Abstract
As the leading cause of morbidity and mortality in patients with diabetes, diabetic cardiomyopathy (DCM) imposes enormous burden on individuals and public health. Therapeutic regimes for DCM treatment have proven to be challenging, with limited efficacy, low compliance, and potential adverse effects. Curcumin, as the most active compound derived from the root of turmeric, exhibits strong anti-inflammation, antioxidant, and anti-apoptosis properties. Recently, clinical trials and preclinical studies have shown that curcumin exerts protective effects against a variety of diseases, including diabetes and its cardiovascular complications. In this review, the clinical trials about curcumin supplementation on diabetes and DCM are presented, and the specific mechanisms by which curcumin might mitigate diabetes and DCM are fully discussed. A better understanding of the pharmacological role of curcumin on diabetes and DCM can provide clinical implications for the intervention of the onset and development of diabetes and DCM.
Keywords: curcumin, curcuminoids, diabetes mellitus, diabetic cardiomyopathy, inflammation, antioxidant, apoptosis
Introduction
The prevalence of diabetes is increasing rapidly during the last three decades, which is becoming one of the most epidemic non-communicable diseases. The International Diabetes Federation [IDF] (2017) estimates that the number of people with diabetes reaches 425 million worldwide in 2017 and it will rise to 629 million by 2045, indicating a 45% increase throughout the world. Globally, about one in eleven adults have diabetes, and 90% of whom are diagnosed as type 2 diabetes mellitus (T2DM). T2DM and its complications have contributed tremendously to the burden of mortality and health cost worldwide. Among the various complications of diabetes, cardiovascular complications are believed to be the leading causes of disability and death among diabetic patients, particularly for diabetic cardiomyopathy (DCM) (Cai and Kang, 2003). Cardiovascular diseases (CVDs) typically develop about 15 years earlier in T2DM (Booth et al., 2006), and T2DM patients are more than twice as likely to develop CVDs as compared with those without T2DM (Sarwar et al., 2010).
Limitations in Therapeutic Strategies for Diabetes and Dcm
In 2016, the Global Burden of Diseases (GBD) study reported that T2DM and its complications accounted for a 22% increase in disability-adjusted life years (DALYs) during the last decade (2016), imposing enormous burden on individuals and public health (GBD 2015 Risk Factors Collaborators, 2016). Despite prominent advances in diabetes prevention, treatment, glucose monitoring and novel glycemic control biomarkers, detrimental cardiovascular complications, especially for DCM still remain rigorous in patients with T2DM (Pirola et al., 2010). Nowadays, therapeutic regimes for diabetes and DCM include several clinical managements, involving lifestyle modifications (diet and exercise), glucose and lipid control (antidiabetic and lipid-lowering drugs), hypertension treatment, and coronary artery diseases intervention. The commonly used therapeutic strategies for CVDs in diabetic patients include cardiac glycoside, Ca2+ antagonist, β-adrenergic blocking agents, angiotensin converting enzyme inhibitor (ACEI), angiotensin receptor blocker (ARB) and diuretics (Marwick et al., 2018). However, the incidence and mortality rate of DCM still remains high, it is imperative to develop novel and effective therapeutic strategies for diabetes and DCM.
Curcumin and Its Protective Effects on Human Health
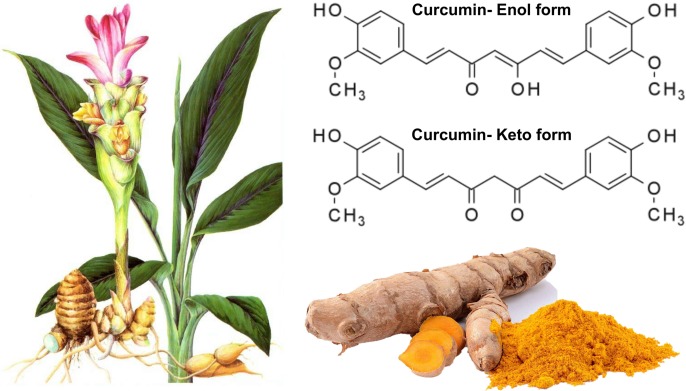
Plants and herbs have historically been widely used for medicinal purposes. Traditional Chinese Medicine (TCM), with a history of more than 2,000 years, includes various forms of herbal medicine and dietary therapy (Hao et al., 2015b). Natural plants and their active derivatives have been deemed as novel therapeutic agents for multiple diseases, such as CVDs (Hao et al., 2017), metabolic disorders (Martel et al., 2017), rheumatic autoimmune diseases (Dahan et al., 2017), and cancer (Seca and Pinto, 2018). A randomized, placebo-controlled study indicated that dietary supplementation with equol and resveratrol can reduce the severity of menopausal symptoms in recently postmenopausal women, that can improve menopause-related quality of life in healthy women (Davinelli et al., 2017). Curcumin, a natural compound, is the most active agent of the polyphenolic curcuminoids derived from the root of turmeric (Curcuma longa). It is a tautomeric compound existing in organic solvents as its enolic form, and in water as a keto form (Manolova et al., 2014) (Figure 1). Traditionally, turmeric, as a member of the ginger family, has been widely used as an herbal medicine, ingredient of cosmetics, and dietary supplement (food flavoring and coloring). In addition to a dietary ingredient, turmeric is also prescribed abundantly for ailments in traditional medicine (Nelson et al., 2017). Numerous studies suggest that curcumin is a potent molecule that can exert a variety of positive pharmacological effects, including anti-inflammation (Sikora et al., 2010; Koeberle and Werz, 2014), antioxidant (Nakmareong et al., 2011), and anti-apoptosis (Topcu-Tarladacalisir et al., 2013) properties. Corbi et al. (2016) also showed that curcumin can exert relevant immunomodulatory and/or anti-inflammatory activities in the context of brain aging. Some phytochemicals, such as curcumin inducing increase in nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and sirtuin 1 (SIRT1) activity could be able to inhibit the nuclear factor kappa-B (NF-κB) activation and then to end the progression of the brain aging (Corbi et al., 2016). In recent years, compelling data indicates that curcumin is a protective compound against insulin resistance (Yekollu et al., 2011), obesity (Hariri and Haghighatdoost, 2018), diabetes mellitus (Arivazhagan et al., 2015), and CVDs (Hashemzaei et al., 2017; Jiang et al., 2017). However, although the evidence alludes to protective effects of curcumin on human health, information about the effect of curcumin on diabetes and DCM is limited. It is speculated that curcumin may be a pleiotropic molecule targeting diabetes and DCM, with a rather diverse array of metabolic, cellular, and molecular activities. Therefore, the current review aimed to provide an overview of the effect of curcumin on diabetes and DCM, and the molecular mechanisms of curcumin in alleviating diabetes and DCM.
