ABSTRACT
Strains of Helicobacter pylori that cause ulcer or gastric cancer typically express a type IV secretion system (T4SS) encoded by the cag pathogenicity island (cagPAI). CagY is an ortholog of VirB10 that, unlike other VirB10 orthologs, has a large middle repeat region (MRR) with extensive repetitive sequence motifs, which undergo CD4+ T cell-dependent recombination during infection of mice. Recombination in the CagY MRR reduces T4SS function, diminishes the host inflammatory response, and enables the bacteria to colonize at a higher density. Since CagY is known to bind human α5β1 integrin, we tested the hypothesis that recombination in the CagY MRR regulates T4SS function by modulating binding to α5β1 integrin. Using a cell-free microfluidic assay, we found that H. pylori binding to α5β1 integrin under shear flow is dependent on the CagY MRR, but independent of the presence of the T4SS pili, which are only formed when H. pylori is in contact with host cells. Similarly, expression of CagY in the absence of other T4SS genes was necessary and sufficient for whole bacterial cell binding to α5β1 integrin. Bacteria with variant cagY alleles that reduced T4SS function showed comparable reduction in binding to α5β1 integrin, although CagY was still expressed on the bacterial surface. We speculate that cagY-dependent modulation of H. pylori T4SS function is mediated by alterations in binding to α5β1 integrin, which in turn regulates the host inflammatory response so as to maximize persistent infection.
KEYWORDS: CagA, CagY, Helicobacter pylori, integrin, pathogenicity island, type IV secretion system
IMPORTANCE
Infection with H. pylori can cause peptic ulcers and is the most important risk factor for gastric cancer, the third most common cause of cancer death worldwide. The major H. pylori virulence factor that determines whether infection causes disease or asymptomatic colonization is the type IV secretion system (T4SS), a sort of molecular syringe that injects bacterial products into gastric epithelial cells and alters host cell physiology. We previously showed that recombination in CagY, an essential T4SS component, modulates the function of the T4SS. Here we found that these recombination events produce parallel changes in specific binding to α5β1 integrin, a host cell receptor that is essential for T4SS-dependent translocation of bacterial effectors. We propose that CagY-dependent binding to α5β1 integrin acts like a molecular rheostat that alters T4SS function and modulates the host immune response to promote persistent infection.
INTRODUCTION
Helicobacter pylori infection most often causes only asymptomatic gastritis, but H. pylori is considered an important human pathogen because it is the major risk factor for development of peptic ulcer disease and gastric adenocarcinoma (1), the third most common cause of cancer death. On the other hand, H. pylori infection may also have beneficial effects, particularly prevention of chronic diseases that have increased in frequency in developed countries as the prevalence of H. pylori has declined (2). The bacterial virulence factor most strongly associated with the outcome of H. pylori infection is the cag pathogenicity island (cagPAI), an ~40-kb DNA segment that encodes a type IV secretion system (T4SS). When H. pylori comes in contact with the gastric epithelium, it assembles the T4SS pilus (3), through which it injects the CagA oncoprotein into host cells (4). Other T4SS-dependent effectors have also been identified, including DNA (5), peptidoglycan (6), and heptose-1,7-bisphosphate, a metabolic precursor in lipopolysaccharide biosynthesis (7–9). Together, T4SS injection of effector molecules results in complex changes in host cell physiology that include cytoskeletal rearrangements, disruption of tight junctions, loss in cell polarity, and production of interleukin-8 (IL-8) and other proinflammatory cytokines (4, 10).
Host cell expression of β1 integrins is required for T4SS-dependent translocation of CagA (11, 12) and presumably other effectors as well. Four cagPAI proteins essential for T4SS function have been found to bind β1 integrins, although the details are unclear and some reports are contradictory. The first to be described was CagL, an RGD-dependent ligand for α5β1 integrin that presumably mimics fibronectin, an intrinsic host integrin ligand (11). An RGD helper motif in CagL (FEANE) may also be important (13). However, other studies have failed to demonstrate CagL binding to β1 integrins (12), have yielded discrepant results about the role of CagL polymorphisms (14–16), or have identified completely different integrin binding partners, including αVβ6 and αVβ8 (17). CagA, CagI, and CagY have also been shown to bind β1 integrin using yeast two-hybrid, immunoprecipitation, and flow cytometry approaches (12). However, H. pylori binding to integrins has only occasionally been performed with intact bacterial cells (12, 18), and the role of the cagPAI-encoded proteins for integrin binding has not yet been examined in the context of a fully assembled T4SS.
It has long been known that passage of H. pylori in mice results in loss of T4SS function (19, 20). We previously demonstrated that this is typically a result of recombination events in cagY (21), a virB10 ortholog that contains in its middle repeat region (MRR) an extraordinary series of direct DNA repeats that are predicted to encode in-frame insertions or deletions in a surface-exposed region of the protein (22). Recombination events in the cagY MRR lead to expression of an alternative CagY allele that can modulate T4SS function, including induction of IL-8 and translocation of CagA (21). This modulation can occur in a graded fashion and cause both gain and loss of T4SS function (21). More recently, we demonstrated that gamma interferon (IFN-γ) and CD4+ T cells are essential for cagY-mediated loss of T4SS function, which can rescue colonization in IL10−/− mice that have an exaggerated inflammatory response to H. pylori infection (23). Together, these results suggest that cagY recombination serves as an immune-sensitive molecular rheostat that “tunes” the host inflammatory response so as to maintain persistent infection.
Here we examined the mechanism by which recombination in cagY alters T4SS function. Since CagY forms the spokes of a T4SS core complex, together with CagX, CagM, CagT, and Cag3 (24, 25), one possibility is that changes in the MRR alter T4SS function by modifying essential protein-protein interactions or changing the pore through which effectors must travel. Alternatively, since CagY recombination occurs in the MRR, which is predicted to extend extracellularly, allelic variation in CagY might alter integrin binding. At first glance, this seemed unlikely since there are multiple cagPAI proteins that bind integrins. Surprisingly, our results demonstrate that indeed recombination in the CagY MRR alters binding to β1 integrin, which in turn modulates T4SS function. Moreover, the CagY MRR is expressed on the bacterial surface, even in the absence of a T4SS pilus. We propose that CagY is a bifunctional protein that contains a VirB10 domain that is an essential part of a complete T4SS structure and an MRR region that mediates close contact with the host cell and modulates T4SS function.
RESULTS
H. pylori binds to α5β1 integrin in a host cell-free assay.
Previous studies analyzed binding of H. pylori to β1 integrins by protein-protein interaction assays, protein to host cell binding, or bacterial colocalization to β1 integrin on host cells in vitro (11, 12). To demonstrate binding of intact live H. pylori cells to β1 integrin, we developed a microfluidic assay in which human recombinant α5β1 integrin was coated onto glass coverslips, which served as the substrate of a flow channel (Fig. 1A). Fluorescently stained bacteria were flowed through the channel at a defined shear stress (~1 dyne/cm2), microscopic images were recorded, and immobilized fluorescent bacteria were counted. To validate the microfluidic assay, we first analyzed binding of Escherichia coli expressing Yersinia invasin, a well-characterized β1 integrin ligand (26). Yersinia InvA was expressed in E. coli after IPTG (isopropyl-β-d-thiogalactopyranoside) stimulation (see Fig. S1A in the supplemental material) and was presented on the bacterial cell surface (Fig. 1B). E. coli harboring the plasmid vector alone served as a negative control. E. coli expressing InvA and fluorescently stained with either DiO or DiD showed markedly increased binding to α5β1 integrin compared to control E. coli with vector alone (Fig. 1C). Similar results were obtained when InvA and control strains were mixed 1:1 and analyzed simultaneously, which permitted direct comparison in the same flow channel and limited variability that might otherwise arise from differences in integrin density or flow disturbances on glass coverslips (Fig. S1B).
FIG 1 .
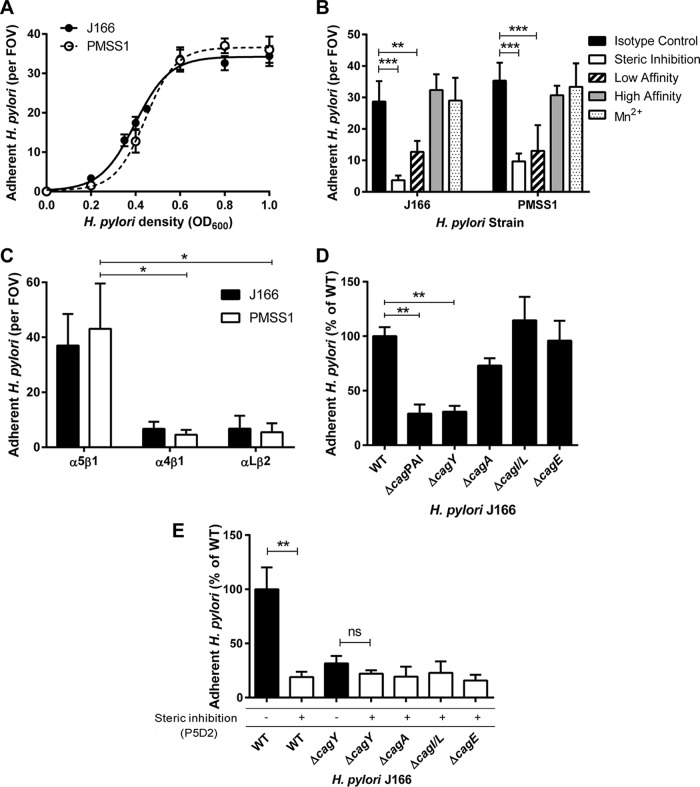
Microfluidic detection of bacterial adherence to recombinant α5β1 integrin. (A) Schematic diagram and photograph of the microfluidic flow cell assembly. (B) Immunofluorescent detection of InvA on the surface of nonpermeabilized IPTG-treated E. coli cells containing the pRI253 plasmid with (+) or without (−) invA. (C) Attachment to α5β1 integrin of IPTG-treated E. coli. Each strain was used at an OD600 of 0.8, labeled with DiD or DiO, and assayed separately. **, P < 0.01; ***, P < 0.001; n.s., not significant. (D) Micrograph of H. pylori J166 labeled with DiO membrane dye, attached to α5β1 integrin in the microfluidic flow cell. Bright-field and fluorescence overlay of the field of view (FOV) demonstrates fluorescent labeling of H. pylori.
