Abstract
Background
The epidemiology and clinical features of human coronaviruses (HCoVs) in children are not fully characterized.
Methods
A retrospective study of children with HCoV detected by reverse-transcriptase polymerase chain reaction (RT-PCR) was performed for a community cohort and a children’s hospital in the same community from January 2013 to December 2014. The RT-PCR assay detected HCoV 229E, HKU1, NL63, and OC43 in nasal swabs from symptomatic children ≤18 years. Factors associated with increased severity of illness in hospitalized children were assessed by multivariable logistic regression.
Results
Human coronavirus was detected in 261 children, 49 and 212 from the community and hospital, respectively. The distribution of HCoV types and seasonal trends were similar in the community and hospital. Community cases were older than hospitalized cases (median age, 4.4 versus 1.7 years, respectively; P < .01), and a minority of community cases (26.5%) sought medical attention. Among the hospitalized children with HCoV detected, 39 (18.4%) received respiratory support and 24 (11.3%) were admitted to the pediatric intensive care unit (PICU). Age <2 years (odds ratio [OR] = 5.0; 95% confidence interval [CI], 1.9–13.1) and cardiovascular (OR = 3.9; 95% CI, 1.6–9.5), genetic/congenital (OR = 2.8; 95% CI, 1.1–7.0), and respiratory chronic complex conditions ([CCCs] OR = 4.5; 95% CI, 1.7–12.0) were associated with receiving respiratory support. Genetic/congenital (OR = 2.8; 95% CI, 1.1–7.4) CCCs were associated with PICU admission. Severity of illness was similar among hospitalized children with different HCoV types.
Conclusions
Children in the community with HCoV detected generally had mild illness as demonstrated by few medically attended cases. In hospitalized children, young age and CCCs, but not HCoV type, were associated with increased severity of illness.
Keywords: community surveillance, medically attended, pediatric ICU, respiratory viral illness
Human coronaviruses (HCoVs) are known causes of human disease, and HCoVs causing severe acute respiratory syndrome and Middle East respiratory syndrome have caused international outbreaks associated with high mortality rates [1]. Human coronavirus 229E and OC43, identified in the 1960s, and NL63 and HKU1, identified more recently [2–4], have been described in upper respiratory tract infections, asthma, bronchiolitis, pneumonia, and croup, with more severe disease in infants, the elderly, and immunocompromised individuals [5–8]. Although HCoV 229E, OC43, and NL63 have been shown to be pathogenic in humans [9, 10], the pathogenicity of HKU1 is less well described.
Until recently, epidemiologic studies for HCoV were limited, in part because of a lack of commercially available diagnostic assays. However, US Food and Drug Adminstration (FDA)-approved assays that use reverse-transcriptase polymerase chain reaction (RT-PCR) technology now enable rapid, sensitive, and specific detection of the HCoV types 229E, OC43, NL63, and HKU1, from respiratory tract specimens and therefore allow more comprehensive studies of the epidemiology and clinical features of persons who have HCoV detected [11].
The current study presents a unique opportunity to expand our understanding of HCoV in ill children from the community and children admitted to a hospital in the same community, who were tested using the same RT-PCR assay. The objectives of this study were to (1) characterize the epidemiology of HCoV detected in children from a community-based cohort and in hospitalized children, (2) assess the clinical features of illnesses with different HCoV types detected, and (3) evaluate potential risk factors associated with increased severity of illness, defined as the use of respiratory support and/or hospitalization in the pediatric intensive care unit (PICU).
METHODS
Study Design, Study Subjects, and Study Sites
From January 2013 to December 2014, a retrospective study was performed to identify children ≤18 years of age with HCoV detected in respiratory tract in nasal or nasopharyngeal swab specimens collected using flocked nylon swabs. If a child had more than 1 positive test for HCoV, subsequent episodes were included if a different HCoV type was detected. There was no minimum time frame between subsequent episodes, but we used chart reviews to confirm these were distinct episodes. The Columbia University Medical Center (CUMC) Institutional Review Board approved this study; the community cohort provided written informed consent to be in the study, verbal consent for swabs, and children were granted a waiver of verbal assent; a waiver of informed consent was granted for hospitalized children.
Children from the community cohort were participants in the MObile Surveillance for ARI/ILI in the Community (MoSAIC) study (Centers for Disease Control and Prevention, Grant number: 1UO1IP000618), based in the Washington Heights and Inwood areas of Northern Manhattan, who tested positive for HCoV during the surveillance period [12]. The MoSAIC study performs prospective community-based surveillance for acute respiratory illness (ARI) using text messaging. Households in the MoSAIC study were selected using a random sample of participants who were previously enrolled in a large, community-based survey (https://www.dbmi.columbia.edu/impact/wicer/, last accessed: April 14, 2017). Households receive twice-weekly text messages inquiring if any members have ARI symptoms, and ill individuals are swabbed in their homes by research staff if they had 2 of the following: fever/feverishness, cough, pharyngitis, rhinorrhea/nasal congestion, and body aches. Children less than 1 year of age meet criteria for swabbing if they have rhinorrhea/congestion only. Swabs were not collected if subjects refused collection or if they were no longer symptomatic at the time of collection.