FIGURE 1.
The molecular structure of curcumin isolated from the root of turmeric. Curcumin, a natural compound, is the most active agent of the polyphenolic curcuminoids derived from the root of turmeric (Curcuma longa). It is a tautomeric compound existing in organic solvents as its enolic form, and in water as a keto form. Turmeric, as a member of the ginger family, has been widely used as an herbal medicine, ingredient of cosmetics, and dietary supplement.
Molecular Mechanisms Underlying the Pathogenesis of Diabetes and Dcm
Diabetic cardiomyopathy, as a severe complication of diabetes, is characterized by cardiac structure and function disorders (Bugger and Abel, 2014), including metabolic deregulation, left ventricular dysfunction, and myocardial cell deterioration (Marwick et al., 2018). DCM is associated with impaired systolic and diastolic functions with prolonged contraction and relaxation duration, and depressed myocardial contractility and relaxation (Boudina and Abel, 2007). Echocardiography revealed shorter left ventricular ejection duration, and increased wall stiffness in diabetic patients (Poirier et al., 2001). The pathogenesis of diabetes and DCM is multifactorial, and evidence indicates that the risks of diabetes and DCM are not limited to traditional factors, such as atherosclerosis, hypertension, and coronary diseases (Boudina and Abel, 2007). To date, a range of molecular and cellular mechanisms have been proposed for the development and progress of diabetes and DCM, including advanced glycation end products (AGEs) accumulation (Goldin et al., 2006), inflammation activation (Diamant et al., 2005), increased oxidative stress (Anderson et al., 2009), higher induction of apoptosis (Frustaci et al., 2000), and impaired autophagy (Nakai et al., 2007).
Clinical Trials With Curcumin in Diabetes and Its-Related Cardiovascular Risks
Accumulating clinical trials have revealed that curcumin has its beneficial effects on rheumatoid arthritis (Mande et al., 2016), inflammatory bowel disease (Taylor and Leonard, 2011), cancer (Shehzad et al., 2013; Naksuriya et al., 2014), Alzheimer’s disease (Hathout et al., 2017), and other diseases (Gupta et al., 2012). However, few studies have been conducted to investigate the effects of curcumin on diabetes and its-related CVDs. Importantly, pharmacological research reveal that curcumin is effective, safe, and without toxicity (Prasad et al., 2014). Dyslipidemia is an established factor and increase the susceptibility of atherosclerotic heart disease in diabetic patients. Panahi et al. (2017b) aimed to examine an anti-atherosclerosis effect of curcumin in diabetic patients. It showed that curcuminoids supplementation (1,000 mg/day) for 12 weeks can reduce serum levels of atherogenic lipid levels, including non-high density lipoprotein (HDL) and lipoprotein(a) [Lp(a)] in patients with T2DM (Panahi et al., 2017b). Chuengsamarn et al. (2014) reported that curcuminoids intake (1,500 mg/day) for 6 months continuously increased insulin sensitivity, decreased pulse wave velocity, triglyceride level, and atherosclerosis incidence in patients with T2DM, indicating that curcuminoids supplementation could contribute to a lower risk of cardiovascular events in dyslipidemic patients with T2DM (Chuengsamarn et al., 2014). A report from the same group found that curcumin intervention (1,500 mg/day) for 12 months significantly decreased the incidence of T2DM in pre-diabetic individuals, with a lower level of insulin resistance and higher adiponectin level (Chuengsamarn et al., 2012). A recent clinical trial showed that curcumin supplementation (1,000 mg/day) for 12 weeks exhibited higher adiponectin level and lower leptin concentration, with decreased leptin/adiponectin ratio (a measure of atherosclerosis) in patients with T2DM (Sahebkar et al., 2018). In addition, Panahi et al. (2017a) showed that curcuminoids supplementation (1,000 mg/day) for 8 weeks significantly increased serum total antioxidant capacity (TAC) and superoxide dismutase (SOD) activities, with reduced malondialdehyde (MDA) concentrations in diabetic patients (Panahi et al., 2017a). Since dyslipidemia, insulin resistance, and oxidative stress principally increases the risks of CVDs in diabetic patients, these studies suggest the protective role of curcumin on diabetes and its-related cardiovascular risks. However, clinical trials about the direct effects of curcumin on DCM in patients with diabetes are needed. The clinical studies about curcumin and its protective effects against diabetes and its-related cardiovascular risks are listed in Table 1.
Table 1.