Adherence of invasin-expressing E. coli. (A) Immunoblot of E. coli with plasmid pRI253 with or without invA, untreated or treated with IPTG to induce InvA expression. Full-length InvA is around 100 kDa, and the lower bands represent degradation products (55, 58). (B) Attachment to α5β1 integrin in a competitive assay. The two strains of E. coli were stained with either DiD or DiO and mixed 1:1 for a total OD600 of 0.8. A parallel dye swap was performed to exclude influence of the dye on the integrin attachment. Download FIG S1, TIF file, 1.3 MB (1.4MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fluorescently stained H. pylori cells were also readily visualized adherent to α5β1 integrin (Fig. 1D). H. pylori strains J166 and PMSS1 both attached to α5β1 integrin in a concentration-dependent manner and reached saturation at an optical density at 600 nm (OD600) of 0.8 (Fig. 2A). This correlates with approximately 4 × 108 bacterial cells per ml and was used for all subsequent experiments. Binding was blocked by preincubating the integrin-coated coverslips with P5D2 anti-β1 antibody, which sterically inhibits integrin-dependent binding (Fig. 2B). Allosterically stabilizing the α5β1 integrin in the low-affinity conformation by preincubation with SG19 antibody decreased H. pylori-integrin binding, while the TS2/16 antibody, which locks α5β1 in the high-affinity conformation, yielded binding similar to that of an isotype control, indicating that the majority of derivatized α5β1 is active (Fig. 2B). This was also supported by the observation that pretreatment of the microfluidic channels with Mn2+ to lock α5β1 integrin in the high-affinity state yielded H. pylori adherence similar to that with the TS2/16 antibody and the isotype control (Fig. 2B). Adherence to α5β1 integrin was greater than that to α4β1 and αLβ2 (Fig. 2C). Thus, live whole-cell H. pylori binds specifically and in a conformation-dependent manner to α5β1 integrin.
FIG 2 .
α5β1 integrin adherence of WT H. pylori J166 and cagPAI deletion mutants. (A) Adherent H. pylori J166 and PMSS1 cells per field of view (FOV) as a function of bacterial optical cell density at 600 nm (OD600). (B) Adherent H. pylori after preincubation of flow cells with B11/6 isotype control antibody, P5D2 antibody to sterically inhibit β1 integrin binding, or antibodies to lock the integrin in the low-affinity (SG19) or high-affinity (TS2/16) conformation, respectively. Treatment of integrin with Mn2+ to stabilize the high-affinity state produced results similar to treatment with TS2/16 and the B11/6 isotype control antibody. (C) Adherence to α5β1, α4β1, and αLβ2 integrins. (D) Adherence to α5β1 integrin of the J166 WT and deletion mutants, which were fluorescently labeled with DiO and DiI, respectively, mixed in a 1:1 ratio, and enumerated by counting fluorescent bacteria per FOV. Results are expressed as the ratio of deletion mutant to WT. (E) Steric inhibition with P5D2 antibody (white bars) demonstrated that adherence is integrin specific. ΔcagY mutant adherence was similar with and without steric inhibition, suggesting that it represents only nonspecific background binding. Results are the mean ± SEM from 3 to 5 independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
H. pylori adherence to α5β1 integrin in a host cell-free assay is dependent on CagY.
To determine if the cagPAI or any of the putative integrin binding partners (CagA, CagI, CagL, or CagY) are responsible for α5β1 integrin binding of intact H. pylori, we compared deletion mutants of H. pylori J166 to the wild-type (WT) control. The number of adherent mutant and WT H. pylori cells per field of view (FOV) was determined, and the results were analyzed as the percentage of adherence of the mutant compared to WT. Initial control experiments demonstrated that WT and selected mutant strains stained with similar efficiency with both dyes (see Fig. S2A in the supplemental material), and levels of adhesion were independent of the dye and were similar whether strains were analyzed individually or competitively (Fig. S2B). Adherence to α5β1 integrin was markedly reduced by deletion of the entire cagPAI (ΔcagPAI), but not by deletion of cagE (ΔcagE) or cagI/L (Δ cagI/L) (Fig. 2D). Deletion of cagA (ΔcagA) produced a small reduction in adherence to α5β1 integrin, but the difference was not statistically significant (P = 0.25). In contrast, integrin adherence by the cagY deletion mutant (ΔcagY [shown schematically in Fig. 3]) was significantly reduced to a level similar to that of the ΔcagPAI mutant (Fig. 2D). Blocking by treatment with anti-β1 antibody demonstrated β1-specific binding in ΔcagA, ΔcagI/L, and ΔcagE mutants (Fig. 2E), which all produced CagY, as demonstrated by immunoblotting (see Fig. S3A in the supplemental material). The ΔcagY mutant showed only residual adherence that was not β1 specific (Fig. 2E). Together, these results demonstrate that in this host cell-free system, adhesion of H. pylori to α5β1 integrin under physiological levels of shear stress is mediated predominantly by CagY.
FIG 3 .
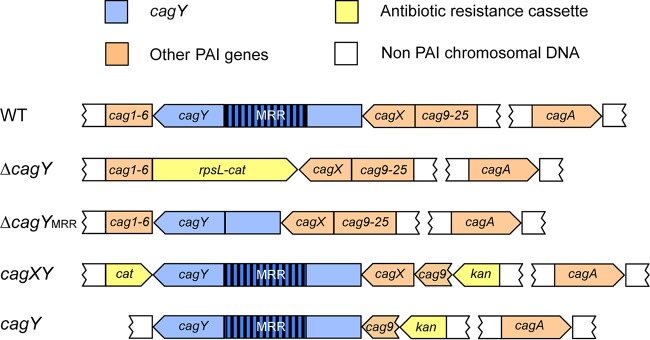
Schematic diagram of the H. pylori J166 cagPAI in the WT and selected deletion mutants. In J166 ΔcagY, the entire cagY gene is replaced by a cat-rpsL cassette (coding for streptomycin susceptibility and chloramphenicol resistance). The ΔcagYMRR mutant has an unmarked, in-frame deletion of the MRR created by contraselection. In J166 cagXY, cag1 to 6 are replaced with cat, and cag9 to 25 are replaced with a kanamycin resistance cassette, starting from after the putative cagY promoter in cag9. J166 cagY has an unmarked deletion of cag1 to 6 and cagX, while cag9 to 25 downstream of the cagX/Y promoter in cag9 are replaced with a kanamycin resistance cassette. cagA is intact in all strains since it is not on the cagPAI in J166 (56).
Fluorophore labeling control experiments. (A) Percentage of cells stained with Dil or DiO [(fluorescently stained cells divided by total cells seen on bright field) ×100] was determined for the H. pylori J166 WT and the ΔcagPAI, ΔcagY, and isogenic strains expressing functional (ΔcagY[Out1]) or nonfunctional (ΔcagY[Out3]) cagY alleles. (B) The H. pylori J166 WT and ΔcagPAI mutant (each at an OD600 of 0.4) were labeled with DiI or DiO, respectively (left), mixed 1:1, and imaged with a 546-nm (DiI) or 488-nm (DiO) light source. Parallel experiments were performed with a dye swap (middle). The WT labeled with DiI was also mixed with the WT labeled with DiO (right). Binding to α5β1 integrin was similar, whether detected with DiO or Dil fluorescent dyes and whether in competition with a strain with low or high binding ability. Results for the WT measured in a competition setting were also very similar to those when measured individually (compare with Fig. 2B). Data represent the mean ± SEM from ≥3 experiments. Download FIG S2, TIF file, 1.9 MB (1.9MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of CagY in the wild-type and H. pylori J166 isogenic knockout mutants. (A) Immunoblot of CagY in WT H. pylori and isogenic cagY, cagA, cagI/L, and cagE knockouts. The immunoblot of UreB is shown as a loading control. (B to D) Quantification of whole-cell CagY MRR fluorescence signal normalized to DAPI in nonpermeabilized WT H. pylori and isogenic knockouts or cagY variants. CagY surface expression in strains with MRR motifs that bind α5β1 integrin and have a functional cagPAI (variants 1 and 2) is similar to that in strains that do not bind integrin and do not have a functional cagPAI (variants 3 and 4), although all are generally lower than J166 WT. Results are expressed as the percentage of the ratio of deletion mutant to WT and represent the mean ± SEM from 3 independent experiments. MFI, mean fluorescence intensity. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, compared to WT. Download FIG S3, TIF file, 3.6 MB (3.7MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
CagY-mediated integrin binding is independent of the T4SS pilus.
