.6 mM, and 10 μM indomethacin. The solution was bubbled with 5% carbon dioxide and 95% oxygen. To equilibrate the isotonic tension at 1 gram for 1 hour, a KH solution was added every fifteen minutes. Pre-contraction of the rings was carried out with 10 μM N-vanillylnonanamide followed by applications of acetylcholine at increasing concentrations (0.1–100 μM) and buffer. The resting tension was reset to 1g in between and after the applications. To reduce the effects histamine receptors and airways nerves, 1 μM Tetrodotoxin and 10 μM pyrilamine were added to the buffer. This was followed by substance P (1 μM) in order to contract the tracheal rings starting from resting tension of 1 g. MIDD0301 or vehicle was given to the organ bath after the highest contraction was established. The percentage of the starting contraction remaining at indicated time points after compound exposure was expressed as a percentage of the remaining contractile force in vehicle-treated tissues and compared between groups.
Assessment of airway hyperresponsiveness
DSI’s Buxco FinePointe NAM (Non-Invasive Airway Mechanics) instrument was used to record AHR in response to methacholine.28 For this experiment, animals were trained five days for fifteen minutes to stay in the measuring chambers. Calibration of the instrument was carried out before data collection. sRaw (specific airway resistance) was computed with a FinePoint software using ventilation parameters recorded during the experiment from a nasal chamber and thoracic chamber. Increasing concentration of methacholine were dissolved in PBS and aerosolized12, 13 to induce airway smooth muscle contraction for one minute. Measurements were carried out for three minutes and the obtained sRaw values were plotted against the concentration of methacholine.
Drug pharmacokinetic studies, rotarod studies and patch clamp studies with transient transfected cells
(see Supporting Information)
Automated patch-clamp with CD4+ T-lymphocytes
Four BALB/c mouse spleens were isolated and excised through a strainer using the plunger of a syringe. The cells were washed through the strainer with PBS and centrifuged for 5 min at 1,600 rpm. After aspiration of the supernatant the cell pellet was resuspended in BD Pharm Lyse lysing solution (BD Biosciences, San Jose, CA), followed by incubation (2 min at 37°C), addition of 30 ml PBS and centrifugation (5 minutes at 1,600 rpm). CD4+ T cells were isolated from splenocytes using an Affymetrix eBiosciences MagniSort mouse CD4+ T cell enrichment kit following manufacturer’s instructions (Thermo Fisher Scientific Inc., Rockford, IL). After isolation, cells were centrifuged at 380g for 2 min and gently suspended in extracellular solution (in mM: NaCl 140, KCl 5, CaCl2 2, MgCl2 1, glucose 5, HEPES 10, pH 7.4 with NaOH) at a concentration of 5x106 cells/ml. Automated patch clamp assays were conducted with the IonFlux16 as described previously.12, 13 Briefly, the IonFlux16 plates consist of 8 patterns, each containing 8 concentration wells, 1 inlet for cell supply, 1 outlet for waste collection, and 2 traps which contain combs that can patch 20 cells per experiment (for a total of 40 cells per pattern). The inlet wells contain intracellular solution (in mM: CsCl 140, CaCl2 1, MgCl2 1, EGTA 11, and HEPES 10, pH 7.2 with CsOH). The cells were suspended in extracellular solution. The 8 concentration wells contained MIDD0301 diluted in DMSO, then diluted in ECS with a final DMSO concentration of 0.1%. Cells are captured in the traps through a pulse of suction, then whole cell recording access is obtained through a second strong pulse of suction which breaks the membrane. Compound application is achieved by applying pressure onto the appropriate well. Cells are voltage clamped at a holding potential of −80 mV.
Quantification of airway inflammatory cells
Bronchoalveolar lavage (BAL) of Balb/c mice was performed with 1 mL of Ca2+ and Mg2+ free PBS. Red blood cells (RBCs) were lysed using BD red blood cell lysis buffer (BD Pharmingen, San Jose, CA). BALF was split into four different tubes and nonspecific binding to Fc receptors was blocked for 5 min using 6 μg/mL of 2.4G2 mouse BD Fc Block (BD Pharmingen, San Jose, CA). BALF cells were stained for 30 min at 4°C in the dark with 100 μL of BSA stain buffer (BD Pharmingen, San Jose, CA) containing the final concentrations of the following antibodies: anti-mouse CD45 APC (1:1000, 30- F11, Affymetrix eBiosciences, San Diego, CA), FITC rat anti-mouse CD4 (1:500, RM4–5, BD Pharmingen, San Jose, CA), FITC anti-mouse F4/80 (1:200, M1/70 Affymetrix eBiosciences, San Diego, CA) and PE rat anti-mouse Siglec-F (1:500, E50-2440, BD Pharmingen, San Jose, CA). Flow cytometric studies were done using the BD FACS Calibur (BD Pharmingen, San Jose, CA). Dead cells were excluded using the live/dead propidium iodide viability stain (BD Pharmingen, San Jose, CA). Data was analyzed subsequently using Cell Quest pro software (BD Pharmingen, San Jose, CA). Gating strategies for the different markers and treatment groups are shown in Supporting Information. The total inflammatory cell count was obtained by running all samples on high (60 μL/min) for 180 s. The gated anti-mouse CD45 positive events in the fourth channel (FL4) were used to calculate the total inflammatory cell count as cells/mL. The frequencies of CD4+, F4/80+ and Siglec-F+ cell populations in their respective gates were multiplied by the total inflammatory cell count (cells/mL) to obtain the differential cell population.
EdU (5-ethynyl-2′-deoxyuridine) staining
In separate treatment groups (100 mg/kg MIDD0301 and vehicle treated ova s/c mice), mice received a single i.p. injection of EdU (Invitrogen, Carlsbad, CA) at a dose of 100 mg/kg before ketamine/xylazine overdose. Mice were euthanized 4 hours after injection and lungs were harvested, formalin fixed, and paraffin embedded. 6 μm lung sections were mounted onto Fisher Superfrost Plus Slides. EdU staining was conducted using Click-iT™ EdU imaging kit (Invitrogen, Carlsbad, CA) per manufacturer’s instructions. Briefly lungs were deparaffinized in Histoclear and rehydrated in graded ethanol. Tissue sections were washed twice with 3% BSA (bovine serum albumin) in PBS followed by 0.5% Triton X-100 in PBS for 20 minutes. The permeabilized tissue slices were washed two times with 3% BSA in PBS followed by incubation with a Click-iT™ reaction cocktail containing Click-iT™ reaction buffer, copper sulfate, Alexa Fluor® 488 azide for 30 minutes in the dark. After that procedure, the tissue slices were treated with 3% BSA in PBS. In order to stain DNA, tissue slices were washed with PBS once followed by 30 minute incubation with 5 μg/mL Hoechst 33342. The sections were washed two times with PBS and cover slipped with Permount mounting media. These steps were all carried out at ambient temperature.
