Abstract
Introduction:
Monosodium glutamate (MSG), an extensively used flavor enhancer, produces degenerative changes in cell and causes neural death which cause imbalance in endocrine system and disturb the body system. While taking consideration of its potency and affinity, it falls under the category of Dooshi Visha, which leads to various diseases among which Kshapayet Shukram is one and can be correlated with disturbance in reproductive system which may be endocrinological or related to gametes. Dooshivishari Agada (DVA) is a herbomineral formulation which is mentioned for the treatment of Dooshi Visha.
Aim:
The aim is to evaluate the efficacy of DVA in MSG-induced female reproductive toxicity in Wistar rats.
Materials and Methods:
DVA was prepared and analyzed for preliminary physicochemical, organic and inorganic tests. Twenty-four rats were divided into four groups. Up to 14 days, MSG (0.20g/kg) was given to all groups except control group. From days 15 to 42, DVA (216mg/200g) was given to the third group and in the fourth group no intervention was given to evaluate auto recovery. The second group was considered as disease control. At the end, Follicle-stimu`lating hormone test and histopathology of ovary were done.
Results:
There was a significant increase in primary follicle count and decrease in atretic follicle count in DVA group. Secondary follicle count, tertiary follicle count and Graafian follicle count were increased in DVA group but the increase was not statistically significant.
Conclusion:
MSG mainly acts as a neurotoxic component by oxidative stress. Mostly, the ingredients of DVA have anti-oxidant properties, which counteract the oxidative stress caused by MSG in cell. Many ingredients such as Pippali, Tagara and Kushtha have neuroprotective property which corrects the neurodegeneration and balances the endocrine system.
Keywords: Anti-oxidant, Dooshi Visha, Follicle, neurotoxic
Introduction
Monosodium glutamate (MSG) is a flavor enhancer, also called as “AJINOMOTO;” is a combination of 78% glutamic acid and 22% sodium and water.[1] Studies have shown that it causes degenerative changes in the cells of brain,[2] liver, kidney,[3] spleen and pancreas,[4] reproductive organs[5,6] and also causes hormonal imbalance.[7]
Dooshi Visha is a unique concept mentioned in ancient Ayurvedic classics and “Kshapayet Shukram” is one of the effects of Dooshi Visha. MSG has the affinity to affect reproductive system and it has reduced potency, so it falls under the category of Dooshi Visha. Hence, this study was conducted to evaluate the effect of Dooshivishari Agada (DVA) in MSG induced female reproductive toxicity.
Materials and Methods
Chemicals
The chemical used in this study was MSG (C5H8NNaO4.H2O) purchased from SDFCL, Mumbai. A stock solution was prepared by dissolving MSG crystals in known milliliters (mL) of distilled water. The dose scheduled was so adjusted that the amount of MSG administration per animal was as per their respective weights. The dose and duration of MSG were taken from previous study done on MSG with special reference to female reproductive toxicity done by Oladipo, Adebayo and Kuye.
Drug
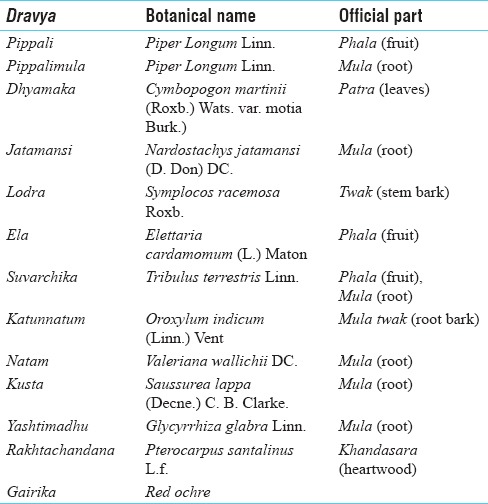
DVA is a herbomineral formulation which is explained in the context of treatment of Dooshi Visha in Ashtanga Hrudayam.[8] Powders of 13 ingredients [Table 1] were taken in equal quantity and trituration was done using decoction of the same ingredients mentioned in DVA as liquid media.