Children in the hospital group were admitted to the NewYork-Presbyterian Morgan Stanley Children’s Hospital (MSCH) and CUMC and were tested for respiratory pathogens according to the medical judgment of their treating providers. Respiratory pathogen testing for inpatients at MSCH is recommended only in the presence of active respiratory symptoms. Hospitalized children had their first positive HCoV test either the day before admission (eg, in the emergency department [ED]) or within the first 2 calendar days of admission, to exclude subjects with healthcare-associated infection.
Viral Diagnostic Testing
Nasal swabs from the community and nasopharyngeal swabs from hospitalized subjects were analyzed by multiplex RT-PCR using the same FDA-approved FilmArray Respiratory panel 1.7 (BioFire Diagnostics, Inc., Salt Lake City, UT) that identifies 20 respiratory pathogens including the following: adenovirus and coronavirus (strains HKU1, NL63, 229E, OC43); human metapneumovirus and rhinovirus/enterovirus; influenza (strains A, A/H1, A/H3, A/H1- 2009, B); parainfluenza virus (strains 1, 2, 3, 4); respiratory syncytial virus (RSV); as well as the bacterial respiratory pathogens Mycoplasma, pertussis, and Chlamydophilia. The community samples were tested in a research laboratory, and the hospital samples were tested in the Clinical Microbiology Laboratory at CUMC.
Associated Clinical Features of Human Coronaviruses
To assess the clinical features associated with acute respiratory infections in the community cohort, research staff asked participants about the presence of fever (defined as ≥37.8°C), their maximum temperature, feeling feverish, cough, sore throat, runny nose/nasal congestion, body aches, chills, headache, and wheezing. Medically attended events were defined as reported visits to the primary care provider or urgent care, admissions to the ED, and/or hospitalizations. Coinfection was defined as the presence of 2 or more viruses in the same PCR test.
To assess the severity of illness in hospitalized children with HCoV detected, their electronic medical records were reviewed to determine the type of respiratory support they received, including oxygen delivered by high-flow nasal cannula or nasal cannula, continuous positive airway pressure, bilevel positive airway pressure, intubation, and mechanical ventilation and/or PICU admission for management of respiratory illness.
Factors Associated With Increased Severity of Illness
To evaluate potential risk factors associated with increased severity of illness in the hospitalized children with HCoV, demographic (eg, age, sex) and clinical characteristics (ie, chronic complex conditions [CCCs]), HCoV types and viral coinfection associated with respiratory support (defined above), and/or PICU admissions were collected from the electronic medical record. Chronic complex conditions included prematurity (<37 weeks gestation in children <2 years of age), cardiovascular, gastrointestinal, genetic/congenital, hematologic/immunologic, malignancy, metabolic, neuromuscular, renal, and respiratory conditions [13, 14].
Data Analysis
Time trends for each HCoV type were generated for the community and hospital groups. Differences in the proportions of HCoV types detected in the community versus the hospital group were compared using χ2 tests. Demographic and clinical characteristics of children in both groups with different HCoV types were compared using analysis of variance for continuous variables and χ2 test for frequencies. Among hospitalized children, bivariate analyses assessed the associations of demographic characteristics, clinical factors, HCoV types, and presence of viral codetections, with increased severity of illness, ie, receiving respiratory support and/or admission to the PICU. To determine the possible impact of viral codetections, severity of illness was compared between hospitalized children with and without viral codetections. Because only 9 children in the community cohort had codetections, an analysis comparing those with and without codetections was not performed.
So that the analysis of severity of illness would not reflect the impact of other clinical factors, children admitted to the PICU and/or to the floor who required respiratory support for postoperative care or to manage CCCs and were subsequently found to have a positive test for HCoV were excluded (n = 16).
Multivariable logistic regression analyses were conducted to examine the associations between any predictor variables with a P value of <.1 in the bivariate analyses and each of 2 severity of illness outcomes, ie, receiving respiratory support and admission to the PICU. Analysis was repeated to adjust for duplicate patients by including a clustering variable for each unique patient to calculate robust standard errors. Risk factors with a P value <.05 in the multivariable analyses were considered significant. Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC) and R 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Among 455 positive RT-PCR tests in pediatric participants in the community cohort, 49 (10.8%) were positive for HCoV. Of 2582 positive RT-PCR tests performed among hospitalized children, 212 (8.2%) were positive for HCoV. Thus, this study included 261 children ≤18 years of age.