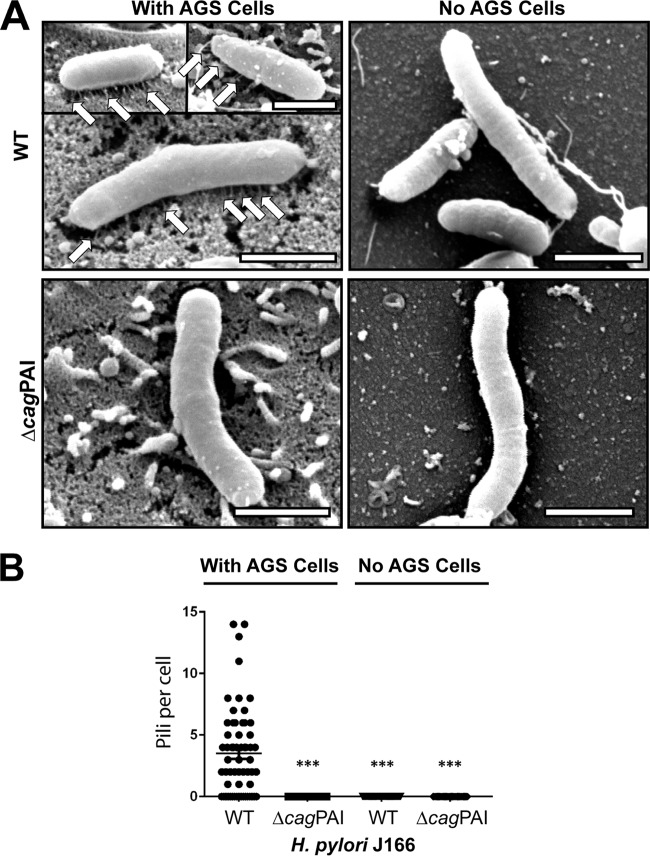
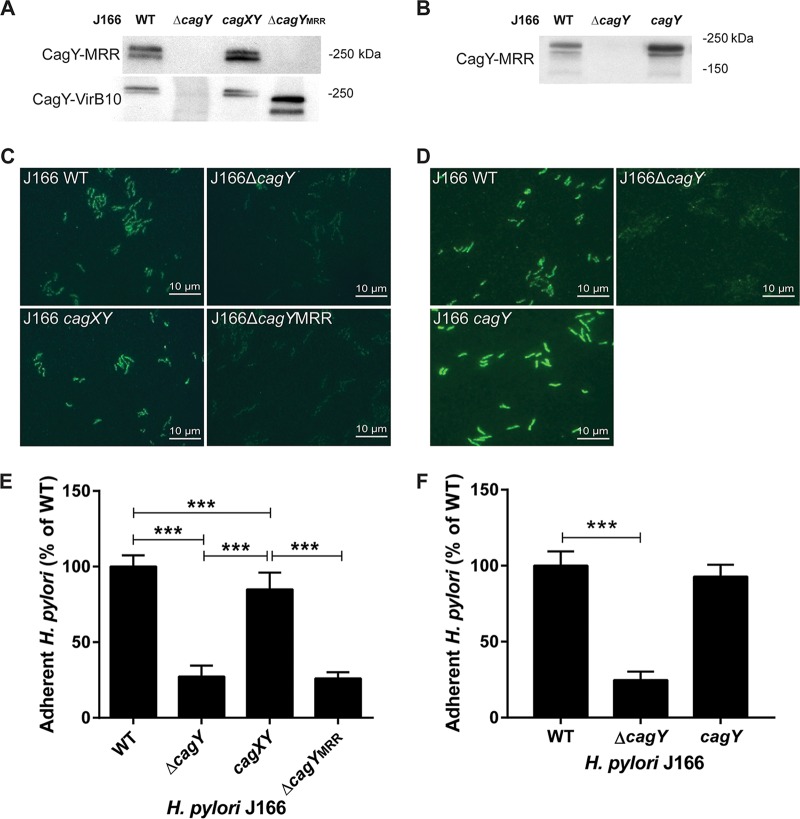
H. pylori T4SS pilus formation is thought to require host cell contact (27), although this has never been formally demonstrated. Since H. pylori attachment to integrin in the flow channel occurs in the absence of host cells, this suggests that H. pylori can bind to α5β1 integrin independent of the T4SS pilus. To examine this further, we used field emission gun scanning electron microscopy (FEG-SEM) to image the T4SS pili in the H. pylori WT and ΔcagPAI mutant, cocultured with or without AGS gastric epithelial cells. Numerous pili were observed on WT H. pylori J166, but only in the presence of AGS cells (Fig. 4). As expected, no pili were detected on J166 ΔcagPAI. The same results were found for the H. pylori PMSS1 WT and ΔcagPAI mutant (see Fig. S4 in the supplemental material). Culture of H. pylori together with α5β1 integrin also failed to induce pilus formation (data not shown). Therefore, under shear flow in this cell-free system, CagY-mediated binding to α5β1 integrin does not require formation of the T4SS pilus. To further demonstrate that CagY is sufficient for integrin binding in the absence of the T4SS pilus, all of the PAI genes were deleted, except cagX and cagY, which are transcribed as an operon from a putative promoter located in cag9, upstream of cagX (28, 29). This mutant, designated cagXY, is shown schematically in Fig. 3 compared to the J166 WT and ΔcagY mutant. J166 cagXY expresses CagY on the bacterial surface (Fig. 5A and C) but fails to induce a robust IL-8 response in AGS cells due to the lack of a T4SS (see Fig. S5 in the supplemental material). In the flow channel α5β1 integrin binding assay, J166 cagXY binds at a level similar to the J166 WT (Fig. 5E). To exclude a role for CagX, we deleted all cagPAI genes and stitched cagY directly to the promoter in cag9, creating J166 cagY. Similar to J166 cagXY, J166 cagY fails to induce IL-8 (Fig. S5), but expresses CagY and binds to α5β1 integrin similarly to the J166 WT (Fig. 5B and D and F). Together these results suggest that in this assay, H. pylori binds to α5β1 integrin predominantly via a CagY-dependent mechanism, but independently of T4SS pilus formation. This conclusion is also supported by the observation that integrin binding in the J166 ΔcagI/L and ΔcagE mutants, which do not form a T4SS pilus (27), is similar to that in the WT (Fig. 2D).
FIG 4 .
Field emission gun scanning electron microscopy (FEG-SEM) of H. pylori demonstrates that host cell contact is required for T4SS pilus formation. (A) FEG-SEM of the H. pylori J166 WT and ΔcagPAI mutant cultured with or without AGS cells. Pili are indicated by white arrows. Scale bar, 1 µm. (B) Enumeration of pili per bacterial cell. ***, P < 0.001, compared with WT.
FIG 5 .
The CagY middle repeat region (MRR), but not the T4SS pilus, is required to bind α5β1 integrin in a host cell-free system and is expressed on the bacterial surface. (A and B) Immunoblot detection of the CagY MRR and VirB10 region in bacterial lysates. (C and D) Immunofluorescent detection of the CagY MRR on the surface of nonpermeabilized H. pylori. (E and F) Flow channel competitive adherence to α5β1 integrin. Results are expressed as the ratio of deletion mutant to WT adherence and represent the mean ± SEM from at least 3 independent experiments. ***, P < 0.001.
Field emission scanning gun electron microscopy (FEG-SEM) of H. pylori PMSS1. (A) FEG-SEM of H. pylori PMSS1 WT and ΔcagPAI mutant cultured with or without AGS cells. Pili are indicated by white arrows. (B) Enumeration of pili per bacterial cell. ***, P < 0.001. Download FIG S4, TIF file, 4.2 MB (4.3MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
T4SS function in various cagPAI H. pylori J166 mutants. Shown is IL-8 induction in AGS cells cocultured with the J166 WT or ΔcagY, cagXY, cagY, and ΔcagYMRR mutants. ***, P < 0.001. Download FIG S5, TIF file, 0.1 MB (105.7KB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The CagY MRR is necessary for integrin binding.
The topography of CagY in the bacterial cell is poorly understood. Proteomic studies suggest that it may be located in the cytoplasmic membrane or perhaps span the inner and outer membranes (18), similar to what has been demonstrated for the Escherichia coli VirB10 (30). However, CagY is much larger than other VirB10 orthologs and includes two membrane-spanning domains that flank the MRR, which previous studies suggested may be localized to the bacterial surface (31). Surface localization is also apparent in cagA, cagI/L, and cagE deletion mutants (Fig. S3B) and in J166 cagXY (Fig. 5A; Fig. S3C) and J166 cagY (Fig. 5D), which do not make a T4SS pilus. We next constructed an unmarked in-frame deletion of the MRR (designated J166 ΔcagYMRR), which is shown schematically in Fig. 3. J166 ΔcagYMRR does not induce IL-8 (Fig. S5) or bind α5β1 integrin in the flow channel (Fig. 5E), and as expected, shows no surface localization of CagY using antibody directed to the MRR (Fig. 5C). However, J166 ΔcagYMRR has an in-frame deletion and produces CagY that can be detected with antibody to the VirB10 portion of CagY (Fig. 5A). Together, these results suggest that the H. pylori CagY MRR is expressed on the bacterial surface, is required for the binding of α5β1 integrin in a T4SS-independent manner, and is essential for T4SS function.
Variation in the motif structure of the CagY MRR alters binding to α5β1 integrin and T4SS function.