Clinical trials about curcumin and its protective effects against diabetes and its-related cardiovascular risks.
| Subjects included | Treatments | Metabolic effects | Reference |
|---|---|---|---|
| 118 subjects with T2DM | Curcuminoids (1,000 mg/day) for 12 weeks | - Reductions in serum total cholesterol, non-HDL-C and Lp(a) levels | Panahi et al., 2017 |
| - Elevations in serum HDL-C levels | |||
| 240 patients with T2DM | Curcuminoids (1,500 mg/day) for 6 months | - Reduced pulse wave velocity | Chuengsamarn et al., 2014 |
| - Increased level of serum adiponectin and decreased level of leptin | |||
| - Reduced levels of HOMA-IR, triglyceride, uric acid, visceral fat, and total body fat | |||
| 240 pre-diabetic individuals | Curcuminoids (1,500 mg/day) for 12 months | - Decreased the number of pre-diabetic individuals who eventually developed T2DM | Chuengsamarn et al., 2012 |
| - Better overall function of β-cells, with higher HOMA-β and lower C-peptide | |||
| - A lower level of HOMA-IR and higher adiponectin | |||
| 118 patients with T2DM | Curcuminoids (1,000 mg/day) for 12 weeks | - Higher adiponectin level | Sahebkar et al., 2018 |
| - Lower leptin concentration | |||
| - Decreased leptin/adiponectin ratio | |||
| - Elevated serum ghrelin level | |||
| 118 subjects with T2DM | Curcuminoids (1,000 mg/day) for 8 weeks | - Elevation in serum TAC and SOD activities | Panahi et al., 2017 |
| - Reduced MDA concentration |
DCM, diabetic cardiomyopathy; T2DM, type 2 diabetes mellitus; Lp(a), lipoprotein(a); HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; HOMA-β, homeostasis model assessment-β; TAC, total antioxidant capacity; SOD, superoxide dismutase; MDA, malondialdehyde.
Preclinical Studies About Curcumin and Its Effects on Diabetes and Dcm
Curcumin and Its Anti-inflammatory Effects on Diabetes and DCM
Inflammation plays a critical role of the development of diabetes and its complications (Pereira and Alvarez-Leite, 2014), and inflammation with increased cytokine levels is shown to be closely related to the onset and development of diabetes and DCM (Xu et al., 2003). It demonstrated that intra-myocardial inflammation in DCM is associated with increased macrophages and leucocytes infiltration, significant increment of vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) expressions, as well as elevated expression of inflammatory cytokines, including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-18 (IL-18), and transforming growth factor-β1 (TGF-β1) (Tschope et al., 2005; Westermann et al., 2007; Rajesh et al., 2012; You et al., 2018). Recent evidence reveals that nucleotide-binding oligomerization domain like receptor (NLR) pyrin domain containing 3 (NLRP3) inflammasome is also a promising molecular marker of diabetes and DCM (Luo et al., 2017).
Curcumin is a potent anti-inflammatory agent and considered beneficial for the amelioration of diabetes and DCM. Curcumin was able to suppress NF-κB signaling pathway, defend against inflammation, cardiac hypertrophy and fibrosis in the heart (He et al., 2015). Curcumin supplementation decreased serum inflammatory factors levels of IL-6, TNF-α and monocyte chemoattractant protein-1 (MCP-1), as well as glucose and glycosylated hemoglobin in diabetic rats. Curcumin incubation also inhibited IL-6, IL-8, TNF-α, and MCP-1 secretion in high glucose-treated monocytes (Jain et al., 2009). Yu et al. (2012) showed that curcumin inhibited AGEs accumulation, decreased inflammatory cytokines of IL-1β and TNF-α, and attenuated diabetes-induced left ventricular dysfunction, cardiomyocyte hypertrophy and interstitial fibrosis in diabetic rats. A similar study reported that curcumin dramatically decreased IL-6 and TNF-α levels in streptozotocin-induced diabetic rats with heart injury (Abo-Salem et al., 2014). A recent study showed that curcumin (300 mg/kg/day for 16 weeks) significantly suppressed collagens deposition in diabetic rat heart, with marked reduction of TGF-β1 production, suppression of type II TGF-β (TβRII) levels and sma- and mad- related protein 2/3 (Smad2/3) phosphorylation, and increment of Smad7 expression in the heart of diabetic rat. They further found that application of curcumin inhibited TGF-β1- or high glucose-induced adenosine monophosphate activated protein kinase (AMPK)/p38 mitogen-activated protein kinase (MAPK) activation in human cardiac fibroblasts in vitro (Guo et al., 2018).
In recent years, curcumin analog has been discovered and widely investigated for their roles in diabetes and DCM. A novel curcumin analog C66 reduces serum and heart hypertriglyceridemia, accompanied by improved cardiac function, inhibition of Jun NH2-terminal kinase (JNK) signaling and cardiac inflammation. They further reported that curcumin analog C66 inhibited a high glucose-induced rise in pro-inflammatory cytokines via inactivation of NF-κB (Wang et al., 2014). J17, another molecule with structural similarities to curcumin, exerts significant inhibitory effects on hyperglycemia-induced inflammation and fibrosis in H9C2 cardiomyocytes and streptozotocin-induced diabetic mouse. The underlying mechanisms may be associated with the inhibition of the P38 and protein kinase B (AKT) signal pathway (Chen et al., 2017). Thus, these studied indicated that curcumin and its analogs can alleviate DCM by attenuating inflammation in vivo and in vitro. The relevant evidence is summarized in Table 2, and the potential mechanism by which curcumin might mitigate diabetes and DCM is exhibited in Figure 2.
Table 2.
Pre-clinical studies about curcumin and its effects on diabetes and DCM.