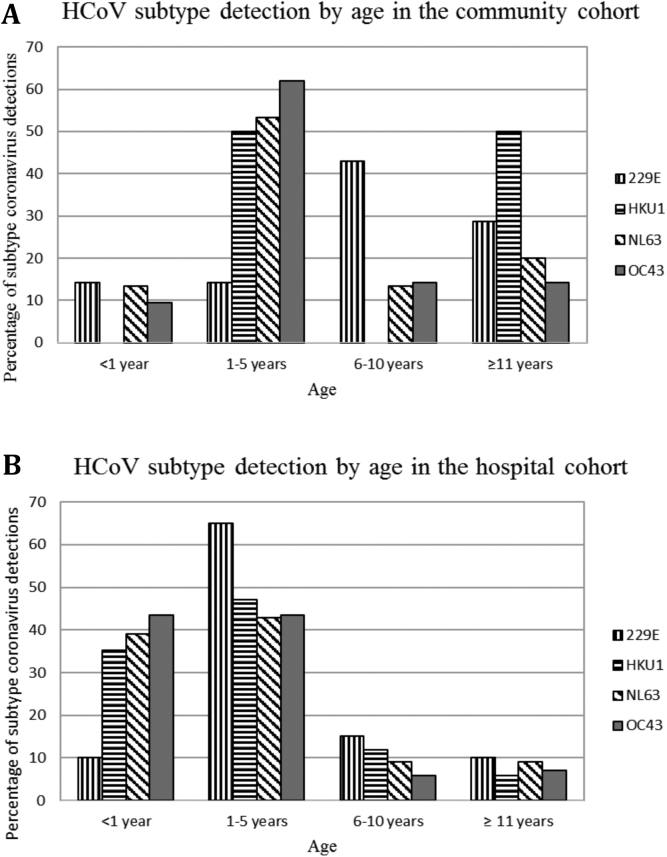
Households (n = 321) in the community cohort had an average of 4.8 members, and of the 1550 participants, 669 were children ≤18 years of age. The baseline distribution of age groups was 0–1 year (37, 5.5%), >1 year–5 years (138, 20.6%), >5 years–10 years (187, 28.0%), and >10 years–18 years (307, 45.9%). Participants were primarily Latino and publically insured. The refusal rate to obtain swabs over the study period was <1%. The majority of specimens (82.3%) were collected within 3 days of symptom onset. The characteristics of the 49 children in the community cohort (median age 4.4 years, mean 6.9 years) with HCoV detected are shown in (Table 1. The most common HCoV types detected were NL63 (30.6%) and OC43 (42.9%). The proportion of all HCoV types detected, except 229E, was highest among 1–5 year olds (Figure 1A). Five (10.2%) children also had other respiratory viruses detected, and 4 children (8.2%) had more than one HCoV type detected (Table 1). Most (73.5%) community cases did not report CCCs other than asthma (20.4%).
Table 1.
Characteristics of Children ≤18 Years Old With HCoV Detected From January 2013 to December 2014 From a Community-Based Cohort From Washington Heights/Inwood Neighborhoods of New York City
| All HCoV | 229Ea | HKU1 | NL63 | OC43 | |
|---|---|---|---|---|---|
| Number of cases | 49 | 7 | 10 | 15 | 21 |
| Age (in years) | |||||
| Average (SD) | 6.9 (5.3) | 8.2 (5.5) | 8.4 (5.7) | 6.4 (5.7) | 5.2 (4.6) |
| <1 year | 4 (8.2%) | 1 (14.3%) | 0 (0.0%) | 2 (13.3%) | 2 (9.5%) |
| 1–5 years | 24 (49.0%) | 1 (14.3%) | 5 (50.0%) | 8 (53.3%) | 13 (61.9%) |
| 6–10 years | 8 (16.3%) | 3 (42.9%) | 0 (0.0%) | 2 (13.3%) | 3 (14.3%) |
| ≥11 years | 13 (26.5%) | 2 (28.6%) | 5 (50.0%) | 3 (20.0%) | 3 (14.3%) |
| Sex | |||||
| Male | 25 (51.0%) | 3 (42.9%) | 6 (60.0%) | 8 (53.3%) | 10 (47.6%) |
| Raceb | |||||
| White | 13 (26.5%) | 0 (0%) | 5 (50.0%) | 2 (13.3%) | 7 (33.3%) |
| Ethnicity | |||||
| Latino/Hispanic | 48 (98.0%) | 7 (100.0%) | 10 (100.0%) | 15 (100.0%) | 20 (95.2%) |
| Chronic Complex Conditions | |||||
| Anyc | 13 (26.5%) | 1 (14.3%) | 1 (10.0%) | 5 (33.3%) | 6 (28.6%) |
| Asthma | 10 (20.4%) | 0 (0.0%) | 1 (10.0%) | 5 (33.3%) | 4 (19.0%) |
| None | 36 (73.5%) | 6 (85.7%) | 9 (90.0%) | 10 (66.7%) | 15 (71.4%) |
| Coinfections | |||||
| HCoV OC43 | 3 (6.1%) | 2 (28.6%) | 0 (0%) | 1 (6.7%) | 0 (0%) |
| HCoV NL63 | 1 (2.0%) | 0 (0%) | 1 (10.0%) | 0 (0%) | 0 (0%) |
| Human rhinovirus/enterovirus | 3 (6.1%) | 0 (0%) | 0 (0%) | 2 (13.3%) | 1 (4.8%) |
| Respiratory syncytial virus | 1 (2.0%) | 0 (0%) | 0 (0%) | 1 (6.7%) | 0 (0%) |
| Influenza A H3 | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.8%) |
| Adenovirus | 1 (2.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (4.8%) |
Abbreviations: HCoV, human coronavirus; SD, standard deviation.
aHCoV strains.
bNo participants reported they were Black/African American or Asian.
cChronic complex conditions other than asthma included diabetes (n = 1), neurologic conditions (n = 4) and prematurity (n = 1).
Figure 1.
(A and B) Distribution of human coronavirus (HCoV) types by age in the community cohort (A) and in hospitalized children (B) from January 2013 to December 2014.