Table 1.
Ingredients of Dooshivishari Agada[8]
Ethics
Ethical clearance was taken from the Institutional Animal Ethical Committee (IAEC) with resolution no. BMK/IAEC/Res-14/01/2015.
Experimental animals
The study was carried out on 24 adult female Wistar rats (150 ± 20 g average weight) which were housed in cages at standard room temperature maintained on 12 h light/dark cycle. Rats were fed to a dry balanced meal (Grower mash) provided for experimental animals, with a continuous source of water. Rats were randomly divided into four groups: control, MSG, MSG+DVA and Auto recovery groups with six rats in each group. Control group was fed only on laboratory diet for 14 days. In the remaining groups, along with food and water, 0.5 ml of distilled water containing MSG 0.20g/kg body weight per day was given by oral gavage tube at 9 am daily for 14 days. In MSG+DVA group, from 15th to 42nd day, DVA (216 mg/200 g) was given and in auto recovery group, no intervention was given. Only normal food and water were given to observe the auto recovery from toxicity. On the 15th day, control group and MSG group and on the 43rd day MSG + DVA group and auto recovery group were sacrificed by anesthetizing with diethyl ether. In semi-conscious stage, 2ml blood was withdrawn and on complete death, animals were dissected and ovary was removed and preserved in 10% formalin. Data were analyzed using SPSS version 20 (IBM, Apache software foundation, immense, Switzerland) and expressed as mean ± standard deviation. Comparisons of the variables were made using the ANOVA followed by Post hoc Bonferroni test. Significance was tested at P ≤ 0.05.
Results
In present study, Follicle-stimulating hormone (FSH) and ovarian reserve were assessed. Ovarian reserve determines the capacity of an ovary to provide egg cells that are capable of fertilization resulting in healthy and successful pregnancy. The quantitative assessment of the ovaries in all groups was done by counting follicle of different stages.
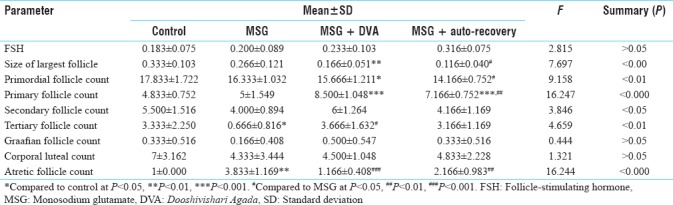
Regarding the FSH hormone, no statistically significant difference was found. Mean difference of size of the largest follicle was found highly significant at the level of P < 0.001 Primordial follicle count significantly decreased in Groups C and D compared to that of control group. Secondary follicle count was significant at P < 0.05. In MSG and auto recovery groups, follicle count was lower than that of the control. Tertiary follicle count was significantly decreased in MSG group (P < 0.01). In all the groups, Graafian follicle count has not shown any significant difference. The mean count of Graafian follicle was decreased in MSG group whereas it was increased in DVA and auto recovery groups. There was no statistically significant difference in corporal luteal count, but mean count was decreased in MSG group compared to the control group. In DVA and auto recovery groups, a slight increase in count was observed but not up to the count of control group. Atretic follicle count was increased at the level of P < 0.001 in MSG group compared to the control group [Table 2].
Table 2.
Result of follicle-stimulating hormone and all follicle count
Histopathology report of ovaries showed mild hypertrophy of theca folliculi in MSG group but it was absent in MSG+DVA and auto-recovery groups. Focal vacuolation was present in control and MSG+DVA groups as it may be normally present in normal histostructure of ovary but it was moderately present in MSG group and showed mild presence in auto recovery group.
Discussion
MSG is a synthetic salt of glutamic acid and used as a flavor enhancer in packed foods. It acts as Kritrima Visha. In higher dose, it will lead to lethality by acting as potent Garavisha and in low dose, it acts as a latent poison and produces the effects like that of Dooshi Visha and hence MSG acts as Kritrima Vishajanya Dooshi Visha.