| Animals/cells | Treatments | Main findings | Reference |
|---|---|---|---|
| Anti-inflammatory effects | |||
| STZ-induced diabetic SD rats | Curcumin (100 mg/kg/day) for 7 weeks | - Decreased blood levels of TNF-α, IL-6, MCP-1 | Jain et al., 2009 |
| - Decreased glucose and glycosylated hemoglobin | |||
| High glucose-treated monocytes | Curcumin incubation (0.01-1 μM) for 24 h | - Lower TNF-α, IL-6, IL-8, and MCP-1 secretion | Jain et al., 2009 |
| STZ-induced diabetic Wistar rats | Curcumin (100 or 200 mg/kg/day) for 8 weeks | - Attenuated diabetes-induced left ventricular dysfunction, cardiomyocyte hypertrophy and interstitial fibrosis | Yu et al., 2012 |
| - Inhibited AGEs accumulation | |||
| - Decreased inflammatory factors (TNF-α and IL-1β) | |||
| - Activated AKT/GSK-3β signaling pathway | |||
| STZ-induced diabetic Wistar rats | Curcumin (200 mg/kg/day) for 6 weeks | - Inhibited IL-6 and TNF-α levels | Abo-Salem et al., 2014 |
| STZ-induced diabetic SD rats | Curcumin (300 mg/kg/day) for 16 weeks | - Reduced TGF-β1 production | Guo et al., 2018 |
| - Suppressed TβR II levels and Smad2/3 phosphorylation | |||
| - Increased Smad7 expression | |||
| High glucose-treated human cardiac fibroblasts | Curcumin incubation (25 μM) for 24 h | - Inhibited TGF-β1- or HG-induced AMPK/p38 MAPK activation | Guo et al., 2018 |
| - Suppressed collagen synthesis in the fibroblasts | |||
| STZ-induced diabetic C57BL/6 mice | Curcumin (5 mg/kg/day) for 3 months | - Reduced hypertriglyceridemia in both serum and hearts | Wang et al., 2014 |
| - Improved cardiac function, inhibition of JNK signaling and cardiac inflammation | |||
| - Inhibited a high glucose-induced rise in pro-inflammatory cytokines via inactivation of NF-κB | |||
| STZ-induced diabetic C57BL/6 mice | Curcumin analog, J17 (10 mg/kg/day) for 42 days | - Suppressed hyperglycemia-induced inflammation, hypertrophy and fibrosis | Chen et al., 2017 |
| - Decreased TNF-α and ICAM-1 | |||
| High glucose-treated H9C2 cardiomyocytes | Curcumin analog, J17 (2.5 or 10 μM) for 30 min | - Decreased pro-inflammatory cytokines (TNF-α and IL-6) and adhesion molecules (VCAM-1 and ICAM-1) expressions | Chen et al., 2017 |
| - Decreased AKT phosphorylation | |||
| - Inhibited the HG-induced increase in fibrotic genes (collagen-IV, TGF-β, and collagen-I) | |||
| Antioxidant properties | |||
| STZ-induced diabetic Wistar rats | Curcumin (100 or 200 mg/kg/day) for 8 weeks | - Attenuated NADP+/NADPH ratio, Rac1 activity and the expression of NADPH oxidase subunits of gp91 phox, p47 phox | Yu et al., 2012 |
| STZ-induced diabetic Wistar rats | Curcumin (200 mg/kg/day) for 6 weeks | - Restored cardiac antioxidant enzymes (catalase, superoxide dismutase, and glutathione-S-transferase) | Abo-Salem et al., 2014 |
| STZ-induced diabetic SD rats | Curcumin (100 mg/kg/day) for 8 weeks | - Decreased NADPH oxidase subunits (p67phox, p22phox, gp91phox) | Soetikno et al., 2012 |
| - Decreased the mRNA expression of transcriptional co-activator p300 and atrial natriuretic peptide | |||
| - Decreased accumulation of ECM protein | |||
| - Reversed the increment of superoxide production | |||
| STZ-induced diabetic C57BL/6 mice | Curcumin (5 mg/kg/day) for 3 months | - Protection against diabetes-induced cardiac fibrosis, oxidative stress, and ER | Wang et al., 2014 |
| STZ-induced diabetic rats | Curcumin (20 mg/kg/day) for 45 days | - Prevented diabetes-induced upregulation of HO-1 expression and activity | Aziz et al., 2013 |
| Anti-apoptotic effects | |||
| STZ-induced diabetic Wistar rats | Curcumin (100 or 200 mg/kg/day) for 8 weeks | - Prevented diabetes-induced cardiomyocytes apoptosis | Yu et al., 2012 |
| STZ-induced diabetic C57BL/6 mice | Curcumin (5 mg/kg/day) for 8 weeks | - Prevented high glucose-induced apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy | Pan et al., 2014 |
| - Inhibition of JNK phosphorylation | |||
| STZ-induced diabetic C57BL/6 mice | Curcumin (5 mg/kg/day) for 3 months | - Protection against diabetes-induced cardiac fibrosis, oxidative stress, and ER; | Wang et al., 2014 |
| High glucose-treated neonatal rat cardiomyocytes | Curcumin incubation (10 μM) for 24 h | - Inhibited the increased Bax/Bcl-2 ratio elicited by high glucose exposure | Yu et al., 2016 |
| - Increased AKT and GSK-3β phosphorylation | |||
DCM, diabetic cardiomyopathy; STZ, streptozotocin; SD, Sprague-Dawley; HG, high glucose; AGEs, advanced glycation end products; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-18, interleukin-18; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor; NF-κB, nuclear factor kappa-B; TβRII, type II transforming growth factor-β; Smad, sma- and mad-related protein; MCP-1, monocyte chemoattractant protein-1; AMPK, adenosine monophosphate activated protein kinase; MAPK, mitogen-activated protein kinase; JNK, Jun NH2-terminal kinase; AKT, protein kinase B; NADPH, nicotinamide adenine dinucleotide phosphate; Rac1, Ras-related C3 botulinum toxin substrate 1; PKC, protein kinase C; HO-1, heme-oxygenase-1; GSK-3β, glycogen synthase kinase 3β; ECM, extracellular matrix; ER, endoplasmic reticulum.
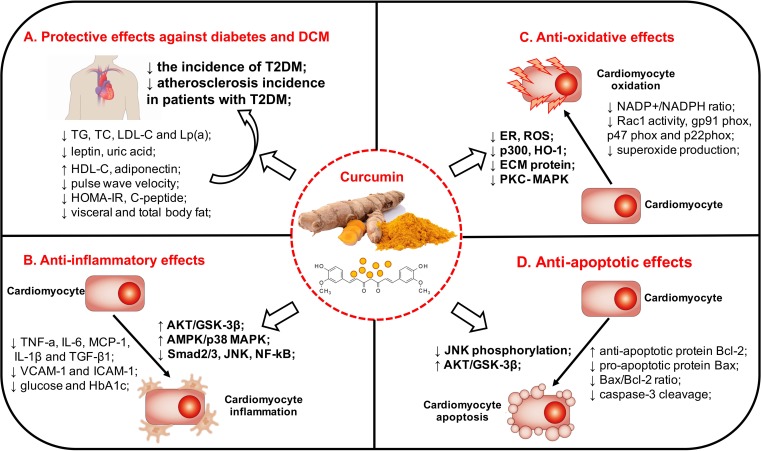
FIGURE 2.
