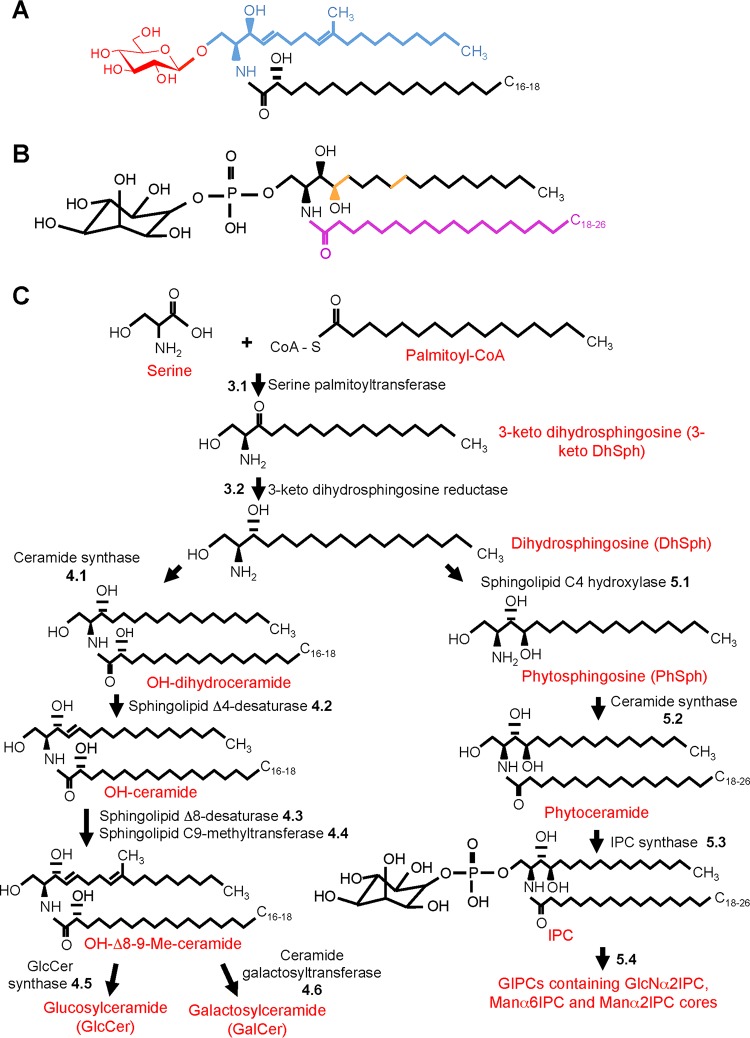
FIG 1 .
Glycosphingolipid structure and biosynthesis. (A) Basic structure of a neutral glycosphingolipid, made up of a sphingoid base (highlighted in blue) and a fatty acid chain (highlighted in black) to form ceramide, which is linked to a sugar residue (highlighted in red). (B) The structure of IPC, a simple acidic glycosphingolipid, is shown. Acidic glycosphingolipids differ from neutral glycosphingolipids in that they contain an additional -OH group at C4 of the sphingoid base and lack C9-methylation and Δ4- and Δ8-unsaturations (highlighted in orange). Another difference is that acidic glycosphingolipids are made up of a very long fatty acid (C18–26, highlighted in purple) instead of the C16–18 chain found in neutral glycosphingolipids. (C) Proposed biosynthetic pathway of glycosphingolipids. The reactions indicated by the number 3 are common to acidic and neutral GSL synthesis, while those indicated by the numbers 4 and 5 are exclusive of neutral (see Neutral GSL Synthesis in the text) and acidic (see Acidic GSL Synthesis in the text) GSLs, respectively.