MSG affects female reproductive system in two ways- by excitotoxicity[9] and by producing oxidative stress.[10] MSG causes arcuate lesions in hypothalamus and decreases catecholamine which are involved in the release of luteinizing hormone-releasing hormone.[11] Hence, MSG by disturbing Hypothalamus Pitutary Ovarian (HPO) axis causes hormonal imbalance.
Follicle-stimulating hormone
FSH showed no significant difference, but in auto recovery group, mean is almost double of that of control group. FSH level inversely co-relates to ovarian reserve. High FSH value indicates the poor ovarian reserve and quality of ovum is not good, which leads to infertility in females. If the ovary has many eggs, FSH in blood will be low because the body does not need to produce much FSH to induce normal ovulation. A high FSH means the egg number is reduced and the body is trying to produce more eggs so that FSH secretion increases.[12]
Ovarian reserve
Ovarian reserve refers to the number of eggs that are available for fertilization. A high ovarian reserve usually indicates good number of viable eggs present in ovaries. A low ovarian reserve may indicate fewer available eggs for fertilization.
Size of largest follicle
Size of the largest follicle was extremely significant with P < 0.001. The size was decreased in MSG group but was not significant and it was significantly decreased in DVA and auto recovery groups. This shows that some degeneration happens in follicle due to which the growth is not occurring normally. Follicles have receptor for FSH[13] which bind with FSH and growth takes place. MSG might be blocking or destroying the FSH receptor and the effect is persisting even with the intervention of DVA or withdrawal of MSG and appears not reversible.
Primordial follicle count
Count of primordial follicles showed highly significant difference at the level of P < 0.001. In DVA and auto recovery groups, significant decrease was seen compared to control group. In MSG group also, count was decreased but was not statistically significant. It might be the starting phase of degeneration and it might continue even after stoppage of MSG, as it establishes the concept of cumulative toxicity. MSG causes oxidative stress and degrades cell membrane due to which apoptosis of primordial follicles takes place.[9]
Primary follicle
The granulosa cells of primordial follicles change from a flat to a cuboidal structure, marking the beginning of the primary follicle. The oocyte genome is activated and genes become transcribed. Primary follicles develop receptors to FSH at this time, but they are gonadotropin independent until the antral stage.[13] FSH accelerates the growth of follicle. Highly significant difference was seen in primary follicles at the level of P < 0.001. Wiwin et al (2014) and Snoor et al (2015) had shown that there was a significant decrease in primary follicle count.
In the present study, MSG produced no change in primary follicle count, but in auto recovery group, the count was significantly increased at P < 0.001 compared to control and MSG groups. It might be because, after stoppage of MSG, the body was trying to reduce the oxidative stress. In DVA group, follicle count was also highly significant compared to control and MSG groups and higher than auto recovery group but not statistically significant. As DVA contains Lodhra,[14] Gokshura,[15] Yashtimadhu[16] and Jatamansi[17] which have anti-oxidant property, they might have acted upon to reduce oxidative stress.
Secondary follicle count
Secondary follicle count was significant at P < 0.05. In MSG and auto recovery groups, follicle count is lower than the control group. It shows that MSG is toxic to follicle and it inhibits the follicle to turn into a mature follicle.
Mitochondrion is the center to all metabolic activities in cells, so any disturbance in its function can lead to altered generation of adenosine triphosphate (ATP). Energy of ATP is essential for gamete formation. As mitochondria is the major site for reactive oxygen species (ROS) production, excessive ROS can affect the function of the mitochondria in oocyte. This mitochondrial dysfunction may lead to arrest of cell division in oocyte. Mitochondrial DNA is more prone to ROS attack due to lack of histone protection and absence of repair mechanism.[18] In DVA group, the count has been increased, this can be attributed to Lodhra[19] as it has been proved to correct female sexual dysfunction and other ingredients which reduced oxidative stress.