The clinical evidence and potential mechanism by which curcumin might mitigate diabetes and DCM. The pathogenesis of diabetes and DCM is multifactorial, and a plethora of molecular mechanisms have been postulated for the onset and development of diabetes and DCM. Clinical trials have shown that curcumin possessed a potency to decrease blood glucose, improve insulin resistance and ameliorate dyslipidemia in patients with diabetes. Curcumin also exerts a variety of positive effects that was able to attenuate inflammatory activation, oxidative stress, and apoptosis in diabetes and DCM through several signaling pathways. DCM, diabetic cardiomyopathy; T2DM, type 2 diabetes mellitus; DCM, diabetic cardiomyopathy; LDL-C, low density lipoprotein cholesterol; Lp(a), lipoprotein(a); HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment-insulin resistance; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-18, interleukin-18; TNF-α, tumor necrosis factor-α; TGF-β1, transforming growth factor; ICAM-1, intercellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule-1; HbA1c, glycosylated hemoglobin A1c; AKT, protein kinase B; GSK-3β, glycogen synthase kinase 3β; AMPK, adenosine monophosphate activated protein kinase; MAPK, mitogen-activated protein kinase; Smad, sma- and mad- related protein; JNK, Jun NH2-terminal kinase; NF-κB, nuclear factor kappa-B; TβRII, type II transforming growth factor-β; MCP-1, monocyte chemoattractant protein-1; NADPH, nicotinamide adenine dinucleotide phosphate; Rac1, Ras-related C3 botulinum toxin substrate 1; HO-1, heme-oxygenase-1; ER, endoplasmic reticulum; ROS, reactive oxygen species; ECM, extracellular matrix; PKC, protein kinase C. (A) Curcumin and its protective effects of against diabetes and DCM. (B) Curcumin and its anti-inflammatory effects on diabetes and DCM. (C) Curcumin and its antioxidant properties on diabetes and DCM. (D) Curcumin and its anti-apoptotic effects on diabetes and DCM.
Curcumin and Its Antioxidant Properties on Diabetes and DCM
Oxidative stress is known to participate in the development of diabetes and DCM. Reactive oxygen species (ROS) have been postulated to play a significant role in the pathogenesis of diabetes and DCM. Anderson et al. firstly showed the evidence of increased oxidative stress, with higher mitochondrial hydrogen peroxide (H2O2) production and elevated 4-hydroxynonenal- and 3-nitrotyrosine-modified proteins levels in atrial tissue in human diabetic hearts (Anderson et al., 2009). Furthermore, Ye et al. (2004) provided the evidence of causality between ROS and DCM. It indicated that overexpression of catalase or manganese SOD partially restored the dysfunction of mitochondrion and cardiomyocyte contractility in diabetic mice.
Curcumin was able to act as a natural free radical scavenger as its chemical structure and has anti-oxidative potency. Abo-Salem et al. (2014) found that curcumin mitigated the reduction of cardiac antioxidant enzymes and glutathione levels in diabetic rats, including SOD, catalase, and glutathione-S-transferase. It showed that curcumin attenuated nicotinamide adenine dinucleotide phosphate (NADPH) oxidase subunits (p47phox and gp91phox) expressions, NADP+/NADPH ratio, and Ras-related C3 botulinum toxin substrate 1 (Rac1) activity in diabetic rats (Yu et al., 2012). Soetikno et al. (2012) also showed that curcumin treatment markedly decreased NADPH oxidase subunits (p67phox, p22phox, gp91phox) expressions and superoxide production in diabetic rats, possibly by inhibiting protein kinase C (PKC)- MAPK signaling pathway. The novel curcumin analog C66 was able to inhibit JNK activation in diabetes, resulting in protection of endoplasmic reticulum (ER) and oxidative stress against induced by diabetes (Wang et al., 2014). Aziz et al. (2013) reported that curcumin protected against diabetes-induced heme-oxygenase-1 (HO-1) upregulation in the cardiac tissue of diabetic rats.
In addition, the heart depends on continuous mitochondrial ATP supply and maintained redox balance to properly develop force. However, myocardial energetic-redox balance is perturbed exposed to hyperglycemia, that is a critical driver of mitochondrial dysfunction in the diabetic myocardium (Aon et al., 2015). Mitochondria control cell respiration and energy production are also closely related to oxidative stress. Wright et al. (2011) found reduced cardiac function and increased myocardial oxygen consumption in db/db diabetic mice, that was possibly regulated by increased mitochondrial ROS generation and lipid and protein peroxidation products in mice hearts. Alleviating oxidative stress targeting mitochondrial function has been confirmed as a potential therapeutic approach to limit ischemia-reperfusion (IR)-induced cardiac injury and protect against diabetes (Yang et al., 2013). Thus, preserving mitochondrial function, especially maintaining mitochondrial redox potential is also an important mechanism underlying the protective effects of curcumin against oxidative stress and diabetes. It showed that curcumin significantly decreased palmitate-induced oxidative stress and increased mitochondrial permeability, thus it was able to promote glucose-induced insulin secretion in pancreatic β-cells (Hao et al., 2015a). Lin et al. (2014) showed that collagen fibers in interstitium and expansion of mitochondria in cytoplasm of myocardium were increased in diabetic rats, that can be ameliorated by curcumin derivative B06 treatment. However, studies about direct effects targeting mitochondrial function of curcumin against DCM are limited, and further investigations about curcumin and its role in preserving mitochondrial function in DCM are needed. Thus, these studied indicated that curcumin can play its antioxidant properties in diabetes and DCM (Table 2 and Figure 2).