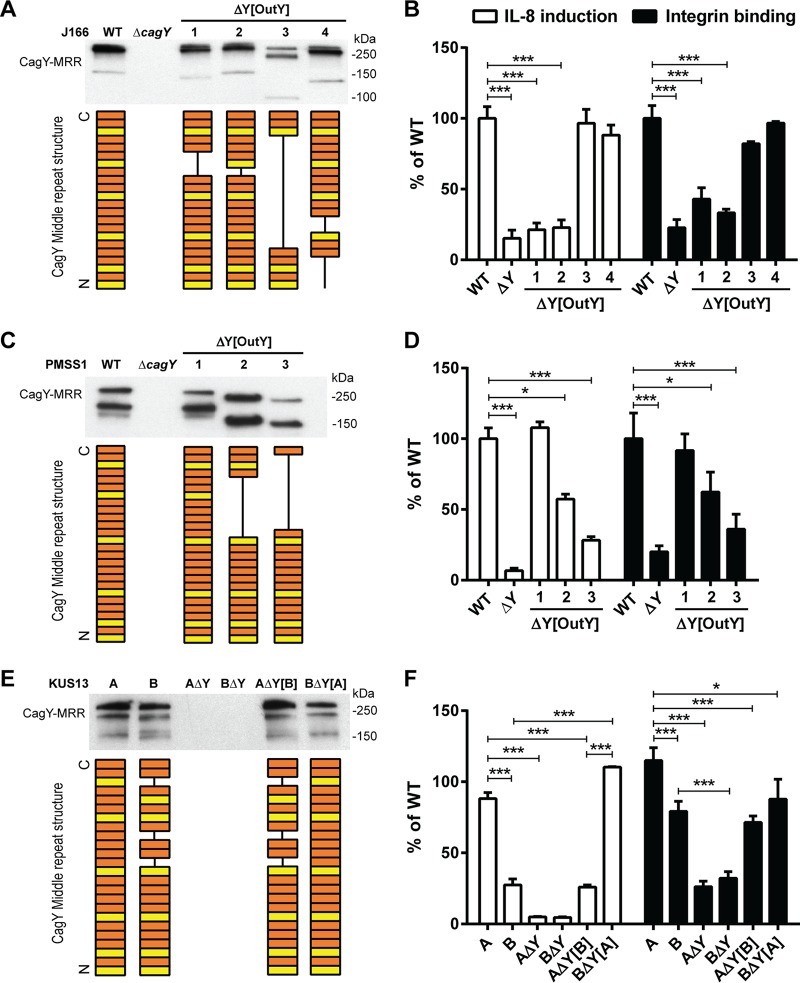
We previously demonstrated, using mouse and nonhuman primate models, that recombination in the cagY MRR regulates T4SS function (21, 23), although the mechanism is unknown. Since we have now shown that the MRR is also required to bind α5β1 integrin in the flow channel, we hypothesized that recombination in cagY modulates T4SS function by altering the efficiency of H. pylori adhesion to α5β1 integrin. To test this hypothesis, we compared IL-8 induction to integrin adhesion, using three groups of H. pylori strains, each with several isogenic variants bearing unique cagY alleles that were previously documented to confer changes in IL-8 induction. First, we examined four isogenic H. pylori J166 strains bearing different cagY alleles, which arose naturally during infection of mice and were transformed into the WT parent strain (21). All four strains express an unmarked CagY that differs only in the motif structure of the MRR (Fig. 6A). Two of the strains induce IL-8 and translocate CagA similarly to WT J166, and two have a nonfunctional T4SS (21). Consistent with our hypothesis, changes in the J166 CagY MRR that reduced IL-8 also showed a marked and commensurate reduction in adhesion to α5β1 integrin (Fig. 6B). Parallel experiments with isogenic strains of H. pylori PMSS1 bearing a unique CagY MRR that altered T4SS function (23) showed similar results (Fig. 6C and D). Finally, we examined the relationship between induction of IL-8 and integrin binding in paired clonal H. pylori isolates recovered from a human patient over a period of 7.4 years (KUS13A and KUS13B) and which differed in the CagY MRR and T4SS function (23). Again we found that MRR-dependent adhesion of each H. pylori isolate to α5β1 integrin was in most cases commensurate with the level of IL-8 induction (Fig. 6E and F). Together these results suggest that recombination in cagY modulates T4SS function by altering H. pylori attachment to α5β1 integrin.
FIG 6 .
Variation in the amino acid motif structure of the CagY MRR regulates T4SS function by altering α5β1 integrin binding. Shown is Western blot detection of the CagY MRR in whole-cell bacterial lysates of H. pylori J166 (A) or PMSS1 (C) isogenic strains, each bearing unique cagY alleles, or their ΔcagY deletion mutants. The corresponding amino acid structure of the MRR is shown schematically as a series of A (orange) or B (yellow) motifs, each 31 to 39 residues, based on DNA sequence analysis as described previously (61). IL-8 induction (white bars) and integrin adhesion (black bars) relative to WT are shown for H. pylori J166 (B) and PMSS1 (D), which correspond to strains shown in panels A and C, respectively. (E) Western blot detection and schematic of the CagY MRR (derived as in panels A and C) from KUS13A, KUS13B, and isogenic variants in which cagY was deleted (ΔcagY) or replaced with that from the variant strain (i.e., KUS13A with cagY13B or KUS13B with cagY13A). (F) IL-8 induction (white bars) and integrin adhesion (black bars) relative to WT for the strains shown in panel E. Quantitative results represent the mean ± SEM from at least 3 independent experiments. *, P < 0.05, and ***, P < 0.001, for comparison of the WT to the isogenic cagY deletion mutant and variants. Results for IL-8 are adapted from references 21 and 23.
Variant CagY amino acid motifs that differ in integrin binding and T4SS function are expressed on the bacterial surface.
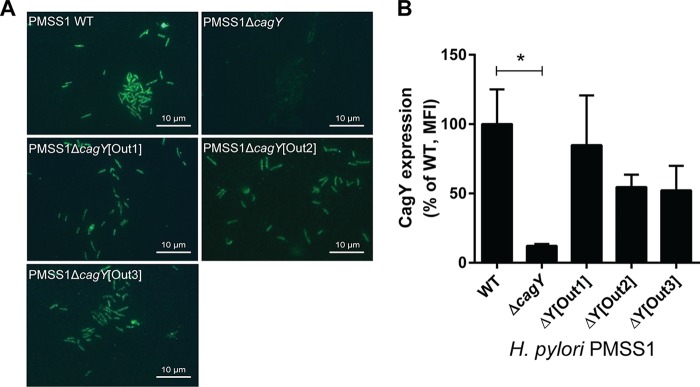
Recombination of cagY could modulate integrin binding by changing its amino acid motif structure, but it might also change its level of expression or surface localization. Although in some strains, the level of CagY expression appears decreased (e.g., Fig. 6A, strain 3), this likely reflects a marked reduction in size of the MRR and reduced antibody recognition. We detected no relationship between MRR expression on Western blotting and either H. pylori adhesion to integrin or induction of IL-8 (Fig. 6). CagY MRR was expressed on the bacterial surface in isogenic H. pylori PMSS1 strains that differed only in their MRR and also showed no relationship to T4SS function or integrin binding (Fig. 7A). Analysis of fluorescence intensity normalized to DAPI (4′,6-diamidino-2-phenylindole) staining demonstrated quantitatively that CagY was expressed on the bacterial surface at similar levels, with no detection in the negative control (Fig. 7B). Quantitation of expression on the bacterial surface of isogenic cagPAI mutants of J166 similarly showed no relationship to T4SS function or integrin binding, although all MRR variants showed reduced expression (Fig. S3D), perhaps related to the reduction in the number of MRR motifs. These results suggest that changes in the motif structure of CagY on the bacterial surface modulate T4SS function by altering bacterial adhesion to α5β1 integrin, rather than altering surface presentation of CagY.
FIG 7 .
Recombination in cagY does not change its surface expression. (A) Immunofluorescent detection of the CagY MRR on the surface of nonpermeabilized H. pylori PMSS1 with distinct cagY alleles from mouse output strains. (B) Quantification of CagY MRR mean fluorescence intensity (MFI) normalized to DAPI. Results are expressed as percentages of the ratios of deletion mutant to WT and represent the mean ± SEM from 3 independent experiments. *, P < 0.05, compared to WT.
DISCUSSION
H. pylori persistence in the gastric mucosa is often attributed to evasion of the innate and adaptive immune responses, including antimicrobial peptides (32), Toll-like receptor signaling (33, 34), and T cell proliferation (35, 36), as well as promotion of a regulatory T cell response (37). However, the very presence in most strains of the cagPAI, which promotes the host immune response (38, 39), and the uniform occurrence of gastritis in infected patients suggest the possibility that the host inflammatory response may at the same time actually promote H. pylori colonization, a concept that has recently been elegantly demonstrated for several enteric pathogens (40). This is supported by observations of functional antagonism between some H. pylori virulence factors, such as the CagA oncoprotein and the VacA cytotoxin (41, 42), and by recent evidence that CagA-dependent inflammation may be important for acquisition of essential nutrients, such as iron (43, 44) and zinc (45). This more nuanced view of the relationship between H. pylori and the host immune response suggests that the overarching strategy used by H. pylori to persist in the stomach might be better characterized as immune regulation rather than simply immune evasion.
CagY is an essential component of the H. pylori T4SS that may be well suited to serve this immune regulatory function. The cagY gene has in its middle repeat region (MRR) a series of direct DNA repeats that in silico predict in-frame recombination events. Recombination in the cagY MRR is in fact common, since variants can be readily detected in vitro, although it remains possible that the frequency is increased in response to unknown host signals. We previously showed that cagY recombination in vivo yields a library of insertions and deletions in the MRR, which maintain CagY expression but frequently alter T4SS function (21). CagY-dependent modulation of T4SS function is graded—more like a rheostat than a switch—and can yield variants that confer both gain and loss of function in vivo (21, 23). Adoptive transfer and knockout mouse experiments demonstrate that development of variant cagY alleles requires a CD4+ T cell- and IFN-γ-dependent immune response (23). Thus, cagY recombination can modulate T4SS function and may be a bacterial strategy to both up- and downregulate the host immune response to promote persistent infection.
Here we addressed the mechanism by which recombination in the MRR alters T4SS function. Since CagY is a ligand for α5β1 integrin, which is essential for T4SS function, we hypothesized that changes in the amino acid motif structure from recombination in the MRR might alter integrin binding and modulate T4SS function. Analysis of whole bacterial cells in a microfluidic assay demonstrated CagY-dependent and integrin conformation-specific binding to α5β1, which correlated closely with T4SS function in isogenic variants that differed only in the MRR region of CagY. This binding was independent of the T4SS pilus, which was not formed under these cell-free conditions, although the MRR was expressed on the bacterial surface as described previously (31). Moreover, we could detect the MRR on the bacterial surface even when the entire cagPAI was deleted, except for cagY and the upstream promoter. Binding to α5β1 integrin was not dependent upon CagA, CagE, or CagL, which was originally identified as the ligand for α5β1 integrin (11). While CagL is clearly essential for T4SS function, more recent studies have suggested that it binds αVβ6 and αVβ8 integrin and not α5β1 integrin (17). The results from yeast two-hybrid studies (12) also identified CagI as a β1 integrin binding partner, which we could not confirm in whole bacterial cells.