Tertiary follicle count
Tertiary follicle count was significantly decreased in MSG group. It supports the findings of Snoor et al (2015) and Wiwin et al (2014). MSG damaged follicles by producing oxidative stress and by destroying the FSH receptor. In DVA group, tertiary follicle count increased significantly. It states that DVA is protecting the cell membrane from damage. For the growth of follicle, availability of steroidal hormone is also necessary. Increased oxidative stress disrupts the balance of these hormones.[20] MSG might be making hormone unavailable due to oxidative stress so that follicles were not grown properly. DVA might have corrected the hormonal balance in ovary by reducing oxidative stress.
Graafian follicle count
Graafian follicle count had not shown any significant difference. The mean count decreased in MSG group. It shows that MSG had acted as a toxic agent for the follicles. Hyperactivation of glutamate receptor causes inhibition of cysteine transport, glutathione (GSH) depletion and lipid peroxidation, which results in depletion of intracellular cysteine and GSH. GSH leads to accumulation of free radical which causes cell injury because GSH plays an important role in maintaining cellular oxidant homeostasis by detoxifying free radical of oxygen.[21] In DVA group, Graafian follicle count is increased which might be because DVA prevented further damage caused by MSG as it contains Chandana which is Vishaghana Darvya and protects the cell from the toxic effects of MSG. Jatamansi which has been proved as an anti-oxidant[17] might also interrupted cysteine inhibition process by not allowing the generation of oxidative stress.
It is well known that two oocyte-specific genes, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), play a key role in the regulation of folliculogenesis. These factors induce the proliferation and differentiation of the follicular cells during follicular development from the primordial stage. MSG has been proved for its toxicity to DNA. It might have downregulated these genes and inhibited the growth of primordial follicle. Gokshura stimulates these genes for normal development of oocytes.[22]
Corporal luteal count
The ruptured follicle will undergo a dramatic transformation into the corpus luteum, a steroidogenic cluster of cells that maintains the endometrium of the uterus by the secretion of large amounts of progesterone and minor amounts of estrogen.[23] In the present study, there was no statistically significant difference in corporal luteal count, but the mean value was decreased in MSG group compared to the control group.
In DVA and auto-recovery groups, slight increase in count was observed but not up to the control group. It shows that less ovulation took place in treated group compared to control group.
Atretic follicle count
Atretic follicle count was highly significant at P < 0.001. In MSG group, atretic follicle count was increased at the level of P < 0.001 compared to control group which was in agreement with the findings of Dian Megawati, Sutarno (2005), and Wiwin Rohmawati (2014) and many others. It shows that MSG prevents ovulation which is evidenced by increased atretic follicle count in MSG group. The growth and development of oocyte is targeted by an increase in reactive oxygen species (ROS) and inhibited by antioxidant, suggesting that there is a complex relationship between ROS and antioxidant in the ovary. Follicle ROS promotes apoptosis whereas GSH and FSH counterbalance this action in the growing follicle.[10] MSG increases ROS which leads to inhibition of follicle growth.
In DVA group, the count was found decreased which was significant at P < 0.001, which was almost nearer to control group. It proved that DVA is accelerating the ovulation by correcting H-P-O axis. In auto recovery group, count is less but not up to normal. It shows that MSG-induced damage to H-P-O axis is not completely corrected just by withdrawal of MSG.
Histopathological Result
In this study, ovarian section of rats treated with MSG showed a high number of degenerated follicles, moderate vacuolation, and congested blood vessels. Vacuolation of different numbers and sizes is present in all groups as this may be normal. These findings were in covenant with Bojanic et al and Oladipo et al. Mild vacuolation was present in auto recovery group but absent in DVA group. Vacuolation occurs in varied ranges of cell line either spontaneous or as induced by a variety of stimuli, the extent to which a cell becomes vacuolated depends on the cell type. Vacuolation is considered a defensive mechanism against harmful oxidative stress and vacuoles may collect the damaging substance, preventing them from interfering with biological activities.[24]
The vacuolation process follows a definite pattern with the number and size of vacuolation increasing gradually. A cell can recover up to a limit, after that, cell death takes place. MSG had been proved for causing oxidative stress by many researchers. MSG causes vacuolation which was confirmed by many other researches. MSG reacts with oxygen species and creates free radicals. These free radicals cause damage to DNA and lipid proteins and lead to cellular damage.[25]
MSG might be affecting the follicle in the following way:
By producing oxidative stress
Blocking or destructing FSH receptor
Suppressing genes such as GDF9 and BMP15
By disturbing mitochondrial function due to increased ROS.