Curcumin and Its Anti-apoptotic Effects on Diabetes and DCM
Higher degree of cell apoptosis can be observed in the hearts of diabetic patients and in rodent models of diabetes. It has been shown that renin–angiotensin–aldosterone system (RAAS) activation, increased production of ROS, and abnormal expressions of apoptosis-related molecules are all associated with cardiomyocyte apoptosis and necrosis in diabetic hearts (Frustaci et al., 2000). Yu et al. (2012) found that curcumin supplementation (200 mg/kg/day) for 8 weeks prevented DM-induced cardiomyocytes apoptosis, evaluated by increased TUNEL-positive cells in diabetic rats. Pan et al. (2014) demonstrated that curcumin analog mitigated high glucose-induced apoptosis in cardiomyocytes and prevented the development of DCM, through the inhibition of JNK phosphorylation in the diabetic heart (Ren and Sowers, 2014). Wang et al. (2014) indicated a marked decrease in anti-apoptotic protein (Bcl-2), as well as an increase in caspase-3 cleavage and pro-apoptotic protein (Bax) in diabetic mice. However, treatment with curcumin analog (5 mg/kg/day) for 3 months significantly reversed the diabetes-induced cardiac cells apoptosis in diabetic mice (Wang et al., 2014). It also indicated that curcumin supplement effectively inhibited the elevated Bax/Bcl-2 ratio and alleviated high glucose-induced cardiomyocyte apoptosis in neonatal rat cardiomyocytes, accompanied by increased AKT and glycogen synthase kinase 3β (GSK-3β) phosphorylation (Yu et al., 2016). Thus, the evidence indicated that curcumin can alleviate cardiomyocyte apoptosis in diabetes (Table 2 and Figure 2).
Conclusion
In summary, as a natural compound, curcumin plays a critical role in human health. Clinical trials and preclinical studies have shown that curcumin possessed a potency to decrease blood glucose, improve insulin resistance and ameliorate dyslipidemia in patients with diabetes. It can exert positive effects on attenuate inflammatory activation, oxidative stress, and apoptosis in diabetes, and it is proposed to have the potential impacts to protect against DCM. Curcumin, which is believed to be pharmacologically safe, effective, and with low adverse effects, it can be considered as a promising agent for alternative therapies for the prevention and treatment of diabetes and its cardiovascular complications. However, clinical trials about the long-term effects and precise mechanisms of curcumin on diabetes and DCM in humans are still lacking. Thus, more randomized controlled trials and pre-clinical experiments are required to confirm the efficacy of curcumin and to elucidate the various mechanisms by which curcumin might mitigate diabetes and DCM, which may have the potential to open new horizons in the early prevention and treatment of the development of diabetes and DCM in the future.
Author Contributions
JZ and JC collected and synthesized the data and wrote the manuscript. QF and SZ reviewed and edited the manuscript. JZ and XX contributed to the design of this review.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was sponsored by National Key R&D Program of China (2017YFC1309603), National Key Research and Development Program of China (2016YFA0101002), National Natural Science Foundation of China (No. 81170736 and 81570715), Beijing Natural Science Foundation (No. 7184252), and the Fund for Fostering Young Scholars of Peking University Health Science Center (No. BMU2017PY008).
References
- Abo-Salem O. M., Harisa G. I., Ali T. M., El-Sayed el S. M., Abou-Elnour F. M. (2014). Curcumin ameliorates streptozotocin-induced heart injury in rats. 28 263–270. 10.1002/jbt.21562 [DOI] [PubMed] [Google Scholar]
- Anderson E. J., Kypson A. P., Rodriguez E., Anderson C. A., Lehr E. J., Neufer P. D. (2009). Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. 54 1891–1898. 10.1016/j.jacc.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aon M. A., Tocchetti C. G., Bhatt N., Paolocci N., Cortassa S. (2015). Protective mechanisms of mitochondria and heart function in diabetes. 22 1563–1586. 10.1089/ars.2014.6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arivazhagan A., Krishna S., Yadav S., Shah H. R., Kumar P., Ambasta R. K. (2015). Synergy of bone marrow transplantation and curcumin ensue protective effects at early onset of diabetes in mice. 7 473–484. 10.1111/1753-0407.12204 [DOI] [PubMed] [Google Scholar]
- Aziz M. T., El Ibrashy I. N., Mikhailidis D. P., Rezq A. M., Wassef M. A., Fouad H. H., et al. (2013). Signaling mechanisms of a water soluble curcumin derivative in experimental type 1 diabetes with cardiomyopathy. 5:13. 10.1186/1758-5996-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth G. L., Kapral M. K., Fung K., Tu J. V. (2006). Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. 368 29–36. 10.1016/S0140-6736(06)68967-8 [DOI] [PubMed] [Google Scholar]
- Boudina S., Abel E. D. (2007). Diabetic cardiomyopathy revisited. 115 3213–3223. 10.1161/CIRCULATIONAHA.106.679597 [DOI] [PubMed] [Google Scholar]
- Bugger H., Abel E. D. (2014). Molecular mechanisms of diabetic cardiomyopathy. 57 660–671. 10.1007/s00125-014-3171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L., Kang Y. J. (2003). Cell death and diabetic cardiomyopathy. 3 219–228. 10.1385/CT:3:3:219 [DOI] [PubMed] [Google Scholar]
- Chen H., Yang X., Lu K., Lu C., Zhao Y., Zheng S., et al. (2017). Inhibition of high glucose-induced inflammation and fibrosis by a novel curcumin derivative prevents renal and heart injury in diabetic mice. 278 48–58. 10.1016/j.toxlet.2017.07.212 [DOI] [PubMed] [Google Scholar]