Histopathological analysis of lung sections
Lung perfusion using 10 % neutral buffered cold formalin was carried out after the collection of BALF using a tracheal cannula. The trachea was closed using a suture, removed from the animals, and fixed for two days at four degrees. The left lobe of the lung was cut transversely and samples were submitted for dehydration, paraffin embedding and sectioning. Six μm slices were put onto positive charged glass, dewaxed with Histoclear and rehydrated with water:ethanol mixtures. Oxidation was carried out with periodic acid (1%) followed by a twenty minute incubation with fluorescent Schiff’s reagent. Water followed by acidic alcohol was used as wash followed by cover-slipping using Canada Balsam and methyl salicylate for mounting.29, 30 Stained slides were investigated using a fluorescence microscope (EVOS, Invitrogen). Random images were analyzed using Image J software to quantify the mucin volume density by measuring the area of the stained glycoprotein in relationship to the length of the basement of the membrane. 29, 30 All images were scaled to the same amplification.
Cytokine expression
Balb/c mouse lungs were isolated, snap frozen in liquid nitrogen, and stored at −80 °C until cytokine analysis. Whole lung was homogenized in 200 μL of T-PER® tissue protein extraction reagent (Thermo Fisher Scientific Inc., Rockford, IL) containing 1x protease inhibitor cocktail using a hand-held tissue homogenizer. Homogenized lung samples were centrifuged at 10,000 RPM for 5 minutes to pellet cell/tissue debris. Tissue supernatant was collected for cytokine analysis using BD cytometric bead array mouse Th1/Th2/Th17 cytokine kit (BD Biosciences, San Jose, CA) following manufacturer’s instructions. Samples were analyzed using FACSCalibur™ (BD Bioscience, San Jose, CA) flow cytometry and CELLQuest™ Software and FCAP Array™ Software (BD Bioscience, San Jose, CA). Individual cytokine concentrations were indicated by their fluorescence intensities.
Statistical Analysis
GraphPad (GraphPad Software, San Diego, CA) was used to analyze data. Statistical analysis was carried out using ANOVA. A Dunnet or Tukey test was used as post hoc test. Multiple parameters were analyzed using a two-way ANOVA followed by a Bonferroni post hoc test. Two groups were compared using a Student’s test and p< 0.05 was defined as statistically significant.
RESULTS
MIDD0301 effectively relaxes airway smooth muscle
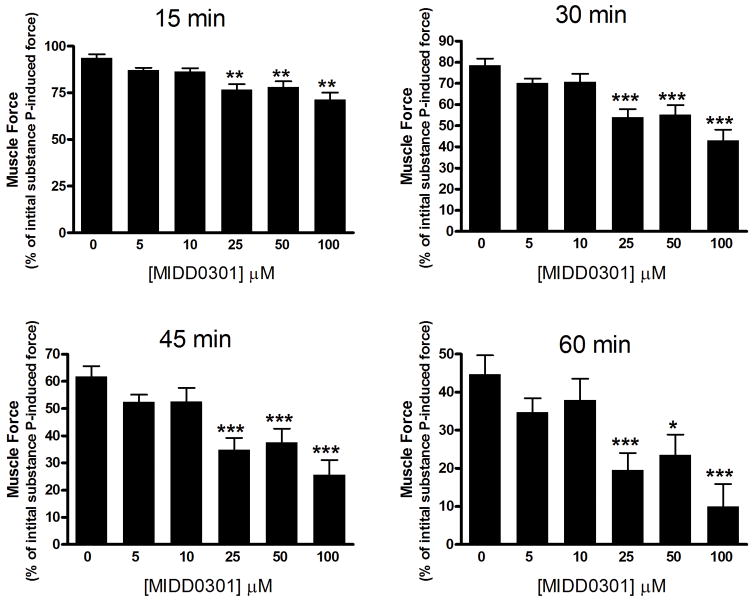
The GABAAR α4 and α5 subunits were identified previously in human and guinea pig airway smooth muscle.18 Furthermore, these GABAAR can be targeted with subtype selective ligands to mediate smooth muscle relaxation.13, 19 We assessed the smooth muscle relaxation potency of MIDD0301 in guinea pig tracheal rings ex vivo contracted with substance P. Direct action of substance P on the neurokinin 1 receptor (NK1R) can lead to a series of GPCR signaling events including Gq coupling, phospholipase C activation, formation of IP3, and DAG, with subsequent Ca2+ mobilization.31 A mechanism of smooth muscle contraction involving a decrease in membrane K+ permeability with subsequent membrane depolarization has also been previously proposed.32 The activity of MIDD0301 is depicted in Figure 2.
Figure 2.
Muscle force in guinea pig airway smooth muscle contracted with 1 μM substance P. MIDD0301 (0–100 μM) induced a significant relaxation of substance P-contracted guinea pig tracheal rings compared to vehicle (0.1% DMSO). Muscle force is expressed as a percent of the initial muscle remaining at various time points. A two-way ANOVA repeated measures analysis was used to determine significance with *, **, and *** equals p < 0.05, 0.01, or 0.001, respectively, compared to vehicle control (n = 33).
The study revealed that significant relaxation of pre-contracted guinea pig smooth muscle occurred at concentrations of 25 μM MIDD0301 and higher. The relaxation effect increased in magnitude over the 60 minute assay period. During the course of the assay, the contractile force induced by substance P diminished (Figure 2, 0 μM MIDD0301). MIDD0301 achieved a more pronounced smooth muscle relaxation than compound 2 using this assay.13
Oral administration of MIDD0301 reduced airway hyperresponsiveness
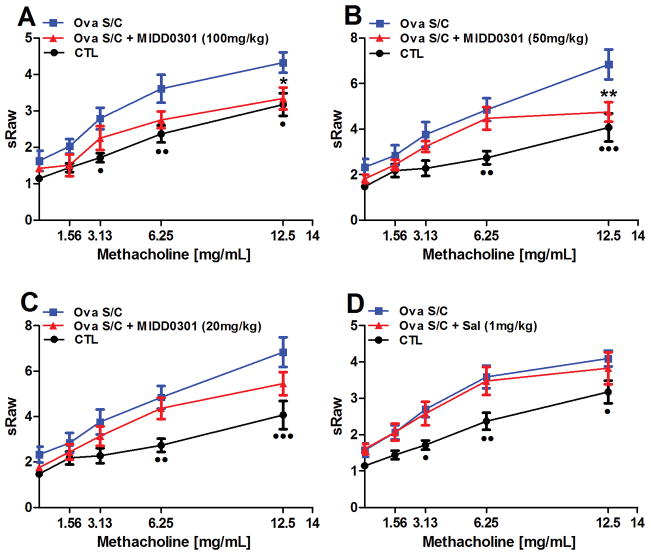
A cardinal measure of asthma severity is airway hyperresponsiveness (AHR) to broncho-constricting agents. A non-invasive airway mechanics instrument was used to quantify AHR in conscious, spontaneously breathing animals treated with increasing doses of methacholine. Consistent with previously published results,12–14 ova s/c mice exhibited higher specific airway resistance (sRaw) values in comparison to control animals. The significance between sRaw values of control and ova s/c mice varied at different methacholine concentrations (Figure 3).
Figure 3.