The characteristics of the 212 hospitalized children (median age 1.7 years, mean 3.2 years) are shown in (Table 2). Hospitalized children with HCoV were significantly younger than the children in the community cohort (3.2 vs 6.9 years, P < .01). The most common HCoV types were OC43 (40.1%) and NL63 (36.3%). The proportion of all HCoV types in the hospital group was highest in children 1–5 years of age (Figure 1B). Fifty-eight (27.4%) children also had other respiratory viruses detected (ie, RSV [17], human rhinovirus/enterovirus [28], human metapneumovirus [3], influenza A H1 2009 virus [2], influenza B virus [1], parainfluenza 2 virus [1], parainfluenza 3 virus [3], or adenovirus [5]) including 4 children with 2 HCoV types detected. Over half (61.3%) of the hospitalized children had 1 or more CCCs, most commonly respiratory (20.3%) and cardiovascular (16.5%) conditions. Significantly more hospitalized children had CCCs than those in the community cohort (61.3% vs 26.5%, respectively; P < .001).
Table 2.
Characteristics of Hospitalized Children ≤18 Years Old Infected With HCoV From January 2013 to December 2014
| All HCoV (n = 212) | 229E (n = 20) | HKU1 (n = 34) | NL63 (n = 77) | OC43 (n = 85) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases | 212 | 20 | 34 | 77 | 85 | ||||||
| Age (in years) | |||||||||||
| Average (SD) | 3.2 (3.9) | 4.8 (4.1) | 3.3 (3.4) | 3.4 (4.4) | 2.8 (3.6) | ||||||
| <1 year | 80 (37.7%) | 2 (10.0%) | 12 (35.3%) | 30 (39.0%) | 37 (43.5%) | ||||||
| 1–5 years | 98 (46.2%) | 13 (65.0%) | 16 (47.1%) | 33 (42.9%) | 37 (43.5%) | ||||||
| 6–10 years | 18 (8.5%) | 3 (15.0%) | 4 (11.8%) | 7 (9.1%) | 5 (5.9%) | ||||||
| ≥11 years | 16 (7.5%) | 2 (10.0%) | 2 (5.9%) | 7 (9.1%) | 6 (7.1%) | ||||||
| Sex | |||||||||||
| Males | 126 (59.4%) | 11 (55.0%) | 19 (55.9%) | 46 (59.7%) | 51 (60.0%) | ||||||
| Racea | White | 107 (50.5%) | 7 (35.0%) | 12 (35.3%) | 36 (46.8%) | 53 (62.4%) | |||||
| Black/African American | 34 (16.0%) | 5 (25.0%) | 7 (20.6%) | 9(11.7%) | 13 (15.3%) | ||||||
| Asian | 7 (3.3%) | 1 (5.0%) | 1 (2.9%) | 5 (6.5%) | 1 (1.2%) | ||||||
| Unknown | 64 (30.2%) | 7 (35.0%) | 14 (41.2%) | 27 (35.1%) | 18 (21.2%) | ||||||
| Chronic Complex Conditions | |||||||||||
| Any | 130 (61.3%) | 16 (80.0%) | 19 (55.9%) | 49 (63.6%) | 48 (56.5%) | ||||||
| None | 82 (38.7%) | 4 (20.0%) | 15 (44.1%) | 28 (36.4%) | 37 (43.5%) | ||||||
| Cardiovascular | 35 (16.5%) | 3 (15.0%) | 7 (20.6%) | 11 (14.3%) | 14 (16.5%) | ||||||
| Gastrointestinal | 23 (10.8%) | 5 (25.0%) | 5 (14.7%) | 9 (11.7%) | 6 (7.1%) | ||||||
| Genetic/congenitalb | 32 (15.1%) | 1 (5.0%) | 5 (14.7%) | 10 (13.0%) | 16 (18.8%) | ||||||
| Hematologic/immunologic | 18 (8.5%) | 3 (15.0%) | 4 (11.8%) | 6 (7.8%) | 6 (7.1%) | ||||||
| Malignancy | 10 (4.7%) | 3 (15.0%) | 2 (5.9%) | 3 (3.9%) | 2 (2.3%) | ||||||
| Metabolic | 16 (7.5%) | 1 (5.0%) | 2 (5.9%) | 6 (7.8%) | 7 (8.2%) | ||||||
| Neuromuscular | 12 (5.7%) | 0 (0.0%) | 0 (0.0%) | 5 (6.5%) | 7 (8.2%) | ||||||
| Prematurity | 8 (3.8%) | 0 (0.0%) | 1 (2.9%) | 6 (7.8%) | 1 (1.2%) | ||||||
| Renal | 6 (2.8%) | 0 (0.0%) | 1 (2.9%) | 4 (5.2%) | 1 (1.2%) | ||||||
| Respiratoryc | 43 (20.3%) | 6 (30.0%) | 6 (17.6%) | 19 (24.7%) | 15 (17.6%) |
Abbreviations: CCC, chronic complex conditions; HCoV, human coronavirus; SD, standard deviation.
aEthnicity is not reported due to inaccurate and incomplete data in the electronic medical records.
bGenetic/congenital CCC include anomalies of bones, joints, chromosomes, diaphragm, abdominal wall, and others not included in any other CCC categories.
cRespiratory CCC include asthma/reactive airway disease, chronic lung disease/respiratory insufficiency, cystic fibrosis, and laryngomalacia/tracheomalacia.