- Chuengsamarn S., Rattanamongkolgul S., Luechapudiporn R., Phisalaphong C., Jirawatnotai S. (2012). Curcumin extract for prevention of type 2 diabetes. 35 2121–2127. 10.2337/dc12-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuengsamarn S., Rattanamongkolgul S., Phonrat B., Tungtrongchitr R., Jirawatnotai S. (2014). Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. 25 144–150. 10.1016/j.jnutbio.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Corbi G., Conti V., Davinelli S., Scapagnini G., Filippelli A., Ferrara N. (2016). Dietary phytochemicals in neuroimmunoaging: a new therapeutic possibility for humans? 7:364. 10.3389/fphar.2016.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan S., Segal Y., Shoenfeld Y. (2017). Dietary factors in rheumatic autoimmune diseases: a recipe for therapy? 13 348–358. 10.1038/nrrheum.2017.42 [DOI] [PubMed] [Google Scholar]
- Davinelli S., Scapagnini G., Marzatico F., Nobile V., Ferrara N., Corbi G. (2017). Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: a randomized, placebo-controlled study. 96 77–83. 10.1016/j.maturitas.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Diamant M., Lamb H. J., Smit J. W., De Roos A., Heine R. J. (2005). Diabetic cardiomyopathy in uncomplicated type 2 diabetes is associated with the metabolic syndrome and systemic inflammation. 48 1669–1670. 10.1007/s00125-005-1821-4 [DOI] [PubMed] [Google Scholar]
- Frustaci A., Kajstura J., Chimenti C., Jakoniuk I., Leri A., Maseri A., et al. (2000). Myocardial cell death in human diabetes. 87 1123–1132. 10.1161/01.RES.87.12.1123 [DOI] [PubMed] [Google Scholar]
- GBD 2015 Risk Factors Collaborators (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. 388 1659–1724. 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A., Beckman J. A., Schmidt A. M., Creager M. A. (2006). Advanced glycation end products: sparking the development of diabetic vascular injury. 114 597–605. 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- Guo S., Meng X. W., Yang X. S., Liu X. F., Ou-Yang C. H., Liu C. (2018). Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. 39 195–204. 10.1038/aps.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S. C., Patchva S., Koh W., Aggarwal B. B. (2012). Discovery of curcumin, a component of golden spice, and its miraculous biological activities. 39 283–299. 10.1111/j.1440-1681.2011.05648.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F., Kang J., Cao Y., Fan S., Yang H., An Y., et al. (2015a). Curcumin attenuates palmitate-induced apoptosis in MIN6 pancreatic beta-cells through PI3K/Akt/FoxO1 and mitochondrial survival pathways. 20 1420–1432. 10.1007/s10495-015-1150-0 [DOI] [PubMed] [Google Scholar]
- Hao P. P., Jiang F., Chen Y. G., Yang J., Zhang K., Zhang M. X., et al. (2015b). Traditional Chinese medication for cardiovascular disease. 12 115–122. 10.1038/nrcardio.2014.177 [DOI] [PubMed] [Google Scholar]
- Hao P., Jiang F., Cheng J., Ma L., Zhang Y., Zhao Y. (2017). Traditional Chinese Medicine for cardiovascular disease: evidence and potential mechanisms. 69 2952–2966. 10.1016/j.jacc.2017.04.041 [DOI] [PubMed] [Google Scholar]
- Hariri M., Haghighatdoost F. (2018). Effect of curcumin on anthropometric measures: a systematic review on randomized clinical trials. 37 215–222. 10.1080/07315724.2017.1392263 [DOI] [PubMed] [Google Scholar]
- Hashemzaei M., Entezari Heravi R., Rezaee R., Roohbakhsh A., Karimi G. (2017). Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. 802 44–51. 10.1016/j.ejphar.2017.02.038 [DOI] [PubMed] [Google Scholar]
- Hathout R. M., El-Ahmady S. H., Metwally A. A. (2017). Curcumin or bisdemethoxycurcumin for nose-to-brain treatment of Alzheimer disease? A bio/chemo-informatics case study. 10.1080/14786419.2017.1385017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- He Y., Yue Y., Zheng X., Zhang K., Chen S., Du Z. (2015). Curcumin, inflammation, and chronic diseases: how are they linked? 20 9183–9213. 10.3390/molecules20059183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Rains J., Croad J., Larson B., Jones K. (2009). Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. 11 241–249. 10.1089/ars.2008.2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Han J., Li T., Xin Z., Ma Z., Di W., et al. (2017). Curcumin as a potential protective compound against cardiac diseases. 119 373–383. 10.1016/j.phrs.2017.03.001 [DOI] [PubMed] [Google Scholar]
- Koeberle A., Werz O. (2014). Multi-target approach for natural products in inflammation. 19 1871–1882. 10.1016/j.drudis.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Lin Z. M., Jiao L. Z., Zheng Y., Wang X. Y., Wang L., Liu W. W., et al. (2014). [The effect and mechanism of curcumin derivative B06 on the myocardium from type 2 diabetic rats]. 30 38–42. [PubMed] [Google Scholar]
- Luo B., Huang F., Liu Y., Liang Y., Wei Z., Ke H., et al. (2017). NLRP3 inflammasome as a molecular marker in diabetic cardiomyopathy. 8:519 10.3389/fphys.2017.00519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mande P. P., Bachhav S. S., Devarajan P. V. (2016). Solid dispersion of curcumin as polymeric films for bioenhancement and improved therapy of rheumatoid arthritis. 33 1972–1987. 10.1007/s11095-016-1934-0 [DOI] [PubMed] [Google Scholar]
- Manolova Y., Deneva V., Antonov L., Drakalska E., Momekova D., Lambov N. (2014). The effect of the water on the curcumin tautomerism: a quantitative approach. 132 815–820. 10.1016/j.saa.2014.05.096 [DOI] [PubMed] [Google Scholar]
- Martel J., Ojcius D. M., Chang C. J., Lin C. S., Lu C. C., Ko Y. F., et al. (2017). Anti-obesogenic and antidiabetic effects of plants and mushrooms. 13 149–160. 10.1038/nrendo.2016.142 [DOI] [PubMed] [Google Scholar]