The ingredients of DVA such as Jatamansi, Pippali and Tagara have antioxidant property with which DVA counteracted the effect of MSG. Gokshura stimulates the suppressed genes. By these activities, DVA has reversed the effect of MSG. However, along with all the above-said properties of individual ingredients of DVA, the whole formulation acts as Vishaghna (antitoxic or antidote) which should not be overlooked. For that purpose, DVA can be tried in comparison with other drugs with antioxidant properties but without Vishaghna property or against standard antioxidant drugs.
Conclusion
MSG produce Oxidative stress which leads to apoptosis of follicle, which was proved in the present study in which MSG has produced ovarian toxicity by decreasing primordial, primary, secondary, tertiary and Graafian follicle counts and corporal luteal counts. In DVA group, increased count of primary, secondary, tertiary and Graafian follicle counts and corporal luteal counts are suggestive of cytoprotective effect of DVA. Ingredients of DVA such as Lodhra, Gokshura, Yashtimadhu and Jatamansi have antioxidant property, through which oxidative stress was decreased and ovum growth was normalized. Gokshur stimulates GDF9 and BMP15 genes, which has a crucial role in the regulation of folliculogenesis. Auto recovery group also showed positive effect to some extent. Hence, these findings of auto recovery group suggest that Nidana Parivarjana itself can prevent the cellular damage to an extent. Above all, the Vishaghna property of DVA should also be kept in mind for the observed results.
Financial support and sponsorship
Institute- KLEU Shri BMK Ayurveda Mahavidyalaya, Belagavi
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Santosh F Patil and Dr. B. R. Tubaki for their help and support.
References
- 1.Samuels A. The toxicity/safety of processed free glutamic acid (MSG): A study in suppression of information. Account Res. 1999;6:259–310. doi: 10.1080/08989629908573933. [DOI] [PubMed] [Google Scholar]
- 2.Swaroop Thonda VS, Harish Kumar S, Handral M, Sonowal A. Neuroprotective evaluation of ethanolic leaf extract of Dalbergia sissoo in monosodium glutamate induced neurotoxicity in rats. Int J Pharm Sci Res. 2014;5:829–38. [Google Scholar]
- 3.Onaolapo AY, Onaolapo OJ, Mosaku TJ, Akanji OO, Abiodun O. A histological study of the hepatic and renal effects of sub chronic low dose oral monosodium glutamate in Swiss albino mice. Br J Med Med Res. 2013;3:294–306. [Google Scholar]
- 4.Ajibade AJ, Fakunle PB, Adetunji MO. Some effects of monosodium glutamate administration on the histo-architecture of the spleen and pancreas of adult Wistar rats. J Pharm Biol Sciences. 2015;3:39–50. [Google Scholar]
- 5.Iamsaard S, Sukhorum W, Samrid R, Yimdee J, Kanla P, Chaisiwamongkol K, et al. The sensitivity of male rat reproductive organs to monosodium glutamate. Acta Med Acad. 2014;43:3–9. doi: 10.5644/ama2006-124.94. [DOI] [PubMed] [Google Scholar]
- 6.Eweka AO, Eweka A, Om'iniabohs FA. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. N Am J Med Sci. 2010;2:146–9. doi: 10.4297/najms.2010.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zia MS, Qamar K, Hanif R, Khalil M. Effect of monosodium glutamate on the serum estrogen and progesterone levels in female rat and prevention of this effect with diltiazem. J Ayub Med Coll Abbottabad. 2014;26:18–20. [PubMed] [Google Scholar]
- 8.Paradkar HS. Varanasi: Chaukhamba Surabharati Prakashan; 2010. Ashtanga Hridaya of acharya Vagbhata, Uttartantra-. Ch. 35, Ver. 39; p. 105. [Google Scholar]
- 9.Liou S. About Glutamate Toxicity, Blog Section, Huntington's Outreach Project for Education, at Stanford. 2011 26 June. [Google Scholar]
- 10.Oladipo IC, Adebayo EA, Kuye OM. Effects of monosodium glutamate in ovaries of female Sprague-Dawley rats. Indian J Curr Microbiol Appl Sci. 2015;4:737–45. [Google Scholar]
- 11.Tafelski TJ, Lamperti AA. The effects of a single injection of monosodium glutamate on the reproductive neuroendocrine axis of the female hamster. Biol Reprod. 1977;17:404–11. doi: 10.1095/biolreprod17.3.404. [DOI] [PubMed] [Google Scholar]
- 12.Tamara Roe L. FSH and AMH determining Ovarian Reserve. [Last accessed on 2017 Jun 26]. Available from: http://www.reproductivewellness.com .