Human Coronaviruses Epidemiology
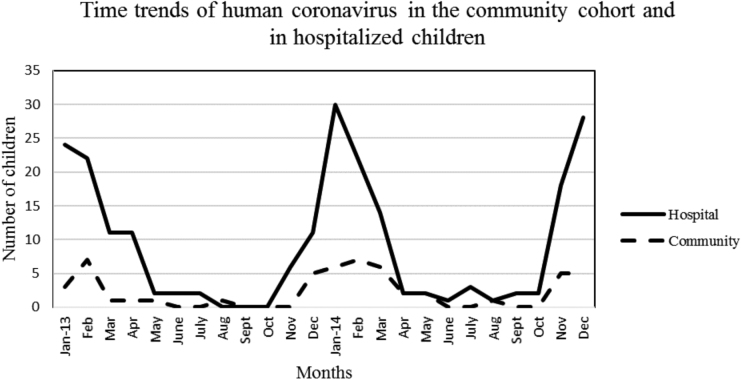
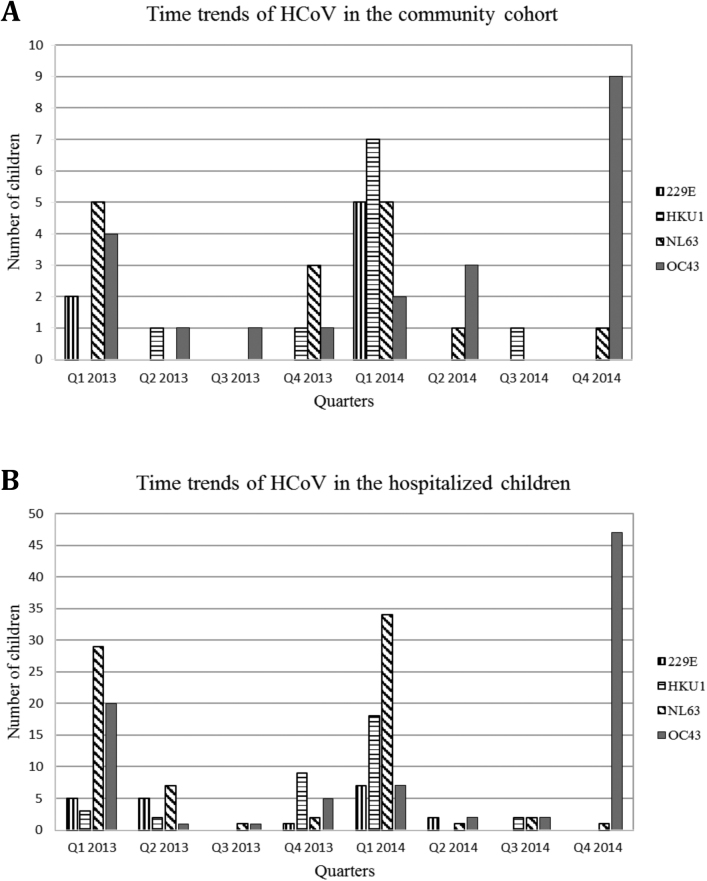
Mostly similar time trends for HCoV were noted among the community cohort and hospitalized children (Figure 2). Human coronavirus was detected predominantly from the late fall to spring, with different types predominating in different years, eg, HKU1 predominated in winter 2013–2014 and OC43 predominated in winter 2014. These trends appeared similar in the community and hospital groups (Figure 3A and B).
Figure 2.
Time trends of human coronavirus in the community cohort and in hospital children from January 2013 to December 2014.
Figure 3.
(A and B) Time trends of human coronavirus (HCoV) types in the community cohort (A) and in hospitalized children (B) from January 2013 to December 2014. Note the differences in the y-axis.
Human Coronaviruses Clinical Features
In the community cohort, the most commonly reported symptoms associated with HCoV were runny nose/nasal congestion (87.8%), cough (85.7%), and sore throat (30.6%). Fever (14.3%), feeling feverish (22.4%), and headache (6.1%) were relatively uncommon, and no participants reported wheezing. The median duration of reported illness was 7 days (interquartile range = 4–14 days). Symptoms associated with the 4 HCoV types were similar across all types, with the exception of cough (P = .02), which was most likely to be reported by those with OC43 (95.2%) followed by HKU1 (90%), NL63 (86.7%), and 229E (42.9%). Thirteen (26.5%) children in the community cohort sought medical attention for their illness; all had visits to their primary care provider, and none were admitted to the ED or were hospitalized. The frequency of medically attended events among the HCoV types was similar (28.5%, 30.0%, 40.0%, and 28.5% with 229E [2 of 7], HKU1 [3 of 10], NL63 [6 of 15], and OC43 [6 of 21], respectively). In 12 (24.5%) cases, children and/or their household members missed a total of 23 days of school and/or work due to the child’s illness.