Effect of orally administrated MIDD0301 and salmeterol on airway hyperresponsiveness. Specific airway resistance (sRaw) was measured at increasing nebulized dosages of methacholine by DSI’s Buxco FinePointe noninvasive airway mechanics instrument (NAM). Ova s/c mice were administered vehicle or (A) 100 mg/kg, (B) 50 mg/kg, (C) 20 mg/kg of MIDD0301 via oral gavage b.i.d. for 5 days or (D) salmeterol also via oral gavage at 1 mg/kg b.i.d. for 5 days. Means ± SEM are presented for groups of 10 BALB/c mice. * and ** indicate p < 0.05 and p < 0.01 significance for the MIDD0301 group and •, ••, and ••• indicate p < 0.05, p < 0.01, and p < 0.001 significance between control mice compared to ova s/c mice using a two-way ANOVA repeated measures.
Treatment of ova s/c mice orally with 100 mg/kg MIDD0301 b.i.d. significantly reduced AHR at 12.5 mg/ml nebulized methacholine (Figure 3, A). At this concentration, the significance between non-asthmatic mice (CTL) and treated asthmatic mice (ova s/c MIDD0301 (100 mg/kg) compared to the non-treated asthmatic mice (ova s/c) was the same (p < 0.5 for • and *). A similar effect was observed with the oral dosage of 50 mg/kg MIDD0301 b.i.d. (Figure 3, B), but with increased significance for control and treatment groups. Reducing the oral dose to 20 mg/kg reflected a downward trend for the sRaw value at 12.5 mg/ml methacholine (p value of 0.507; Figure 3, C). Furthermore, we compared MIDD0301 with LABA salmeterol using the same route and frequency of administration at 1 mg/kg (Figure 3D). Interestingly, no anti-AHR effect was observed for this approved inhaled asthma medication. In addition, a single high oral dose of salmeterol at 10 mg/kg was given acutely before the measurement without any significant effect on AHR (data not shown).
MIDD0301 is well distributed but with limited CNS exposure
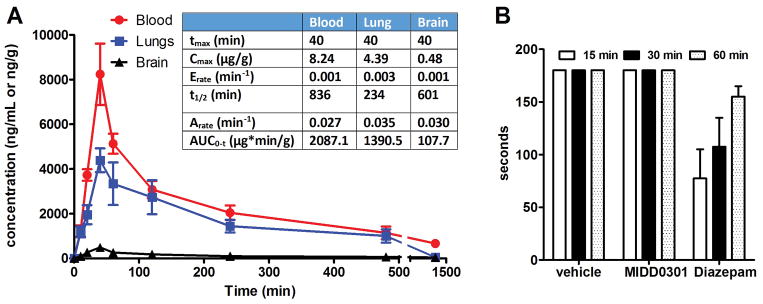
The pharmacokinetic profile of MIDD0301 was investigated in mice over a period of 24 hours. The concentrations of MIDD0301 were quantified by LCMS/MS in blood, brain, and lung following a single oral administration of 25 mg/kg (Figure 4).
Figure 4.
Pharmacokinetic profile of MIDD0301 in mouse blood, lungs, and brain. A) Time-dependent systemic distribution of MIDD0301 (25 mg/kg via oral gavage, vehicle 2% hydroxypropyl methylcellulose and 2.5% polyethylene glycol) and summary of calculated pharmacokinetic parameters; B) Sensorimotor coordination study using a rotarod apparatus with mice treated orally with 100 mg/kg MIDD0301 (vehicle 2% hydroxypropyl methylcellulose and 2.5% polyethylene glycol) (n = 9). 5 mg/kg diazepam was administered i.p. (vehicle 5% DMSO, 35% propylene glycol and 60% PBS) as control. The time that each treated mouse remained balanced on the rotating rod (15 rpm for up to 3 minutes) was recorded.
Within 40 minutes (Tmax), MIDD0301 reached maximum absorption in blood and lung. The Cmax for blood was 8.24 μg/g and 4.39 μg/g for lungs. The compound was well distributed in blood and lung with an AUC of 2087.1 and 1390.5 μg*min/g, respectively. The rate of elimination for MIDD0301 in the blood was slow at 0.001 min−1 resulting in a long half-life of 836 minutes. The elimination rate of MIDD0301 was somewhat faster in lung tissue. Further in vitro microsomal stability studies revealed that MIDD0301 is significantly more stable in human (t1/2 = 1546 minutes) than in mouse (t1/2 = 549 minutes) (Supporting Information). The mouse plasma protein binding of MIDD0301 is 88% (Supporting Information). Poor blood brain barrier penetration of MIDD0301 resulted in an extremely low AUC of 107.7 μg*min/g in the brain. Although this study showed negligible brain exposure to the compound, the absence of any possible adverse CNS effects such as sedation or ataxia caused by MIDD0301 was confirmed by a rotarod study (Figure 4, B). Here, groups of mice were treated orally with vehicle or 100 mg/kg of MIDD0301 and evaluated on a rotating rod for periods of three minutes at three different time points. All treated mice were able to successfully stay on the rotating rod during these time periods confirming the absence of sensorimotor inhibition by MIDD0301 in contrast to brain permeable positive control diazepam.
MIDD0301 is acting through the GABAAR
Immune cells express multiple GABAAR subunits and react electrophysically when exposed to GABA and GABAAR ligands.22–24 Recently we showed that α4β3γ2, and to a lesser extent α5β3γ2, subtype selective GABAAR ligands increase the current response of T lymphocytes in the presence of GABA.12, 13 Accordingly, we investigated the dose dependent electrophysiological effect of MIDD0301 on CD4+ T lymphocytes isolated from ova s/c mouse spleen using automated patch clamp (Figure 5).
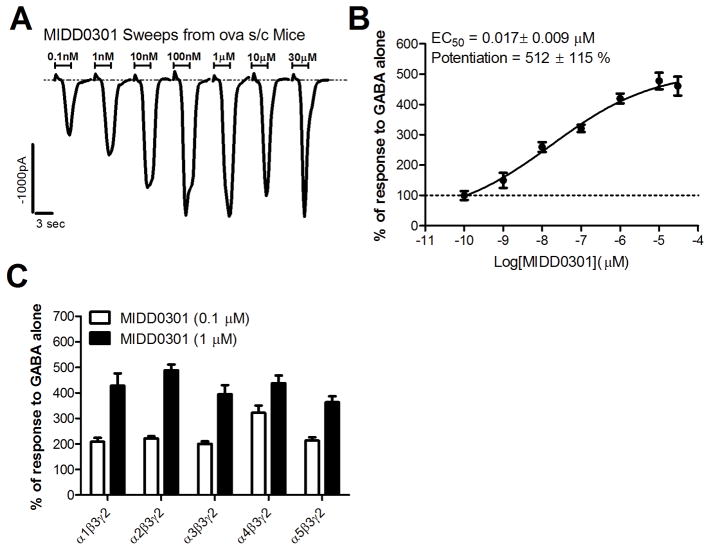
Figure 5.