- 13.Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–73. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- 14.Acharya N, shah U, Hingorani L, acharya S. Anti-Oxidant and anti-Cancer Potential of Symplocos Racemosa Bark against Hep3b Cell Line. Int J Pharm Sci Res. 2015;6(10):4529. doi: 1013040/IJPSR0975-82326(10)4529-33. [Google Scholar]
- 15.Mishra SL, Sinhamahapatra PK, Nayak A, Das R, Sannigrahi S. In vitro antioxidant potential of different parts of Oroxylum indicum: A Comparative study. Indian J Pharm Sci. 2010;72:267–9. doi: 10.4103/0250-474X.65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lateef M, Iqbal L, Fatima N, Siddiqui K, Afza N, Zia-ul-Haq M, et al. Evaluation of antioxidant and urease inhibition activities of roots of Glycyrrhiza glabraS. Pak J Pharm Sci. 2012;25:99–102. [PubMed] [Google Scholar]
- 17.Rasheed AS, Venkataraman S, Jayaveera KN, Fazil AM, Yasodha KJ, Aleem MA, et al. Evaluation of toxicological and antioxidant potential of Nardostachys jatamansiS in reversing haloperidol-induced catalepsy in rats. Int J Gen Med. 2010;3:127–36. doi: 10.2147/ijgm.s9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saraswathi CD, Gupta SK, Sreemantula S. Protective effect of Symplocos racemosa bark on cold restraint stress induced reproductive changes in female rats. J Nat Prod. 2012;5:251–8. [Google Scholar]
- 20.Rajagopal SS, Chidamaram K, Ramamurthy S. Neuroprotective potential of Ocimum sanctum (Linn) leaf extract in monosodium glutamate induced excitotoxicity. Afr J Pharm Pharmacol. 2013;7:1894–906. [Google Scholar]
- 21.Ismail NH. Assessment of DNA damage in testes from young Wistar male rat treated with monosodium glutamate. Life Sci J. 2012;9:930–9. [Google Scholar]
- 22.Abadjieva D, Kistanova E. Tribulus terrestris alters the expression of growth differentiation factor 9 and bone morphogenetic protein 15 in rabbit ovaries of mothers and F1 female offspring. PLoS One. 2016;11:e0150400. doi: 10.1371/journal.pone.0150400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gougeon A, Chainy GB. Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil. 1987;81:433–42. doi: 10.1530/jrf.0.0810433. [DOI] [PubMed] [Google Scholar]
- 24.Henics T, Wheatley DN. Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features. Biol Cell. 1999;91:485–98. doi: 10.1016/s0248-4900(00)88205-2. [DOI] [PubMed] [Google Scholar]
- 25.Singh K, Ahluwalia P. Studies on the effect of monosodium glutamate [MSG] administration on some antioxidant enzymes in the arterial tissue of adult male mice. J Nutr Sci Vitaminol (Tokyo) 2003;49:145–8. doi: 10.3177/jnsv.49.145. [DOI] [PubMed] [Google Scholar]