Previous studies have found that the VirB10 ortholog at the C terminus of CagY bound to α5β1 integrin, but not the MRR region. However, these studies examined protein-protein interactions by yeast two-hybrid assay and immunoprecipitation or by surface plasmon resonance (12, 46), which may not reflect binding in a whole bacterial cell. Since the isogenic cagY variants examined here differed only in the MRR, and deletion of the MRR eliminated α5β1 integrin binding, our results suggest that the H. pylori MRR is required for binding to α5β1 integrin in an intact bacterial cell. However, we have not directly examined MRR binding to α5β1 integrin, so the MRR may not itself be an integrin ligand but instead may modulate binding of the VirB10 domain of CagY. We have been unable to demonstrate CagY-dependent adherence to α5β1 integrin on AGS gastric epithelial cells in our microfluidic assay (data not shown), which may reflect the multiple binding partners, including cagPAI components, as well as HopQ, BabA, SabA, and other outer membrane adhesins (47, 48). Others have also found no difference in binding to AGS cells between the WT and ΔcagPAI mutant (12).
The topology of CagY in the bacterial membrane and the accessibility to the α5β1 integrin also remain areas of uncertainty. Integrins are generally found in the basolateral compartment, which would not normally be accessible to H. pylori on the apical cell surface. However, H. pylori binds preferentially at tight junctions in cell culture and in gastric tissue, leading to disruption of the integrity of the epithelial layer (49). Moreover, recent studies suggest that H. pylori HtrA, an essential serine protease, cleaves occludin, claudin-8, and E-cadherin, which opens cell-cell junctions and may explain how H. pylori could bind integrins in vivo (50–52). H. pylori binding to CEACAMs (53, 54) or other yet identified cell receptors may also induce redistribution of integrins from the basolateral to the apical cell surface, making them accessible to CagY. It also remains unclear precisely how CagY is localized in the bacterial cell membrane. Elegant cryo-electron microscopy studies have demonstrated that the VirB10 orthologue in the Escherichia coli plasmid conjugation T4SS forms part of a core complex that spans the inner and outer bacterial membranes (30). However, the topology in H. pylori appears different, as recent electron microscopy studies suggest that the core complex is much larger than that in E. coli and is composed of 5 proteins (rather than 3), including CagX, CagY, CagM, CagT, and Cag3 (25).
In conclusion, these studies demonstrate that CagY modulates attachment to α5β1 integrin independently of the T4SS pilus in a manner that depends on the MRR motif structure. It is tempting to speculate that CagY-mediated alteration in integrin binding is also mechanistically linked to T4SS function, since they are strongly correlated (Fig. 6). For example, surface expression of an integrin-binding motif may promote intimate epithelial cell contact, which in turn serves as a nucleation signal to promote expression of the T4SS pilus, further enhancing integrin binding and injection of effector molecules. Such a scenario might entail MRR-dependent integrin signaling, including activation of focal adhesion kinase (FAK) and the Src family kinase, although others have shown that only the extracellular domains of the β1 integrin are important for CagA translocation (12). On the other hand, it is logically possible that changes in the MRR affect integrin binding and T4SS function independently. Although the details remain to be elucidated, we hypothesize that CagY-dependent binding to α5β1 integrin serves as a molecular rheostat that “tunes” the optimal balance between the competing pressures of gastric inflammation, which serves a metabolic function for the bacterium on the one hand, but comes at a cost of exposure to immune pressure, decreased bacterial load, and decreased possibility of transmission to a new host.
MATERIALS AND METHODS
Construction and culture of E. coli expressing InvA.
Plasmid pRI253 (kindly provided by Ralph Isberg, Tufts University, Boston, MA) contains the invA gene from Yersinia pseudotuberculosis under control of a phage T7 RNA polymerase promoter (55). To create a negative control, the invA gene was cut out from the plasmid using restriction enzymes EcoRI and HindIII. The recircularized plasmid, pRI253 ΔinvA, and the original plasmid, pRI253, were transformed into competent E. coli BL21 (Invitrogen) according to the manufacturers’ instructions. E. coli strains were cultured overnight at 37°C in Luria-Bertani (LB) broth supplemented with 5 mg/liter carbenicillin. Overnight cultures were diluted to an OD600 of 0.05 and cultured for an additional 2 to 3 h, followed by addition of 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) and another 2 h of incubation to induce InvA expression.
H. pylori strains and culture conditions.
Wild-type H. pylori strains were cultured on brucella agar or in brucella broth (BBL/Becton, Dickinson, Sparks, MD) supplemented with 5% heat-inactivated newborn calf serum (Invitrogen, Carlsbad, CA) and antibiotics (trimethoprim, 5 mg/liter; vancomycin, 10 mg/liter; polymyxin B, 2.5 IU/liter; amphotericin B, 2.5 mg/liter). H. pylori mutant strains were cultured as for the wild type, but with the addition of kanamycin (25 mg/liter), chloramphenicol (5 mg/liter), or streptomycin (10 mg/liter) as appropriate (all antibiotics from Sigma). H. pylori liquid cultures were grown overnight to an optical density at 600 nm (OD600) of approximately 0.3 to 0.4. All H. pylori cultures were grown at 37°C under microaerophilic conditions generated by a fixed 5% O2 concentration (Anoxomat; Advanced Instruments, Norwood, MA). A complete list of strains is shown in Table 1.
TABLE 1 .
E. coli and H pylori strains used in this study
| Strain | Relevant characteristic(s) | Antibiotic resistancea |
Reference or source |
|---|---|---|---|
| E. coli | |||
| BL21 invA + | E. coli BL21 with plasmid pRI253 | Amp | 55 |
| BL21 invA − | E. coli BL21 with plasmid pRI253ΔinvA | Amp | This study |
| H. pylori | |||
| WT | |||
| J166 | Wild type | 62 | |
| PMSS1 | Wild type | 38 | |
| KUS13A | Clinical isolate from patient KUS13 | 63 | |
| KUS13B | Isolate from patient KUS13 obtained 7.4 yr after isolate A | 63 | |
| Deletion mutants | |||
| J166 ΔcagPAI | Deletion of the entire cagPAI | Cm Km | 39 |
| J166 ΔcagA | J166 ΔcagA::aphA | Km | This study |
| J166 ΔcagI/L | J166 ΔcagI/L::aphA | Km | This study |
| J166 ΔcagY | J166 Strr ΔcagY::cat_rpsL | Cm | 21 |
| J166 ΔcagE | J166 ΔcagE::aphA | Km | This study |
| J166 cagXY | J166 Δcag1–6::cat Δcag9–25::aphA | Cm Km | This study |
| J166 cagY | J166 Δcag1–6 Δcag8 Δcag9–25::aphA | Km | This study |
| J166 ΔcagYMRR | J166 Strr ΔcagYMRR | Str | This study |
| J166 cagY replacements | |||
| J166 ΔcagY[mOut1] | J166 ΔcagY replaced with cagY from mOut1 | Str | 21 |
| J166 ΔcagY[mOut2] | J166 ΔcagY replaced with cagY from mOut2 | Str | 21 |
| J166 ΔcagY[mOut3] | J166 ΔcagY replaced with cagY from mOut3 | Str | 21 |
| J166 ΔcagY[mOut4] | J166 ΔcagY replaced with cagY from mOut4 | Str | 21 |
| PMSS1 cagY replacements | |||
| PMSS1 ΔcagY[Out1] | PMSS1 ΔcagY replaced with cagY from Out1 | Str | 23 |
| PMSS1 ΔcagY[Out2] | PMSS1 ΔcagY replaced with cagY from Out2 | Str | 23 |
| PMSS1 ΔcagY[Out3] | PMSS1 ΔcagY replaced with cagY from Out3 | Str | 23 |
| CagY replacement clinical isolates | |||
| KUS13A ΔcagY | KUS13A ΔcagY::cat_rpsL | Cm | 23 |
| KUS13B ΔcagY | KUS13B ΔcagY::cat_rpsL | Cm | 23 |
| KUS13A ΔcagY[KUS13B] | KUS13A ΔcagY replaced with cagY from KUS13B | Str | 23 |
| KUS13B ΔcagY[KUS13A] | KUS13B ΔcagY replaced with cagY from KUS13A | Str | 23 |
Amp, ampicillin; Cm, chloramphenicol; Km, kanamycin; Str, streptomycin.
Construction of H. pylori mutants.
Six H. pylori J166 mutants were constructed (Table 1). For J166 ΔcagA, J166 ΔcagI/L, and J166 ΔcagE, DNA fragments upstream and downstream of the respective gene deletion were PCR amplified using primers (see Table S1 in the supplemental material) with restriction sites that permitted ligation to a kanamycin resistance gene (aphA) and insertion into the multiple cloning site of pBluescript (Stratagene, La Jolla, CA). The resulting plasmid was transformed into E. coli TOP10 (Invitrogen) according to the manufacturers’ instructions, and transformants were grown overnight on Luria-Bertani (LB) plates containing kanamycin. Resistant colonies were inoculated into selective LB broth, and plasmids from the resulting culture were purified with a QIAprep spin miniprep kit (Qiagen). Plasmids were sequenced and digested with appropriate enzymes for verification of correct construction prior to natural transformation of H. pylori with kanamycin selection. J166 cagXY was created in a similar fashion, but in two steps: first deleting cag1 to 6 with a chloramphenicol resistance cassette (cat) and selection on chloramphenicol and then deleting cag9 to 25 with a kanamycin cassette, leaving only cagX, cagY (and its promoter), and cagA, which in strain J166 is not on the cagPAI (56). J166 cagY was made in a series of 3 steps. First an unmarked deletion of cag1 to 6 was constructed using contraselection. The region was replaced by a cat-rpsL casette, resulting in streptomycin-sensitive (rpsL encodes dominant streptomycin sensitivity) and chloramphenicol-resistant transformants. Then, upstream and downstream fragments were stitched together, and the PCR product was used to replace the cassette, leaving an unmarked deletion. Next, cag9 to 25 were deleted as in the cagXY construct and replaced with a kanamycin cassette. Finally, contraselection was again used to excise the cagX gene, bringing 313 bp upstream of cagX (putatively containing its promoter) immediately upstream of cagY.