Induced electrophysiological responses by MIDD0301. A) Current responses of CD4+ T lymphocytes isolated from ova s/c mice (n = 12) in the presence of 600 nM GABA and increasing concentration of MIDD0301 applied for 3 seconds, as determined by automated patch clamp. B) Normalized current responses of isolated CD4+ T lymphocytes (ova s/c mice) in the presence of 600 nM GABA (100 %) and increasing concentrations of MIDD03101 for eight independent measurements with an n = 12. Data was fitted to a Y=Bottom + (Top-Bottom)/(1+10^((LogIC50-X)*HillSlope)) to determine EC50 and top of the curve (potentiation); C) Average enhancement of current evoked to GABA by 0.1 μM or 1 μM of MIDD0301 using transiently transfected cells with α GABAAR subunits, as indicated, along with β3 and γ2L subunits measured by patch clamp. Data represent mean ± SEM with an n = 5.
MIDD0301 potentiated a current response in CD4+ T cells at very low concentrations in the presence of 600 nM GABA, exhibiting a fast on-rate and rapid current decrease during the washout phase (Figure 5, A). The current change for MIDD0301 saturated at a concentration of 100 nM. The data showed an EC50 of 17 nM for MIDD0301 and maximal potentiation of 512%. Patch clamp measurements with cells transfected with various GABAAR subunits indicated that MIDD0301 is activating GABAARs strongly among the alpha subtypes tested. Testing by the NIMH Psychoactive Drug Screening Program (PDSP) showed no significant binding at the peripheral GABAAR at 10 μM MIDD0301.33
MIDD0301 has anti-inflammatory properties in the lung
Chronic airway inflammation is a hallmark feature of asthma and can be measured by quantification of immune cell subtypes in the bronchoalveolar lavage fluid (BALF). We analyzed BALF from vehicle and MIDD0301 treated mice by flow cytometry using differential counts for eosinophils, macrophages, and CD4+ T cells, using Siglec F,34 F4/80, and CD4 antibodies, respectively. Total inflammatory cells in BALF were quantified with anti-CD45. The CD45 marker is also referred to as the leukocyte common factor, which is a 180–240 kD glycoprotein expressed on all hematopoietic cells except mature erythrocytes and platelets.35 The results are depicted in Figure 6.
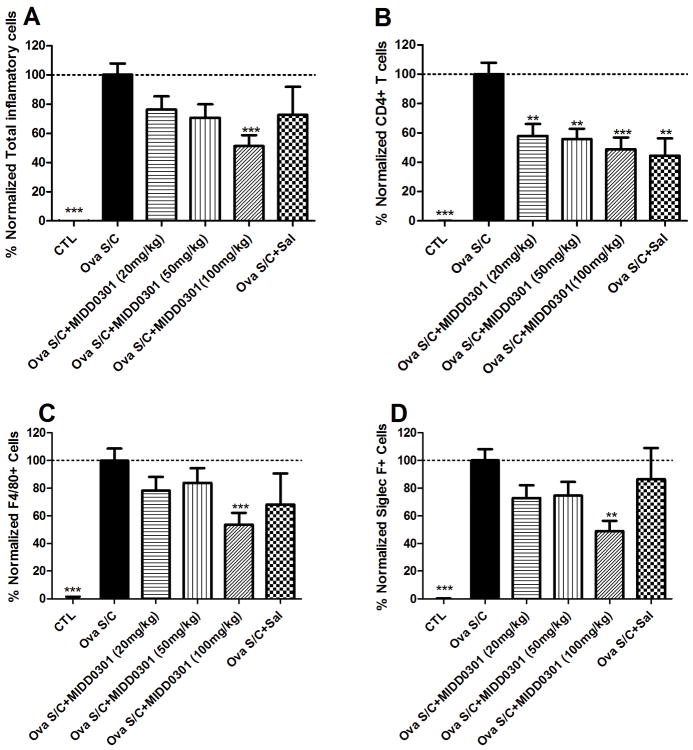
Figure 6. Effect of MIDD0301 and salmeterol on airway inflammatory cells.
Groups of ova s/c mice were administered vehicle, MIDD0301 (20, 50 or 100 mg/kg), or salmeterol (1 mg/kg) via oral gavage b.i.d. for 5 days. BALF was harvested from each animal and used for (A) quantification of total inflammatory cells using anti-CD45 APC antibody and flow cytometry. (B) CD4+ T cell, (C) F4/80+ cell, and (D) Siglec F+ cell populations were quantified by flow cytometry. Data represent mean ± SEM from 10 mice in each group. One way ANOVA was used to calculate significance indicated as *, **, and *** for p < 0.05, p < 0.01, and p < 0.001 compared to vehicle treated ova s/c mice. The gated positive events are depicted in the Supporting Information.
We observed significant suppression of total inflammatory cells in BALF following 5 day oral administration with MIDD0301 at 100 mg/kg (Figure 6, A). Salmeterol at 1 mg/kg p.o. did not significantly change the leukocyte numbers. Efficacy of oral MIDD0301 treatment was also observed for the BALF Siglec F+ cell population that include eosinophils/alveolar macrophages36 and F4/80+ cells that represent the murine macrophage population, though, only the 100 mg/kg dosage reduced their numbers significantly (Figure 6, C and D). In line with the sensitivity of CD4+ T cells toward MIDD0301 determined by patch clamp, 20, 50 and 100 mg/kg oral MIDD0301 treatments significantly reduced the number of BALF CD4+ T cells (Figure 6, B). Although salmeterol reduced BALF CD4+ T cells (Figure 4B), there was no significant effect observed for macrophage and eosinophil BALF populations (Figures 4, C and D).
Effects of MIDD0301 on the mouse lung
Lung inflammation is characterized by infiltration of leukocytes and proliferation of airway smooth muscle cells.37 To visualize this effect in the mouse lung, a thymidine analogue EdU was i.p. injected into vehicle and MIDD0301 (100 mg/kg) treated ova s/c mice and control mice, allowing its incorporation into DNA during S phase of DNA replication. Mice were euthanized after four hours and lungs slices prepared following a standard histology protocol. The EdU labelled DNA was made visible via its conjugation with an Alexa Fluor® 488 azide using a copper catalyzed “Click” reaction.38 The nuclei of all cells were counterstained with Hoechst 33342, and superimposed and individual images presented in Figure 7.
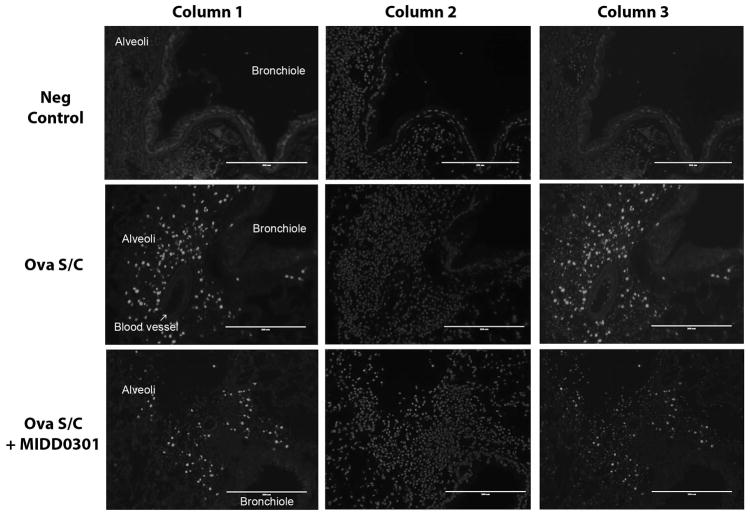
Figure 7. Cellular changes of the asthmatic mouse lung due to MIDD0301 treatment.