Among hospitalized children, the most common symptoms reported were cough (66.9%), nasal congestion (55.2%), fever (54.2%), runny nose (38.2%), and wheezing (19.3%). Symptoms associated with the 4 types were similar, with the exception of nasal congestion (P = .02), which was most commonly noted in those with OC43 (65.9%) followed by HKU1 (61.8%), 229E (45.0%), and NL63 (42.8%). Croup was diagnosed in 11 cases, 7 (63.6%) of them appearing in those with NL63 and 4 (36.4%) in those with OC43. Overall, 45 (21.2%) hospitalized children had increased severity of illness; 39 (18.4%) received respiratory support and 24 (11.3%) were admitted to the PICU. Among children without viral coinfections, 32 (20.3%) had increased severity of illness; 29 (18.4%) received respiratory support and 15 (9.5%) were admitted to the PICU (Table 3). The proportion of children receiving respiratory support and/or admitted to the PICU was similar among the HCoV types (P = .9). The median hospital length of stay was 11 days (mean, 17.1 days) for those admitted to the PICU and 3 days (mean, 4.8 days) for those not admitted to the PICU. A similar proportion of those with (n = 58) and without (n = 154) viral codetections received respiratory support (10 of 58 [17.2%] vs 29 of 154 [18.8%], P = .79) and/or were admitted to the PICU (9 of 58 [15.5%] vs 15 of 154 [9.7%], P = .23), as shown in (Table 4).
Table 3.
Severity of Illness Among Hospitalized Children ≤18 Years Old With HCoV, With and Without Viral Codetections, From January 2013 to December 2014
| HCoV | 229E | HKU1 | NL63 | OC43 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Alla (n = 212) | No CIb (n = 158) | All (n = 20) | No CI (n = 14) | All (n = 34) | No CI (n = 24) | All (n = 77) | No CI (n = 61) | All (n = 85) | No CI (n = 63) | |
| Respiratory support and/or PICU admission | 45 (21.2%) | 32 (20.3%) | 3 (15.0%) | 3 (21.4%) | 7 (20.6%) | 5 (20.8%) | 18 (23.4%) | 14 (23.0%) | 17 (20.0%) | 10 (15.9%) |
| Respiratory supportc | 39 (18.4%) | 29 (18.4%) | 3 (15.0%) | 3 (21.4%) | 5 (14.7%) | 4 (16.7%) | 16 (20.8%) | 13 (21.3%) | 15 (17.6%) | 9 (14.3%) |
| Pediatric ICU Admission | 24 (11.3%) | 15 (9.5%) | 0 (0.0%) | 0 (0.0%) | 4 (11.8%) | 2 (8.3%) | 9 (11.7%) | 6 (9.8%) | 11 (12.9%) | 7 (11.1%) |
| Average PICU LOS, days (SD) | 1.3 (7.1) | 1.4 (8.0) | 0 (0) | 0 (0) | 0.4 (1.1) | 0.3 (1.2) | 1.2 (4.9) | 1.3 (5.4) | 1.9 (10.1) | 2.1 (11.5) |
| Average hospital LOS, days (SD) | 6.2 (9.4) | 6.5 (9.8) | 4.0 (3.6) | 4.6 (4.1) | 7.0 (8.6) | 7.7 (9.2) | 6.0 (7.7) | 6.3 (8.3) | 6.6 (11.7) | 6.4 (11.9) |
| Median hospital LOS, days | 3 | 3 | 3 | 3 | 4 | 4 | 3 | 4 | 3 | 3 |
Abbreviations: BiPAP, bilevel positive airway pressure; CI, coinfection; CPAP, continuous positive airway pressure; HCoV, human coronavirus; ICU intensive care unit; LOS, length of stay; PICU, pediatric intensive care unit; SD, standard deviation.
aIncludes children with and without viral coinfection.
bOnly children without viral coinfection.
cTwenty-one children receiving respiratory support were not admitted to the PICU, 10 of whom received CPAP and 3 received BiPAP. Eighteen children receiving respiratory support were hospitalized in the PICU.
Table 4.