Primers used for PCR. Download TABLE S1, DOCX file, 0.1 MB (21.4KB, docx) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
J166 ΔcagYMRR, with an in-frame markerless deletion of the MRR, was constructed using modifications of contraselection described previously (21). Briefly, the MRR was first replaced by insertion of the cat-rpsL cassette in streptomycin-resistant H. pylori J166. Fragments upstream and downstream of the MRR were then each amplified with overlapping primers that permitted stitching of the two products in a second PCR. The stitched product was ligated into pBluescript and used in a second transformation reaction to replace cat-rpsL, with selection on streptomycin. All H. pylori deletion mutants were sequence verified to confirm the correct construction.
Microfluidic adhesion assay.
Microfluidic adhesion assays were assembled as previously described (57). In brief, 25-mm-diameter no. 1.5 glass coverslips were piranha etched to remove organic molecules and treated with 1% 3-aminopropyltriethoxysilane to add aminosilane groups. Recombinant human α4β1, α5β1, or αLβ2 integrin (R&D Systems, Minneapolis, MN) was adsorbed at a 10-mg/liter concentration overnight at 4°C, resulting in approximately 2,000 sites/µm2. Coverslips were then washed and blocked with Hanks’ balanced salt solution with 0.1% human serum albumin. Where indicated, the blocking solution was supplemented with 5 mg/liter of anti-integrin β1 blocking antibody P5D2 (Abcam, Inc., San Francisco, CA), low-affinity locking anti-β1 integrin antibody SG19, high-affinity locking anti-β1 integrin antibody TS2/16 (both from BioLegend, San Diego, CA), isotype control antibody B11/6 (Abcam, Inc., San Francisco, CA), or 2 mM MnCl2 (Mn2+) to activate integrin. A custom multichannel microfluidic device (57) was vacuum sealed, outlets were attached to Exigo pumps to provide the negative pressure necessary to induce shear, and inlet reservoirs were loaded with E. coli or H. pylori. Prior to loading, liquid-cultured bacteria were stained at an OD600 of 0.8 with 2% Vybrant DiI, DiD, or DiO cell-labeling solution (Grand Island, NY) in brucella broth for 20 min at 37°C in the dark. Stained bacteria were washed twice with phosphate-buffered saline (PBS) and then resuspended in brucella broth to the desired final OD600. Competitive binding assays were performed by mixing differently labeled WT and mutant bacteria at an OD600 of 0.4 (total OD600 of 0.8). Shear was induced at 1 dyne/cm2 for 3 min followed by a 3-min period of no-shear incubation to allow attachment. Then, shear was increased to 1 dyne/cm2, and 10-s videos were taken along the centerline of the channel in four field of views using an inverted total internal reflection fluorescence (TIRF) research microscope (Nikon) equipped with a 60× numerical aperture 1.5 immersion TIRF objective and a 120-W arc lamp to capture epifluorescence images with the appropriate filter sets (488 nm for DiO, 510 nm for DiD, and 543 nm for DiI). Images were captured using a 16-b digital complementary metal oxide semiconductor Zyla camera (Andor, Belfast, United Kingdom) connected to a PC (Dell) with NIS Elements imaging software (Nikon, Melville, NY). Images were collected with 2-by-2 binning at a resolution of 1,024 by 1,024 at a rate of 2 frames per s. Adherent bacteria were identified by the presence of fluorescence, which was cross-checked with an overlaid bright-field image to eliminate fluorescent noise. Small numbers of bacteria that were unstained (typically ~10%) were not counted. Bacteria that remained stationary or tethered after 10 s were counted visually in 3 fields of view, and the results were averaged for each biological replicate. To assess reliability, two observers (one “blind”) independently scored adherent bacteria at 488 nm and 543 nm in 9 fields of view that contained competitive binding assays (WT and mutant). Mean similarity for the 18 observations was 0.94, which was calculated as 1 − [|O1 − O2|/1/2(O1 + O2)], where O1 and O2 are the independent scores for the two observers and a value of 1.0 indicates perfect agreement. Data on integrin binding are representative of at least three biological replicates, which in most cases examined three fields of view in duplicate technical replicates.
Sequencing of cagY.
The DNA sequences of cagY from H. pylori PMSS1 and KUS13A and -B were determined using single-molecule real-time sequencing (Pacific Biosciences, Menlo Park, CA). Briefly, cagY was amplified as previously described (21), and purified PCR products were submitted to the DNA Technologies Core at the UC Davis Genome Center. The amplicons were sequenced using a PacBio RSII sequencer with P6C4 chemistry. Data were analyzed using PacBio’s SMRTportal Analysis 2.3.0. cagY sequences of H. pylori J166 were previously published (21).
Assessment of protein expression by fluorescence microscopy.
Liquid cultures of H. pylori or IPTG-induced E. coli strains were centrifuged (3,000 × g, 3 min) and resuspended in blocking buffer (PBS with 1% bovine serum albumin and 0.05% Tween 20) at an OD600 of 0.4. Each culture was spotted onto two microscope slides using Cytofunnels in a Cytospin centrifuge at 1,000 rpm for 15 min. Air-dried slides were incubated for 1 h with blocking buffer in a humid chamber followed by 1 h of incubation with anti-H. pylori CagY MRR antibody (31) diluted 1:1,000 or anti-Yersinia invasin antibody (58) diluted 1:5,000 in blocking buffer. Slides were washed 3 times with PBS and incubated for 1 h in the dark with Alexa Fluor 488 goat anti-rabbit IgG (R37116; Life Technologies, Inc.) diluted 1:10 in blocking buffer. After further washing, the slides were mounted with Fluoroshield with DAPI (Sigma). The slides were stored in the dark and imaged the next day. Photos of all slides were captured with the same exposure time for each antibody and DAPI. Fluorescence intensity was analyzed with the ImageJ software, normalizing the total CagY fluorescence at a given threshold determined by the positive WT sample to the area of the DAPI fluorescence of the bacterial particles.
Immunoblots.
Expression of E. coli invasin and H. pylori CagY MRR was detected by electrophoresis of lysates of liquid-cultured bacteria as described previously (21), using polyclonal rabbit antisera to invasin (1:15,000) or CagY MRR (1:10,000) as the primary antibodies. Detection of CagY expression in the ΔcagYMRR mutant, which contains an in-frame deletion of the MRR, was performed using antiserum from rabbits immunized with the VirB10 portion at the C terminus of CagY (1:1,000). To generate the antiserum, DNA encoding the C terminus of H. pylori J166 CagY was PCR amplified (Table S1), cloned into pGEX-4T-3 vector, and transformed into E. coli BL21 (both from GE Healthcare). Expression of the glutathione S-transferase (GST)-fusion protein and preparation of cell extracts were performed according to the manufacturer’s instructions. The GST-fusion protein was bound to glutathione Sepharose 4B (GE Healthcare) in a column, and the GST was cleaved off by thrombin. The eluate was run on SDS-PAGE, and the purified CagY C-terminus protein was cut out from the gel and used to generate rabbit antisera according to standard protocols (Antibodies, Inc., Davis, CA).
IL-8 ELISA.
IL-8 was measured essentially as described previously (59). Human AGS gastric adenocarcinoma cells (ATCC, Manassas, VA) were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin in 5% CO2 at 37°C. All antibiotics were excluded from the growth media 24 h prior to H. pylori coculture. Approximately 5 × 105 human AGS gastric adenocarcinoma cells were seeded into six-well plates with 1.8 ml RPMI–10% fetal bovine serum, incubated overnight, and then cocultured with bacteria diluted in 200 µl brucella broth to give a multiplicity of infection (MOI) of 100:1. Brucella broth with no bacteria served as a baseline control. Supernatants were harvested after 20 to 22 h of culture (37°C, 5% CO2), stored at −80°C, and then diluted 1:8 prior to IL-8 enzyme-linked immunosorbent assay (ELISA; Invitrogen, Camarillo, CA) performed according to the manufacturer’s protocol. WT H. pylori J166 or PMSS1 and its isogenic cagY deletion mutant were included on every plate as positive and negative controls, respectively. IL-8 values were normalized to WT H. pylori determined concurrently.
High-resolution field-emission gun scanning electron microscopy analyses.
Bacteria were cultured alone or with AGS cells for 4 h at an MOI of 100:1. Bacteria were prepared for scanning electron microscopy as previously described (27, 60). Briefly, samples were cultured on poly-l-lysine-coated glass coverslips and fixed for 1 h with 2.0% paraformaldehyde–2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer. Cells were washed three times in 0.05 M sodium cacodylate buffer before secondary fixation with 0.1% osmium tetroxide for 15 min. Three additional 0.05 M sodium cacodylate buffer washes were performed before subjecting the samples to sequential ethanol dehydration. Cells were dried at the critical point and carbon coated before imaging with an FEI Quanta 250 FEG-SEM. Pili were enumerated in a blind fashion using ImageJ software.
Statistical analysis.
Data are reported as the mean ± standard error of the mean (SEM). Multiple groups were compared using analysis of variance (ANOVA), with Tukey’s or Bonferroni’s post hoc test, or with Dunnett’s post hoc test compared only to WT. Two group comparisons were performed using Student’s t test. All analyses were carried out using GraphPad Prism 5.01 for Windows (GraphPad Software, Inc., San Diego, CA). A P value of <0.05 was considered statistically significant.
Accession number(s).
Sequences have been deposited in GenBank and are available under accession no. KY613376 to KY613380.
ACKNOWLEDGMENTS
We thank Jordan Feeney, UC Davis, CA, for drawing assistance with Fig. 1A, Ralph Isberg, Tufts University, Boston, MA, for providing pRI253 plasmid, and Virginia Miller, University of North Carolina, Chapel Hill, NC, for providing anti-invasin antibody.