Representative images of lungs from mice that were injected i.p. with EdU, a thymidine analog, and harvested four hours later. After standard histology processing, sections were treated with a fluorescent azide under “Click” chemistry conditions enabling conjugation of incorporated EdU to visualize cells that underwent the S phase during a four hour period (column 1). Slides were counterstained with Hoechst 33342 (column 2) and superimposed images are presented in column 3. Row 1 presents lung images of control mice. Row 2 depicts lung images of vehicle-treated ova s/c mice and row 3 images of MIDD0301 (100 mg/kg) treated ova s/c mice.
EdU visualization of lung sections from control mice (non-asthmatic) revealed no cell staining except for faint non-specific background (Figure 7, column 1 and row 1). In contrast, lung sections from vehicle-treated ova s/c mice showed intensive staining in the alveolar region (Figure 7, column 1 and row 2). Cell layers of blood vessels (lamina and smooth muscle cells) and bronchiole (mucosa and smooth muscle cells) were not stained. Alveolar cells (pneumocytes) have not been shown to proliferate quickly in the asthmatic lung, thus infiltrating alveolar leukocytes such as eosinophils, alveolar macrophages or monocytes were probably visualized.39 Importantly, lungs sections from MIDD0301 treated ova s/c mice (Figure 7, column 1 and row 3) showed reduced cell staining in comparison to vehicle-treated ova s/c mice. Thus, the reduction of inflammatory cells observed in the BALF of MIDD0301 treated animals (Figure 6, A) is consistent with the reduction of alveolar inflammatory cells visualized by EdU.
Mucous metaplasia is unchanged for MIDD0301 treated asthmatic mice
Marked mucus accumulation is a key pathological feature of asthma resulting from mucus cell metaplasia (change in epithelial cell phenotype) and goblet cell hyperplasia (increase in goblet cell number). These histologic changes in the asthmatic lung can be visualized by staining sections with periodic acid fluorescent Schiff’s stain (Figure 8).
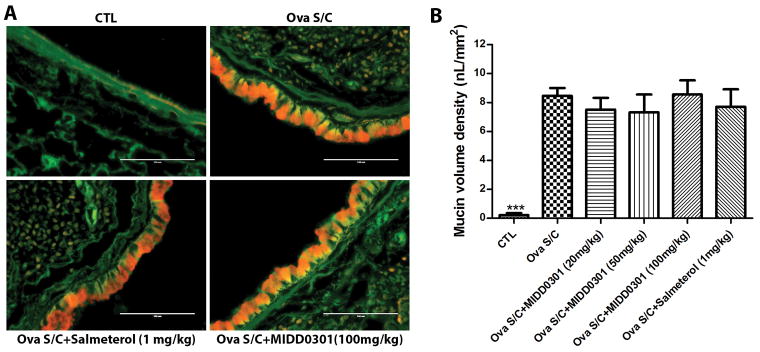
Figure 8. Effect of MIDD0301 and salmeterol on mucin production.
A) Representative images of lung section of control mice (non-asthmatic) and ova s/c mice treated orally with vehicle, MIDD0301 (100 mg/kg, b.i.d. 5 days) or salmeterol (1 mg/kg, b.i.d. 5 days). Scale bar represents 100 μm. Slices were stained with periodic acid fluorescent Schiff’s stain coloring airway epithelium green and mucin red. B) Morphometric quantification of mucin volume density. Data represent mean ± SEM mucin volume density from six mice in each group. One-way ANOVA was used for the analysis.
The stained lung sections revealed significant increases in mucous metaplasia in ova s/c mice compared to control mice (Figure 8, A and B). MIDD0301 at 20, 50 and 100 mg/kg b.i.d. for 5 days did not produce a significant change in mucous production in the airways compared to ova s/c mice. Similarly, a 5 day b.i.d treatment with salmeterol at 1 mg/kg showed no effect on mucous levels in the airways.
Cytokine expression in mouse lung homogenate is significantly reduced by MIDD0301
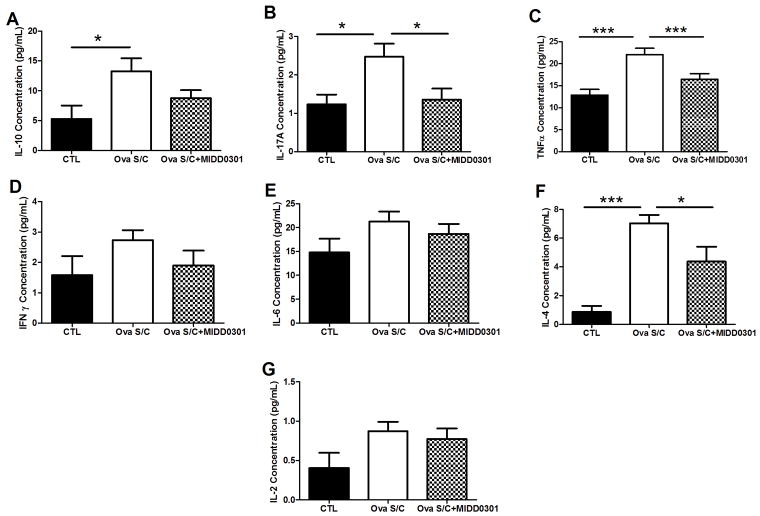
Cytokines play critical functions and serve pleiotropic roles in asthma; hence concentrations of mouse TH1 (IL-2, IFN-γ and TNFα), TH2 (IL-4, IL-6 and IL-10), and TH17 (IL-17A) cytokines were quantified in lung homogenates using flow cytometry (Figure 9).40, 41
Figure 9. Effects of MIDD0301 on cytokine expression in the lung.
Mouse Th1/Th2/Th17 cytokines were quantified in mouse tissue homogenates using the BD mouse Th1/Th2/Th17 cytometric bead array kit. Ova s/c mice were administered vehicle or 100 mg/kg MIDD0301 (vehicle 2% hydroxypropyl methylcellulose and 2.5% polyethylene glycol) via oral gavage twice daily for 5 days. Data represent mean ± SEM from 10 mice in each group. *, **, and *** indicate p < 0.05, p < 0.01, and p < 0.001 significance determined by one-way ANOVA, compared to vehicle treated ova s/c mice.
Ova s/c mice had significantly higher concentrations of IL-4, IL-10, IL-17A, and TNFα compared to control mice (Figures 6A, 6B, 6C and 6F, p<0.05). The concentrations of IL-2, IL-6, and IFN-γ in lung homogenates of ova s/c mice were not significantly different from control mice (Figures 6C, 6D, 6E and 6C). Cytokine levels that were significantly increased in the lung of ova s/c mice in comparison to the control mice, were in turn significantly reduced with the treatment of MIDD0301, except for IL-10. The cytokine concentrations that did not change between the control and ova s/c mice were not altered significantly by MIDD0301 treatments.