Bivariate Analysis of Factors Associated With Increased Severity of Illness in Hospitalized Children ≤18 Years Old With Human Coronavirus From January 2013 to December 2014
| Outcomes | ||||||
|---|---|---|---|---|---|---|
| PICU Admission | Respiratory Support | |||||
| Variables | Yes (n = 24) | No (n = 188) | P Value | Yes (n = 39) | No (n = 173) | P Value |
| Age (<2 years) | 16 (66.7%) | 101 (53.7%) | .230 | 28 (71.8%) | 89 (51.4%) | .021 |
| Sex—Male | 11 (45.8%) | 115 (61.2%) | .149 | 18 (46.2%) | 108 (62.4%) | .061 |
| Chronic Complex Conditions | ||||||
| Any | 19 (79.2%) | 111 (59.0%) | .057 | 31 (79.5%) | 99 (57.2%) | .010 |
| Cardiovascular | 8 (33.3%) | 28 (14.9%) | .023 | 14 (35.9%) | 22 (12.7%) | .0004 |
| Gastrointestinal | 1 (4.2%) | 22 (11.7%) | .263 | 6 (15.4%) | 17 (9.8%) | .390 |
| Genetic/congenital | 8 (33.3%) | 26 (13.8%) | .014 | 13 (33.3%) | 21 (12.1%) | .001 |
| Hematologic/immunologic | 1 (4.2%) | 17 (9.0%) | .700 | 2 (5.1%) | 16 (9.2%) | .537 |
| Malignancy | 1 (4.2%) | 9 (4.8%) | .999 | 0 (0.0%) | 10 (5.8%) | .213 |
| Metabolic | 3 (12.5%) | 13 (6.9%) | .400 | 5 (12.8%) | 11 (6.4%) | .182 |
| Neuromuscular | 3 (12.5%) | 10 (5.3%) | .170 | 6 (15.4%) | 7 (4.0%) | .017 |
| Prematurity | 1 (4.2%) | 7 (3.7%) | .999 | 3 (7.7%) | 5 (2.9%) | .165 |
| Renal | 1 (4.2%) | 5 (2.7%) | .518 | 2 (5.1%) | 4 (2.3%) | .304 |
| Respiratory | 7 (29.2%) | 36 (19.1%) | .250 | 14 (35.9%) | 29 (16.8%) | .007 |
| Viral Pathogens | ||||||
| Coinfections | 9 (37.5%) | 49 (26.1%) | .236 | 10 (25.6%) | 48 (27.7%) | .789 |
| HCoV-229E | 0 (0.0%) | 20 (10.6%) | .138 | 3 (7.7%) | 17 (9.8%) | .999 |
| HCoV-HKU1 | 4 (16.7%) | 30 (16.0%) | .928 | 5 (12.8%) | 29 (16.8%) | .544 |
| HCoV-NL63 | 9 (37.5%) | 68 (36.2%) | .898 | 16 (41.0%) | 61 (35.3%) | .499 |
| HCoV-OC43 | 11 (45.8%) | 74 (39.4%) | .542 | 15 (38.5%) | 70 (40.5%) | .817 |
Abbreviations: HCoV, human coronavirus; PICU, pediatric intensive care unit. Statistically significant P values (P < .1) are indicated in bold.
Factors Associated With Increased Severity of Illness
In bivariate analyses, children <2 years of age and those with any CCC or specifically with cardiovascular, genetic/congenital, neuromuscular, or respiratory CCCs were more likely to receive respiratory support (Table 4). Those with cardiovascular or genetic/congenital CCCs were more likely to be admitted to the PICU. In the multivariable analyses, children <2 years of age and those with cardiovascular, genetic/congenital, or respiratory CCCs were more likely to receive respiratory support, and those with genetic/congenital CCCs were more likely to be admitted to the PICU (Table 5). Clustered regression by patient to account for duplicates in the sample produced similar results.
Table 5.
Multivariable Analysis of Factors Associated With Increased Severity of Illness in Hospitalized Children ≤18 Years Old With Human Coronavirus From January 2013 to December 2014
| Outcomes | Variables | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| Respiratory Support | Age (<2 years) | 4.7 (1.8–12.0) | .001 |
| Chronic Complex Conditions | |||
| Cardiovascular | 4.0 (1.7–9.7) | .002 | |
| Genetic/congenital | 3.3 (1.3–7.9) | .009 | |
| Respiratory | 4.9 (1.9–12.9) | .001 | |
| PICU Admission | Chronic Complex Conditions | ||
| Cardiovascular | 2.6 (0.9–6.7) | .054 | |
| Genetic/congenital | 2.8 (1.1–7.4) | .035 | |
Abbreviations: CI, confidence interval; PICU, pediatric intensive care unit. Statistically significant P values (P < .05) are indicated in bold.
DISCUSSION
We had 2 complementary datasets to provide new characterization of HCoV epidemiology as well as severity of illness among both community-dwelling and hospitalized children with respiratory infections that tested positive for HCoV. We found that hospitalized children with HCoV were younger than those in the community cohort and more likely to have CCCs. Seasonal trends as well as the distribution of 4 HCoV types were similar in the community and hospitalized group; OC43 was most common followed by NL63 in both settings. This is similar to findings from other studies conducted in children in the community or the hospital [15–17]. As others have previously reported, we noted that different types predominated in different winters [18, 19].
In both the community and hospitalized groups, we found that HCoV types generally were associated with similar clinical features. There were relatively few medically attended events in the community group, and no participants were admitted to the ED or hospitalized. Among hospitalized children, no type was significantly associated with an increased likelihood of receiving respiratory support and/or PICU admission. However, in the hospitalized group, we did find that demographic and clinical factors were associated with increased severity of illness including age <2 years and cardiovascular, genetic/congenital, and respiratory conditions. Others have also observed a substantial burden of disease associated with HCoV NL63 or OC43 in children <5 years of age with severe disease occurring more often in the presence of underlying medical conditions [16]. Our results supported an association between HCoV NL63 and croup, which has been noted in multiple studies [20, 21].
Our findings suggest that HCoV HKU1 could be a pathogen; this type was detected in 10 (20.4%) children in the community group and 34 (16.0%) in the hospitalized group including 24 without codetections. There were no discernible differences in the host factors, clinical symptoms, severity of illness, or frequency of coinfections associated with HKU1 compared with the other 3 types.