This work was supported by grants from the National Institutes of Health to S.S. (AI047294) and J.S. (AI108713). V.M. was supported by a National Institutes of Health T32 training grant (AI060555) to J.S. J.G. was supported by the Department of Veterans Affairs Office of Medical Research Career Development award (IK2BX001701). Core Services, including use of the Cell Imaging Shared Resource, were performed through the Vanderbilt University Digestive Disease Research Center supported by National Institutes of Health grant P30DK058404. B.L. was supported by National Natural Science Foundation of China (31672606). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Citation Skoog EC, Morikis VA, Martin ME, Foster GA, Cai LP, Hansen LM, Li B, Gaddy JA, Simon SI, Solnick JV. 2018. CagY-dependent regulation of type IV secretion in Helicobacter pylori is associated with alterations in integrin binding. mBio 9:e00717-18. https://doi.org/10.1128/mBio.00717-18.
REFERENCES
- 1.Wroblewski LE, Peek RM Jr, Wilson KT. 2010. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaser MJ, Falkow S. 2009. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol 7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson EM, Gaddy JA, Voss BJ, Hennig EE, Cover TL. 2014. Genes required for assembly of pili associated with the Helicobacter pylori cag type IV secretion system. Infect Immun 82:3457–3470. doi: 10.1128/IAI.01640-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odenbreit S, Püls J, Sedlmaier B, Gerland E, Fischer W, Haas R. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 5.Varga MG, Shaffer CL, Sierra JC, Suarez G, Piazuelo MB, Whitaker ME, Romero-Gallo J, Krishna US, Delgado A, Gomez MA, Good JA, Almqvist F, Skaar EP, Correa P, Wilson KT, Hadjifrangiskou M, Peek RM. 2016. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated cag type IV secretion system. Oncogene 35:6262–6269. doi: 10.1038/onc.2016.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, Athman R, Mémet S, Huerre MR, Coyle AJ, DiStefano PS, Sansonetti PJ, Labigne A, Bertin J, Philpott DJ, Ferrero RL. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 7.Gall A, Gaudet RG, Gray-Owen SD, Salama NR. 2017. TIFA signaling in gastric epithelial cells initiates the cag type 4 secretion system-dependent innate immune response to Helicobacter pylori infection. mBio 8:e1168-17. doi: 10.1128/mBio.01168-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann S, Pfannkuch L, Al-Zeer MA, Bartfeld S, Koch M, Liu J, Rechner C, Soerensen M, Sokolova O, Zamyatina A, Kosma P, Mäurer AP, Glowinski F, Pleissner KP, Schmid M, Brinkmann V, Karlas A, Naumann M, Rother M, Machuy N, Meyer TF. 2017. ALPK1- and TIFA-dependent innate immune response triggered by the Helicobacter pylori type IV secretion system. Cell Rep 20:2384–2395. doi: 10.1016/j.celrep.2017.08.039. [DOI] [PubMed] [Google Scholar]
- 9.Stein SC, Faber E, Bats SH, Murillo T, Speidel Y, Coombs N, Josenhans C. 2017. Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog 13:e1006514. doi: 10.1371/journal.ppat.1006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Censini S, Stein M, Covacci A. 2001. Cellular responses induced after contact with Helicobacter pylori. Curr Opin Microbiol 4:41–46. doi: 10.1016/S1369-5274(00)00162-4. [DOI] [PubMed] [Google Scholar]
- 11.Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, Misselwitz R, Berger J, Sewald N, König W, Backert S. 2007. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature 449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez-Soto LF, Kutter S, Sewald X, Ertl C, Weiss E, Kapp U, Rohde M, Pirch T, Jung K, Retta SF, Terradot L, Fischer W, Haas R. 2009. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog 5:e1000684. doi: 10.1371/journal.ppat.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conradi J, Tegtmeyer N, Woźna M, Wissbrock M, Michalek C, Gagell C, Cover TL, Frank R, Sewald N, Backert S. 2012. An RGD helper sequence in CagL of Helicobacter pylori assists in interactions with integrins and injection of CagA. Front Cell Infect Microbiol 2:70. doi: 10.3389/fcimb.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeh YC, Chang WL, Yang HB, Cheng HC, Wu JJ, Sheu BS. 2011. H. pylori CagL amino acid sequence polymorphism Y58E59 induces a corpus shift of gastric integrin alpha5beta1 related with gastric carcinogenesis. Mol Carcinog 50:751–759. doi: 10.1002/mc.20753. [DOI] [PubMed] [Google Scholar]
- 15.Tegtmeyer N, Lind J, Schmid B, Backert S. 2014. Helicobacter pylori CagL Y58/E59 mutation turns-off type IV secretion-dependent delivery of CagA into host cells. PLoS One 9:e97782. doi: 10.1371/journal.pone.0097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tafreshi M, Zwickel N, Gorrell RJ, Kwok T. 2015. Preservation of Helicobacter pylori CagA translocation and host cell proinflammatory responses in the face of CagL hypervariability at amino acid residues 58/59. PLoS One 10:e0133531. doi: 10.1371/journal.pone.0133531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barden S, Niemann HH. 2015. Adhesion of several cell lines to Helicobacter pylori CagL is mediated by integrin αVβ6 via an RGDLXXL motif. J Mol Biol 427:1304–1315. doi: 10.1016/j.jmb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Sause WE, Keilberg D, Aboulhouda S, Ottemann KM. 2017. The Helicobacter pylori autotransporter ImaA tempers the bacterium’s interaction with alpha5beta1 integrin. Infect Immun 85:e00450-16. doi: 10.1128/IAI.00450-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philpott DJ, Belaid D, Troubadour P, Thiberge JM, Tankovic J, Labigne A, Ferrero RL. 2002. Reduced activation of inflammatory responses in host cells by mouse-adapted Helicobacter pylori isolates. Cell Microbiol 4:285–296. doi: 10.1046/j.1462-5822.2002.00189.x. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree JE, Ferrero RL, Kusters JG. 2002. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter 7:139–141. [DOI] [PubMed] [Google Scholar]
- 21.Barrozo RM, Cooke CL, Hansen LM, Lam AM, Gaddy JA, Johnson EM, Cariaga TA, Suarez G, Peek RM Jr, Cover TL, Solnick JV. 2013. Functional plasticity in the type IV secretion system of Helicobacter pylori. PLoS Pathog 9:e1003189. doi: 10.1371/journal.ppat.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aras RA, Fischer W, Perez-Perez GI, Crosatti M, Ando T, Haas R, Blaser MJ. 2003. Plasticity of repetitive DNA sequences within a bacterial (type IV) secretion system component. J Exp Med 198:1349–1360. doi: 10.1084/jem.20030381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrozo RM, Hansen LM, Lam AM, Skoog EC, Martin ME, Cai LP, Lin Y, Latoscha A, Suerbaum S, Canfield DR, Solnick JV. 2016. CagY is an immune-sensitive regulator of the Helicobacter pylori type IV secretion system. Gastroenterology 151:1164–1175.e3 doi: 10.1053/j.gastro.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutter S, Buhrdorf R, Haas J, Schneider-Brachert W, Haas R, Fischer W. 2008. Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies. J Bacteriol 190:2161–2171. doi: 10.1128/JB.01341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frick-Cheng AE, Pyburn TM, Voss BJ, McDonald WH, Ohi MD, Cover TL. 2016. Molecular and structural analysis of the Helicobacter pylori cag type IV secretion system core complex. mBio 7:e02001-15. doi: 10.1128/mBio.02001-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isberg RR, Leong JM. 1990. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell 60:861–871. doi: 10.1016/0092-8674(90)90099-Z. [DOI] [PubMed] [Google Scholar]
- 27.Shaffer CL, Gaddy JA, Loh JT, Johnson EM, Hill S, Hennig EE, McClain MS, McDonald WH, Cover TL. 2011. Helicobacter pylori exploits a unique repertoire of type IV secretion system components for pilus assembly at the bacteria-host cell interface. PLoS Pathog 7:e1002237. doi: 10.1371/journal.ppat.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermüller J, Reinhardt R, Stadler PF, Vogel J. 2010. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature 464:250–255. doi: 10.1038/nature08756. [DOI] [PubMed] [Google Scholar]
- 29.Ta LH, Hansen LM, Sause WE, Shiva O, Millstein A, Ottemann KM, Castillo AR, Solnick JV. 2012. Conserved transcriptional unit organization of the cag pathogenicity island among Helicobacter pylori strains. Front Cell Infect Microbiol 2:46. doi: 10.3389/fcimb.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandran V, Fronzes R, Duquerroy S, Cronin N, Navaza J, Waksman G. 2009. Structure of the outer membrane complex of a type IV secretion system. Nature 462:1011–1015. doi: 10.1038/nature08588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohde M, Püls J, Buhrdorf R, Fischer W, Haas R. 2003. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol Microbiol 49:219–234. doi: 10.1046/j.1365-2958.2003.03549.x. [DOI] [PubMed] [Google Scholar]