DISCUSSION
Among the various alpha GABAAR subunits, only α4 and α5 subunit containing GABAAR are expressed in airway smooth muscle.18 Immune cells also express receptors containing these two alpha GABAAR subunits in addition to α2 and α3.13 Thus, the presence of an overlapping subset of discrete GABAARs comprising these alpha subunits enables a novel drug design strategy to target two hallmarks of asthma: airway smooth muscle constriction and lung inflammation. To prove this rationale, prototype GABAAR ligands possessing α4β3γ2 and the α5β3γ2 efficacy were shown to alleviate both of these asthma symptoms in vitro and in vivo.13, 14 Historically, GABAAR ligands based on the benzodiazepine scaffold have been developed to treat various CNS disorders such as anxiety and seizures. A critical innovation of MIDD0301, and earlier GABAAR ligands developed for asthma, is their altered chemical structure that restricts brain exposure but facilitates pharmacological activity to readily permeable lung tissues. Pharmacokinetic studies have confirmed negligible concentrations of MIDD0301 in the brain, which is protected by tight junctions between endothelial cells creating the blood brain barrier. In addition, MIDD0301 caused no sensorimotor impairment in rotarod studies, as would be observed if CNS adverse effects were present. Furthermore, this study also showed that MIDD0301 did not diminish skeletal muscle coordination necessary for the animal to stay balanced on the rotating rod. This finding was observed for midazolam, which has been shown to block inactivated Na channels in skeletal muscle fibers.42
Importantly, MIDD0301 has been shown to relax airway smooth muscle using ex-vivo substance P mediated pre-contracted guinea pig tracheal rings. Treatment with 25 μM MIDD0301 was sufficient to partially relax muscle constriction within 15 minutes. Earlier experiments with an analog of MIDD0301 have shown that the actual concentration of compound in the trachea is only 10% of the organ bath concentration due to limited passive diffusion;13 thus MIDD0301 is pharmaceutically active at single digit micromolar concentration in this assay. Furthermore, the effect of MIDD0301 is reversible, as demonstrated by observing similar contraction of ASM that was washed with buffer and contracted again with substance P (see Supporting Information, Figure S7). The increased potency of MIDD0301 in comparison to previous GABAAR ligands developed for asthma was also observed by the alleviation of the ova s/c induced asthma phenotype in BALB/c mice. Oral treatment of MIDD0301 at 50 mg/kg b.i.d. for 5 days was sufficient to overcome induced AHR at a 12.5 mg/kg dose of nebulized methacholine. The therapeutic effective dose range of MIDD0301 is between 20 and 50 mg/kg because 20 mg/kg p.o. did not reduce AHR significantly after the 5 day b.i.d. treatment.
Another critical feature of asthma is persistent goblet cell hyperplasia, mucus cell metaplasia, and mucus hypersecretion.43 Mucus hypersecretion from hyperplastic goblet cells causes mucus plugging, particularly in the peripheral airways and is a major pathologic finding in asthma mediated deaths.44, 45 Ovalbumin and nicotine exposure have shown to induce mucus secretion and increased expression of glutamic acid decarboxylase (GAD) and GABAAR subunits in lung epithelia cells.21, 46–48 In these studies, bicuculline, a GABAAR antagonist that also blocks small-conductance calcium-activated potassium channels,49 has been shown to reduce GABA-induced transmembrane current and mucin 5A expression in lung epithelia cells, as well as mucus secretion and AHR. The administration of imidazobenzodiazepine GABAAR ligands, however, did not alter the mucus production in the lung of ova s/c mice at 100 mg/kg.12, 13 Similar results were obtained with MIDD0301 when administrated at 20, 50 or 100 mg/kg b.i.d. for 5 days.
A major pathological feature of persistent asthma is chronic allergic inflammation leading to airway eosinophilia.50 We demonstrated a significant reduction for BALF eosinophil numbers in ova s/c mice following MIDD0301 treatment with 100 mg/kg twice daily for 5 days. In asthma, airway macrophages are one of the major cell types involved in the chronic inflammatory process and can be divided into three classes: bronchial macrophages (BMs), alveolar macrophages (AMs), and interstitial macrophages (IMs).51 MIDD0301 at 100 mg/kg b.i.d. for 5 days reduced the number of macrophages in the BALF of ova s/c mice. Cell staining with EdU confirmed the reduction of cells the alveoli region of the lung, which might be recruited eosinophils, alveolar macrophages or monocytes, because resident alveolar macrophage proliferate very slowly or not at all.52 Further studies will be conducted to confirm this hypothesis.
Lipids, such as prostaglandins, play an important role in lung inflammation and asthma.53 Many drug candidates have been developed for lung inflammation that inhibit prostaglandin synthesis or binding to their corresponding receptors. Singulair, a leukotriene receptor antagonist is one example.54 Further studies on the pharmacological effects of MIDD0301 will investigate possible changes in inflammatory lipid homeostasis.
In contrast to compound 2, inflammation is reduced by MIDD0301 by direct interaction with CD4+ T cells as demonstrated by potentiation of its transmembrane current in the presence of GABA. We hypothesize that this effect is mediated by the ability of MIDD0301 to activate the α2 subunit containing GABAAR, which has been identified on CD4+ T cells.55 In vivo, airway CD4+ T cell numbers were significantly reduced in ova s/c mice following treatment with 20 mg/kg MIDD0301 b.i.d. for 5 days. In asthma, CD4+T cells produce several TH2 interleukins such as IL-4, IL-5, and IL-13.56 As expected, IL-4 levels in lung homogenates were reduced with the treatment of MIDD0301 in comparison to vehicle-treated ova s/c mice. IL-4 is a key cytokine in the development of allergic inflammation and major mediator of isotype switching and secretion of IgE, which in turn promotes eosinophil transmigration across endothelium, mucus secretion, and differentiation of TH2 lymphocytes leading to cytokine release.57 Activated CD4+ T cells also produce IL-17, which mediates multiple aspects of asthma pathogenesis and has been found in extremely high levels in sputum and bronchial biopsies of patients with severe asthma.58 Importantly, we demonstrated that MIDD0301 treatment significantly reduced IL-17A levels in lung homogenates of ova s/c mice. Low IL-17 levels might be one reason for decreased lung TNF-α levels observed for MIDD0301 treated ova s/c mice due to the fact that IL-17 has been shown to stimulate TNF-α expression by macrophages.59 The lower number of macrophages found in BALF of MIDD0301 treated ova s/c mice might contribute to the reduction of TNF-α. Low TNF-α levels are important therapeutically, because emerging evidence suggests that this pro-inflammatory cytokine plays an important role in severe refractory disease and in many aspects of the airway pathology of asthma.60 The modulation of other cytokines such as IFN-γ, IL-6, and IL-2 is unclear because the ova s/c phenotype did not demonstrate levels significantly different from control mice. Importantly, the anti-inflammatory cytokine IL-10 expression in the lung was not significantly altered by MIDD0301 treatment.