Our study had limitations. The findings from the community cohort may not be generalizable because this population was largely Latino/Hispanic and under active surveillance for respiratory illnesses. Findings from our hospital sample are likely to reflect referral center bias because CUMC is a tertiary pediatric care center, and, as such, there is an overrepresentation of children with CCCs. Collection of nasal swabs for the community surveillance component of the study versus nasopharyngeal swabs in the hospital could have impacted viral detection. We did not capture healthcare-acquired infections, and we could have missed additional HCoV detections [22]. We collected symptoms differently in the 2 groups; symptoms in the community cohort were reported by the participants, whereas symptoms in hospitalized children were extracted from the medical records. Subjective symptoms may have been underreported for younger children in the community cohort leading to bias. Due to the small sample, our analysis did not assess the impact of specific types of viral codetections or bacterial coinfections with Mycoplasma, Bordetella, or Chlamydophila pneumoniae. Although testing was done only for symptomatic individuals in this study, respiratory infections could have been incidental to the reason a child was hospitalized. We most likely did not accurately determine the percentage of children with asymptomatic shedding of HCoV compared with those for whom HCoV was the primary cause of their symptoms. There could also have been other confounding conditions, eg, presence of bacterial superinfection, which contributed to the clinical picture that were not assessed. We lacked healthy controls with which to compare our cohorts. Finally, our analysis was limited by relatively few cases with increased severity of illness.
CONCLUSIONS
In conclusion, children from the community with HCoV detections were mostly 5 years of age and younger with nonmedically attended illness. Among hospitalized children with HCoV detected, a significant number received respiratory support and/or were admitted to the PICU, and this was the same for children without coinfection. Children less than 2 years of age and those with CCCs were at risk of increased severity of illness. Clinical manifestations and severity of illness were similar among the 4 HCoV types studied. In this study, we demonstrated that coronaviruses were detected in a significant proportion of children in both community and hospital settings and could be a contributor to clinically significant respiratory illnesses. Future multicenter studies with representative study samples conducted over a longer time period should be done to provide additional insights into the epidemiology and clinical features of HCoV.
Notes
Author contributions. Investigators from the Centers for Disease Control and Prevention (CDC) took part in designing and conducting the study, analyses and interpretation of the data, and review and approval of the manuscript.
Acknowledgments. We thank the rest of the MObile Surveillance for ARI/ILI in the Community (MoSAIC) study team: Hilbania Diaz, Yaritza Castellanos de Belliard, Maria Morban, Othanya Garcia, and Liqun Wang.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was funded by the Centers for Disease Control and Prevention (Grant U01IP000618) and the National Institutes of Health (Grant T32A1007531; to P. Z.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed..
References
- 1. Coleman CM, Frieman MB. Coronaviruses: important emerging human pathogens. J Virol 2014; 88:5209–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Walsh EE, Shin JH, Falsey AR. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis 2013; 208:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van der Hoek L Pyrc K Jebbink MF, et al. Identification of a new human coronavirus. Nat Med 2004; 10:368–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woo PC Lau SK Chu CM, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol 2005; 79:884–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McIntosh K Ellis EF Hoffman LS, et al. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. J Pediatr 1973; 82:578–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graat JM Schouten EG Heijnen ML, et al. A prospective, community-based study on virologic assessment among elderly people with and without symptoms of acute respiratory infection. J Clin Epidemiol 2003; 56:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh EE, Shin JH, Falsey AR. Clinical impact of human coronaviruses 229E and OC43 infection in diverse adult populations. J Infect Dis 2013; 208:1634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pene F Merlat A Vabret A, et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis 2003; 37:929–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradburne AF, Somerset BA. Coronative antibody tires in sera of healthy adults and experimentally infected volunteers. J Hyg (Lond) 1972; 70:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiu SS Chan KH Chu KW, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis 2005; 40:1721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caliendo AM. Multiplex PCR and emerging technologies for the detection of respiratory pathogens. Clin Infect Dis 2011; 52:S326–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stockwell MS Reed C Vargas CY, et al. MoSAIC: mobile surveillance for acute respiratory infections and influenza-like illness in the community. Am J Epidemiol 2014; 180:1196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simon TD Berry J Feudtner C, et al. Children with complex chronic conditions in inpatient hospital settings in the United States. Pediatrics 2010; 126:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics 2000; 106:205–9. [PubMed] [Google Scholar]
- 15. Dijkman R Jebbink MF Gaunt E, et al. The dominance of human coronavirus OC43 and NL63 infections in infants. J Clin Virol 2012; 53:135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee J, Storch GA. Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J 2014; 33:814–20. [DOI] [PubMed] [Google Scholar]
- 17. Talbot HK Shepherd BE Crowe JE Jr, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J 2009; 28:682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaunt ER Hardie A Claas EC, et al. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol 2010; 48:2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vabret A Dina J Gouarin S, et al. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health 2008; 44:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van der Hoek L Sure K Ihorst G, et al. Croup is associated with the novel coronavirus NL63. PLoS Med 2005; 2:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sung JY Lee HJ Eun BW, et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J 2010; 29:822–6. [DOI] [PubMed] [Google Scholar]
- 22. Gagneur A Sizun J Vallet S, et al. Coronavirus-related nosocomial viral respiratory infections in a neonatal and paediatric intensive care unit: a prospective study. J Hosp Infect 2002; 51:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]