- 32.Bauer B, Pang E, Holland C, Kessler M, Bartfeld S, Meyer TF. 2012. The Helicobacter pylori virulence effector CagA abrogates human beta-defensin 3 expression via inactivation of EGFR signaling. Cell Host Microbe 11:576–586. doi: 10.1016/j.chom.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. 2005. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 102:9247–9252. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cullen TW, Giles DK, Wolf LN, Ecobichon C, Boneca IG, Trent MS. 2011. Helicobacter pylori versus the host: remodeling of the bacterial outer membrane is required for survival in the gastric mucosa. PLoS Pathog 7:e1002454. doi: 10.1371/journal.ppat.1002454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. 2003. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science 301:1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 36.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. 2004. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci U S A 101:7727–7732. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. 2013. Helicobacter pylori gamma-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci U S A 110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, Müller A. 2011. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 140:199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornsby MJ, Huff JL, Kays RJ, Canfield DR, Bevins CL, Solnick JV. 2008. Helicobacter pylori induces an antimicrobial response in rhesus macaques in a cag pathogenicity island-dependent manner. Gastroenterology 134:1049–1057. doi: 10.1053/j.gastro.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bäumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535:85–93. doi: 10.1038/nature18849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. 2012. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Yokoyama K, Higashi H, Ishikawa S, Fujii Y, Kondo S, Kato H, Azuma T, Wada A, Hirayama T, Aburatani H, Hatakeyama M. 2005. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc Natl Acad Sci U S A 102:9661–9666. doi: 10.1073/pnas.0502529102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, Tan S, Morgan DR, Wilson KT, Bravo LE, Correa P, Cover TL, Amieva MR, Peek RM Jr.. 2013. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tan S, Noto JM, Romero-Gallo J, Peek RM Jr, Amieva MR. 2011. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog 7:e1002050. doi: 10.1371/journal.ppat.1002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaddy JA, Radin JN, Loh JT, Piazuelo MB, Kehl-Fie TE, Delgado AG, Ilca FT, Peek RM, Cover TL, Chazin WJ, Skaar EP, Scott Algood HM. 2014. The host protein calprotectin modulates the Helicobacter pylori cag type IV secretion system via zinc sequestration. PLoS Pathog 10:e1004450. doi: 10.1371/journal.ppat.1004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koelblen T, Berge C, Cherrier MV, Brillet K, Jimenez-Soto L, Ballut L, Takagi J, Montserret R, Rousselle P, Fischer W, Haas R, Fronzes R, Terradot L. 2017. Molecular dissection of protein-protein interactions between integrin alpha5beta1 and the Helicobacter pylori Cag type IV secretion system. FEBS J 284:4143–4157. doi: 10.1111/febs.14299. [DOI] [PubMed] [Google Scholar]
- 47.Aspholm-Hurtig M, Dailide G, Lahmann M, Kalia A, Ilver D, Roche N, Vikström S, Sjöström R, Lindén S, Bäckström A, Lundberg C, Arnqvist A, Mahdavi J, Nilsson UJ, Velapatiño B, Gilman RH, Gerhard M, Alarcon T, López-Brea M, Nakazawa T, Fox JG, Correa P, Dominguez-Bello MG, Perez-Perez GI, Blaser MJ, Normark S, Carlstedt I, Oscarson S, Teneberg S, Berg DE, Borén T. 2004. Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305:519–522. doi: 10.1126/science.1098801. [DOI] [PubMed] [Google Scholar]
- 48.Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA, Altraja S, Wadström T, Kersulyte D, Berg DE, Dubois A, Petersson C, Magnusson KE, Norberg T, Lindh F, Lundskog BB, Arnqvist A, Hammarström L, Borén T. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573–578. doi: 10.1126/science.1069076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. 2003. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science 300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt TP, Perna AM, Fugmann T, Böhm M, Jan Hiss H, Haller S, Götz C, Tegtmeyer N, Hoy B, Rau TT, Neri D, Backert S, Schneider G, Wessler S. 2016. Identification of E-cadherin signature motifs functioning as cleavage sites for Helicobacter pylori HtrA. Sci Rep 6:23264. doi: 10.1038/srep23264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tegtmeyer N, Moodley Y, Yamaoka Y, Pernitzsch SR, Schmidt V, Traverso FR, Schmidt TP, Rad R, Yeoh KG, Bow H, Torres J, Gerhard M, Schneider G, Wessler S, Backert S. 2016. Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Mol Microbiol 99:925–944. doi: 10.1111/mmi.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tegtmeyer N, Wessler S, Necchi V, Rohde M, Harrer A, Rau TT, Asche CI, Boehm M, Loessner H, Figueiredo C, Naumann M, Palmisano R, Solcia E, Ricci V, Backert S. 2017. Helicobacter pylori employs a unique basolateral type IV secretion mechanism for CagA delivery. Cell Host Microbe 22:552–560.e5. doi: 10.1016/j.chom.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Javaheri A, Kruse T, Moonens K, Mejías-Luque R, Debraekeleer A, Asche CI, Tegtmeyer N, Kalali B, Bach NC, Sieber SA, Hill DJ, Königer V, Hauck CR, Moskalenko R, Haas R, Busch DH, Klaile E, Slevogt H, Schmidt A, Backert S, Remaut H, Singer BB, Gerhard M. 2016. Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat Microbiol 2:16189. doi: 10.1038/nmicrobiol.2016.189. [DOI] [PubMed] [Google Scholar]
- 54.Königer V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, Bonsor DA, Roth A, Kengmo-Tchoupa A, Smith SI, Mueller S, Sundberg EJ, Zimmermann W, Fischer W, Hauck CR, Haas R. 2016. Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol 2:16188. doi: 10.1038/nmicrobiol.2016.188. [DOI] [PubMed] [Google Scholar]
- 55.Isberg RR, Leong JM. 1988. Cultured mammalian cells attach to the invasin protein of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A 85:6682–6686. doi: 10.1073/pnas.85.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linz B, Windsor HM, McGraw JJ, Hansen LM, Gajewski JP, Tomsho LP, Hake CM, Solnick JV, Schuster SC, Marshall BJ. 2014. A mutation burst during the acute phase of Helicobacter pylori infection in humans and rhesus macaques. Nat Commun 5:4165. doi: 10.1038/ncomms5165. [DOI] [PubMed] [Google Scholar]
- 57.Dixit N, Yamayoshi I, Nazarian A, Simon SI. 2011. Migrational guidance of neutrophils is mechanotransduced via high-affinity LFA-1 and calcium flux. J Immunol 187:472–481. doi: 10.4049/jimmunol.1004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pepe JC, Badger JL, Miller VL. 1994. Growth phase and low pH affect the thermal regulation of the Yersinia enterocolitica inv gene. Mol Microbiol 11:123–135. doi: 10.1111/j.1365-2958.1994.tb00295.x. [DOI] [PubMed] [Google Scholar]
- 59.Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S, Peek RM Jr.. 2001. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest 107:611–620. doi: 10.1172/JCI11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haley KP, Blanz EJ, Gaddy JA. 2014. High resolution electron microscopy of the Helicobacter pylori Cag type IV secretion system pili produced in varying conditions of iron availability. J Vis Exp 93:e52122. doi: 10.3791/52122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delahay RM, Balkwill GD, Bunting KA, Edwards W, Atherton JC, Searle MS. 2008. The highly repetitive region of the Helicobacter pylori CagY protein comprises tandem arrays of an alpha-helical repeat module. J Mol Biol 377:956–971. doi: 10.1016/j.jmb.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubois A, Berg DE, Incecik ET, Fiala N, Heman-Ackah LM, Perez-Perez GI, Blaser MJ. 1996. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun 64:2885–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morelli G, Didelot X, Kusecek B, Schwarz S, Bahlawane C, Falush D, Suerbaum S, Achtman M. 2010. Microevolution of Helicobacter pylori during prolonged infection of single hosts and within families. PLoS Genet 6:e1001036. doi: 10.1371/journal.pgen.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adherence of invasin-expressing E. coli. (A) Immunoblot of E. coli with plasmid pRI253 with or without invA, untreated or treated with IPTG to induce InvA expression. Full-length InvA is around 100 kDa, and the lower bands represent degradation products (55, 58). (B) Attachment to α5β1 integrin in a competitive assay. The two strains of E. coli were stained with either DiD or DiO and mixed 1:1 for a total OD600 of 0.8. A parallel dye swap was performed to exclude influence of the dye on the integrin attachment. Download FIG S1, TIF file, 1.3 MB (1.4MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Fluorophore labeling control experiments. (A) Percentage of cells stained with Dil or DiO [(fluorescently stained cells divided by total cells seen on bright field) ×100] was determined for the H. pylori J166 WT and the ΔcagPAI, ΔcagY, and isogenic strains expressing functional (ΔcagY[Out1]) or nonfunctional (ΔcagY[Out3]) cagY alleles. (B) The H. pylori J166 WT and ΔcagPAI mutant (each at an OD600 of 0.4) were labeled with DiI or DiO, respectively (left), mixed 1:1, and imaged with a 546-nm (DiI) or 488-nm (DiO) light source. Parallel experiments were performed with a dye swap (middle). The WT labeled with DiI was also mixed with the WT labeled with DiO (right). Binding to α5β1 integrin was similar, whether detected with DiO or Dil fluorescent dyes and whether in competition with a strain with low or high binding ability. Results for the WT measured in a competition setting were also very similar to those when measured individually (compare with Fig. 2B). Data represent the mean ± SEM from ≥3 experiments. Download FIG S2, TIF file, 1.9 MB (1.9MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Expression of CagY in the wild-type and H. pylori J166 isogenic knockout mutants. (A) Immunoblot of CagY in WT H. pylori and isogenic cagY, cagA, cagI/L, and cagE knockouts. The immunoblot of UreB is shown as a loading control. (B to D) Quantification of whole-cell CagY MRR fluorescence signal normalized to DAPI in nonpermeabilized WT H. pylori and isogenic knockouts or cagY variants. CagY surface expression in strains with MRR motifs that bind α5β1 integrin and have a functional cagPAI (variants 1 and 2) is similar to that in strains that do not bind integrin and do not have a functional cagPAI (variants 3 and 4), although all are generally lower than J166 WT. Results are expressed as the percentage of the ratio of deletion mutant to WT and represent the mean ± SEM from 3 independent experiments. MFI, mean fluorescence intensity. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, compared to WT. Download FIG S3, TIF file, 3.6 MB (3.7MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Field emission scanning gun electron microscopy (FEG-SEM) of H. pylori PMSS1. (A) FEG-SEM of H. pylori PMSS1 WT and ΔcagPAI mutant cultured with or without AGS cells. Pili are indicated by white arrows. (B) Enumeration of pili per bacterial cell. ***, P < 0.001. Download FIG S4, TIF file, 4.2 MB (4.3MB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
T4SS function in various cagPAI H. pylori J166 mutants. Shown is IL-8 induction in AGS cells cocultured with the J166 WT or ΔcagY, cagXY, cagY, and ΔcagYMRR mutants. ***, P < 0.001. Download FIG S5, TIF file, 0.1 MB (105.7KB, tif) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used for PCR. Download TABLE S1, DOCX file, 0.1 MB (21.4KB, docx) .
Copyright © 2018 Skoog et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.