In conclusion, following earlier development of α4β3γ2 and the α5β3γ2 selective GABAAR ligands to treat asthma, we now report a more potent, orally available asthma drug candidate MIDD0301. This compound has significantly improved anti-inflammatory properties in the lung in addition to its ability to rapidly relax constricted airway smooth muscle at low concentrations. Current studies are focused on better understanding the respiratory immune modulating effects of this novel class of compounds in addition to future IND enabling studies with MIDD0301.
Supplementary Material
Acknowledgments
Funding Sources. This work was supported by the University of Wisconsin-Milwaukee, the NIH R03DA031090 (L.A.A.), R01MH096463 (J.M.C.,.A.A.), R01NS076517 (J.M.C., L.A.A.), R01GM065281 (C.W.E., G.T.Y., J.M.C.), R01HL118561 (J.M.C., L.A.A., C.W.E., D.C.S.), and R01HL122340 (C.W.E.) as well as the University of Wisconsin Milwaukee Research Foundation (Catalyst grant), the Richard and Ethel Herzfeld Foundation, the Lynde and Harry Bradley Foundation, the Foundation for Anesthesia Education and Research (G.T.Y.) and the Stony Wold-Herbert Fund (G.T.Y.).
We thank Dr. Beryl R. Forman and Jennifer L. Nemke (Animal Facility at UWM) for their guidance and support.
Abbreviation
- GABAAR
GABAA receptor
- AHR
airway hyperresponsiveness
- CNS
central nervous system
- NMR
nuclear magnetic resonance
- HRMS
high resolution mass spectrometry
- ECS
external cell solution
- ICS
internal cell solution
- DMSO
dimethylsulfoxide
- BALF
bronchoalveolar lavage fluid
- GABA
gamma aminobutyric acid
- sRaw
specific airway resistance
- PK
pharmacokinetic studies
- LABA
long acting β2 adrenergic receptor agonists
- NK1R
neurokinin 1 receptor
- BM
bronchial macrophages
- AM
alveolar macrophages
- IM
interstitial macrophages
- PAFS
Periodic Acid Fluorescent Schiff’s Stain
Footnotes
Supporting Information. The Supporting Information includes Supplemental Experimental Procedures and six figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Pascual RM, Peters SP. Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol. 2005;116(3):477–86. doi: 10.1016/j.jaci.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ Centers for Disease C, Prevention. National surveillance for asthma--United States 1980–2004. MMWR Surveill Summ. 2007;56(8):1–54. [PubMed] [Google Scholar]
- 3.Masoli M, Fabian D, Holt S, Beasley R Global Initiative for Asthma P. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59(5):469–78. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 4.Myers TR. Guidelines for asthma management: a review and comparison of 5 current guidelines. Respir Care. 2008;53(6):751–67. [PubMed] [Google Scholar]
- 5.Kelloway JS, Wyatt RA, Adlis SA. Comparison of patients’ compliance with prescribed oral and inhaled asthma medications. Arch Intern Med. 1994;154(12):1349–52. [PubMed] [Google Scholar]
- 6.Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. J Asthma. 2003;40(1):93–101. doi: 10.1081/jas-120017212. [DOI] [PubMed] [Google Scholar]
- 7.Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, Wang J, Sylvester J, Holbrook J, Wise R, Weiss ST, Barnes K. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173(4):379–85. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Pineiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130(6):487–95. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Israel E, Chervinsky PS, Friedman B, Van Bavel J, Skalky CS, Ghannam AF, Bird SR, Edelman JM. Effects of montelukast and beclomethasone on airway function and asthma control. J Allergy Clin Immunol. 2002;110(6):847–54. doi: 10.1067/mai.2002.129413. [DOI] [PubMed] [Google Scholar]
- 10.Schumann C, Kropf C, Wibmer T, Rudiger S, Stoiber KM, Thielen A, Rottbauer W, Kroegel C. Omalizumab in patients with severe asthma: the XCLUSIVE study. Clin Respir J. 2012;6(4):215–27. doi: 10.1111/j.1752-699X.2011.00263.x. [DOI] [PubMed] [Google Scholar]
- 11.Abonia JP, Putnam PE. Mepolizumab in eosinophilic disorders. Expert Rev Clin Immunol. 2011;7(4):411–7. doi: 10.1586/eci.11.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forkuo GS, Guthrie ML, Yuan NY, Nieman AN, Kodali R, Jahan R, Stephen MR, Yocum GT, Treven M, Poe MM, Li G, Yu OB, Hartzler BD, Zahn NM, Ernst M, Emala CW, Stafford DC, Cook JM, Arnold LA. Development of GABAA Receptor Subtype-Selective Imidazobenzodiazepines as Novel Asthma Treatments. Mol Pharm. 2016;13(6):2026–38. doi: 10.1021/acs.molpharmaceut.6b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forkuo GS, Nieman AN, Yuan NY, Kodali R, Yu OB, Zahn NM, Jahan R, Li G, Stephen MR, Guthrie ML, Poe MM, Hartzler BD, Harris TW, Yocum GT, Emala CW, Steeber DA, Stafford DC, Cook JM, Arnold LA. Alleviation of Multiple Asthmatic Pathologic Features with Orally Available and Subtype Selective GABAA Receptor Modulators. Mol Pharm. 2017;14(6):2088–2098. doi: 10.1021/acs.molpharmaceut.7b00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahan R, Stephen MR, Forkuo GS, Kodali R, Guthrie ML, Nieman AN, Yuan NY, Zahn NM, Poe MM, Li G, Yu OB, Yocum GT, Emala CW, Stafford DC, Cook JM, Arnold LA. Optimization of substituted imidazobenzodiazepines as novel asthma treatments. Eur J Med Chem. 2017;126:550–560. doi: 10.1016/j.ejmech.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olsen RW, Sieghart W International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60(3):243–60. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortensen M, Patel B, Smart TG. GABA Potency at GABA(A) Receptors Found in Synaptic and Extrasynaptic Zones. Front Cell Neurosci. 2011;6:1–10. doi: 10.3389/fncel.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clayton T, Poe MM, Rallapalli S, Biawat P, Savic MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook JM. A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model. Int J Med Chem. 2015:430248. doi: 10.1155/2015/430248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallos G, Yim P, Chang S, Zhang Y, Xu D, Cook JM, Gerthoffer WT, Emala CW., Sr Targeting the restricted alpha-subunit repertoire of airway smooth muscle GABAA receptors augments airway smooth muscle relaxation. Am J Physiol Lung Cell Mol Physiol. 2012;302(2):248–56. doi: 10.1152/ajplung.00131.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallos G, Yocum GT, Siviski ME, Yim PD, Fu XW, Poe MM, Cook JM, Harrison N, Perez-Zoghbi J, Emala CW., Sr Selective targeting of the alpha5-subunit of GABAA receptors relaxes airway smooth muscle and inhibits cellular calcium handling. Am J Physiol Lung Cell Mol Physiol. 2015;308(9):931–42. doi: 10.1152/ajplung.00107.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW., Sr GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):1206–16. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13(7):862–7. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 22.Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW, Steinman L. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107(6):2580–5. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjurstom H, Wang J, Ericsson I, Bengtsson M, Liu Y, Kumar-Mendu S, Issazadeh-Navikas S, Birnir B. GABA, a natural immunomodulator of T lymphocytes. J Neuroimmunol. 2008;205(1–2):44–50. doi: 10.1016/j.jneuroim.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Dionisio L, Jose De Rosa M, Bouzat C, del Esandi MC. An intrinsic GABAergic system in human lymphocytes. Neuropharmacology. 2011;60(2–3):513–9. doi: 10.1016/j.neuropharm.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Tian J, Lu Y, Zhang H, Chau CH, Dang HN, Kaufman DL. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J Immunol. 2004;173(8):5298–304. doi: 10.4049/jimmunol.173.8.5298. [DOI] [PubMed] [Google Scholar]
- 26.Cook CM, Clayton T, Jain HD, Rallipalli SK, Johnson YT, Yang J, Poe MM, Namjoshi O, Wang Z. GABAergic receptor subtype selective ligands and their uses. 20120295892 US. 2012
- 27.Forkuo GS, Kim H, Thanawala VJ, Al-Sawalha N, Valdez D, Joshi R, Parra S, Pera T, Gonnella PA, Knoll BJ, Walker JK, Penn RB, Bond RA. Phosphodiesterase 4 Inhibitors Attenuate the Asthma Phenotype Produced by beta2-Adrenoceptor Agonists in Phenylethanolamine N-Methyltransferase-Knockout Mice. Am J Respir Cell Mol Biol. 2016;55(2):234–42. doi: 10.1165/rcmb.2015-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res. 2007;8:63. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31(4):382–94. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol. 2012;842:279–95. doi: 10.1007/978-1-61779-513-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Recio S, Gascon P. Biological and Pharmacological Aspects of the NK1-Receptor. Biomed Res Int. 2015:495704. doi: 10.1155/2015/495704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall JM, Morton IK. Mechanism of action of substance P in guinea-pig ileum longitudinal smooth muscle: a re-evaluation. J Physiol. 1990;431:529–41. doi: 10.1113/jphysiol.1990.sp018345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492(7428):215–20. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JQQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Glycobiology. 2004;14(11):1175–1184. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 35.Yu YR, O’Koren EG, Hotten DF, Kan MJ, Kopin D, Nelson ER, Que L, Gunn MD. A Protocol for the Comprehensive Flow Cytometric Analysis of Immune Cells in Normal and Inflamed Murine Non-Lymphoid Tissues. PLoS One. 2016;11(3):e0150606. doi: 10.1371/journal.pone.0150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens WW, Kim TS, Pujanauski LM, Hao X, Braciale TJ. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327(1–2):63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164(3):474–7. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 38.Zeng C, Pan F, Jones LA, Lim MM, Griffin EA, Sheline YI, Mintun MA, Holtzman DM, Mach RH. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8(3):183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 40.Wei H, Tan K, Sun R, Yin L, Zhang J, Pu Y. Aberrant production of Th1/Th2/Th17-related cytokines in serum of C57BL/6 mice after short-term formaldehyde exposure. Int J Environ Res Public Health. 2014;11(10):10036–50. doi: 10.3390/ijerph111010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, Lowe L, Chen R, Shivraj L, Agadir A, Campos R, Ernst D, Gaur A. Cytometric bead array: a multiplexed assay platform with applications in various areas of biology. Clin Immunol. 2004;110(3):252–66. doi: 10.1016/j.clim.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Duval A, Malecot CO, Perchenet L, Piek T. The benzodiazepine midazolam preferentially blocks inactivated Na channels in skeletal muscle fibre. Naunyn Schmiedebergs Arch Pharmacol. 1993;347(5):541–7. doi: 10.1007/BF00166748. [DOI] [PubMed] [Google Scholar]
- 43.Curran DR, Cohn L. Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease. Am J Respir Cell Mol Biol. 2010;42(3):268–75. doi: 10.1165/rcmb.2009-0151TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101(4):916–21. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 45.Rao M, Kravath RE, Abadco D, Arden J, Steiner P. Childhood asthma mortality: the Brooklyn experience and a brief review. J Assoc Acad Minor Phys. 1991;2(3):127–30. [PubMed] [Google Scholar]
- 46.Fu XW, Wood K, Spindel ER. Prenatal Nicotine Exposure Increases GABA Signaling and Mucin Expression in Airway Epithelium. American Journal of Respiratory Cell and Molecular Biology. 2011;44(2):222–229. doi: 10.1165/rcmb.2010-0109OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo Z, Costy-Bennett S, Fregosi RE. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. Journal of Physiology-London. 2004;561(2):387–393. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gundavarapu S, Wilder JA, Mishra NC, Rir-Sima-Ah J, Langley RJ, Singh SP, Saeed AI, Jaramillo RJ, Gott KM, Pena-Philippides JC, Harrod KS, McIntosh JM, Buch S, Sopori ML. Role of nicotinic receptors and acetylcholine in mucous cell metaplasia, hyperplasia, and airway mucus formation in vitro and in vivo. J Allergy Clin Immunol. 2012;130(3):770–780. doi: 10.1016/j.jaci.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch. 1999;438(3):314–21. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- 50.Possa SS, Leick EA, Prado CM, Martins MA, Tiberio IF. Eosinophilic inflammation in allergic asthma. Front Pharmacol. 2013;4:46. doi: 10.3389/fphar.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5(6):605–9. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 52.Chanez P, Vago P, Demoly P, Cornillac L, Godard P, Bureau JP, Michel FB, Bousquet J. Airway macrophages from patients with asthma do not proliferate. J Allergy Clin Immunol. 1993;92(6):869–77. doi: 10.1016/0091-6749(93)90065-n. [DOI] [PubMed] [Google Scholar]
- 53.Claar D, Hartert TV, Peebles RS., Jr The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev Respir Med. 2015;9(1):55–72. doi: 10.1586/17476348.2015.992783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, Becker A. Montelukast for chronic asthma in 6- to 14-year-old children: a randomized, double-blind trial. Pediatric Montelukast Study Group. JAMA. 1998;279(15):1181–6. doi: 10.1001/jama.279.15.1181. [DOI] [PubMed] [Google Scholar]
- 55.Yocum GT, Turner DL, Danielsson J, Barajas MB, Zhang Y, Xu D, Harrison NL, Homanics GE, Farber DL, Emala CW. GABAA receptor alpha4-subunit knockout enhances lung inflammation and airway reactivity in a murine asthma model. Am J Physiol Lung Cell Mol Physiol. 2017;313(2):L406–L415. doi: 10.1152/ajplung.00107.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nature Reviews Immunology. 2010;10(12):838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine. 2015;75(1):89–116. doi: 10.1016/j.cyto.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Wang YH, Wills-Karp M. The potential role of interleukin-17 in severe asthma. Curr Allergy Asthma Rep. 2011;11(5):388–94. doi: 10.1007/s11882-011-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160(7):3513–21. [PubMed] [Google Scholar]
- 60.Brightling C, Berry M, Amrani Y. Targeting TNF-alpha: a novel therapeutic approach for asthma. J Allergy Clin Immunol. 2008;121(1):5–10. doi: 10.1016/j.jaci.2007.10.028. quiz 11–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.