- Marwick T. H., Ritchie R., Shaw J. E., Kaye D. (2018). Implications of underlying mechanisms for the recognition and management of diabetic cardiomyopathy. 71 339–351. 10.1016/j.jacc.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Nakai A., Yamaguchi O., Takeda T., Higuchi Y., Hikoso S., Taniike M., et al. (2007). The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. 13 619–624. 10.1038/nm1574 [DOI] [PubMed] [Google Scholar]
- Nakmareong S., Kukongviriyapan U., Pakdeechote P., Donpunha W., Kukongviriyapan V., Kongyingyoes B., et al. (2011). Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. 383 519–529. 10.1007/s00210-011-0624-z [DOI] [PubMed] [Google Scholar]
- Naksuriya O., Okonogi S., Schiffelers R. M., Hennink W. E. (2014). Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. 35 3365–3383. 10.1016/j.biomaterials.2013.12.090 [DOI] [PubMed] [Google Scholar]
- Nelson K. M., Dahlin J. L., Bisson J., Graham J., Pauli G. F., Walters M. A. (2017). The essential medicinal chemistry of curcumin. 60 1620–1637. 10.1021/acs.jmedchem.6b00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Wang Y., Zhao Y., Peng K., Li W., Wang Y., et al. (2014). Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. 63 3497–3511. 10.2337/db13-1577 [DOI] [PubMed] [Google Scholar]
- Panahi Y., Khalili N., Sahebi E., Namazi S., Karimian M. S., Majeed M., et al. (2017a). Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. 25 25–31. 10.1007/s10787-016-0301-4 [DOI] [PubMed] [Google Scholar]
- Panahi Y., Khalili N., Sahebi E., Namazi S., Reiner Z., Majeed M., et al. (2017b). Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial. 33 1–5. 10.1016/j.ctim.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Pereira S. S., Alvarez-Leite J. I. (2014). Low-grade inflammation, obesity, and diabetes. 3 422–431. 10.1007/s13679-014-0124-9 [DOI] [PubMed] [Google Scholar]
- Pirola L., Balcerczyk A., Okabe J., El-Osta A. (2010). Epigenetic phenomena linked to diabetic complications. 6 665–675. 10.1038/nrendo.2010.188 [DOI] [PubMed] [Google Scholar]
- Poirier P., Bogaty P., Garneau C., Marois L., Dumesnil J. G. (2001). Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. 24 5–10. 10.2337/diacare.24.1.5 [DOI] [PubMed] [Google Scholar]
- Prasad S., Tyagi A. K., Aggarwal B. B. (2014). Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. 46 2–18. 10.4143/crt.2014.46.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M., Batkai S., Kechrid M., Mukhopadhyay P., Lee W. S., Horvath B., et al. (2012). Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. 61 716–727. 10.2337/db11-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Sowers J. R. (2014). Application of a novel curcumin analog in the management of diabetic cardiomyopathy. 63 3166–3168. 10.2337/db14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebkar A., Panahi Y., Khalili N., Sahebi E., Namazi S., Atkin S. L., et al. (2018). Curcuminoids plus piperine modulate adipokines in type 2 diabetes mellitus. 12 253–258. 10.2174/1574884713666180104095641 [DOI] [PubMed] [Google Scholar]
- Sarwar N., Gao P., Seshasai S. R., Gobin R., Kaptoge S., Di Angelantonio E., et al. (2010). Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. 375 2215–2222. 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seca A. M. L., Pinto D. C. G. A. (2018). Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. 19:E263. 10.3390/ijms19010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad A., Lee J., Lee Y. S. (2013). Curcumin in various cancers. 39 56–68. 10.1002/biof.1068 [DOI] [PubMed] [Google Scholar]
- Sikora E., Scapagnini G., Barbagallo M. (2010). Curcumin, inflammation, ageing and age-related diseases. 7:1. 10.1186/1742-4933-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetikno V., Sari F. R., Sukumaran V., Lakshmanan A. P., Mito S., Harima M., et al. (2012). Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC-MAPK signaling pathway. 47 604–614. 10.1016/j.ejps.2012.04.018 [DOI] [PubMed] [Google Scholar]
- Taylor R. A., Leonard M. C. (2011). Curcumin for inflammatory bowel disease: a review of human studies. 16 152–156. [PubMed] [Google Scholar]
- The International Diabetes Federation [IDF] (2017). , 8th Edn. Brussels: International Diabetes Federation. [Google Scholar]
- Topcu-Tarladacalisir Y., Akpolat M., Uz Y. H., Kizilay G., Sapmaz-Metin M., Cerkezkayabekir A., et al. (2013). Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: the roles of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. 16 296–305. 10.1089/jmf.2012.2550 [DOI] [PubMed] [Google Scholar]
- Tschope C., Walther T., Escher F., Spillmann F., Du J., Altmann C., et al. (2005). Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. 19 2057–2059. 10.1096/fj.05-4095fje [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhou S., Sun W., Mcclung K., Pan Y., Liang G., et al. (2014). Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. 306 E1239–E1247. 10.1152/ajpendo.00629.2013 [DOI] [PubMed] [Google Scholar]
- Westermann D., Rutschow S., Jager S., Linderer A., Anker S., Riad A., et al. (2007). Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. 56 641–646. 10.2337/db06-1163 [DOI] [PubMed] [Google Scholar]
- Wright E. M., Loo D. D., Hirayama B. A. (2011). Biology of human sodium glucose transporters. 91 733–794. 10.1152/physrev.00055.2009 [DOI] [PubMed] [Google Scholar]
- Xu H., Barnes G. T., Yang Q., Tan G., Yang D., Chou C. J., et al. (2003). Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. 112 1821–1830. 10.1172/JCI200319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Duan W., Lin Y., Yi W., Liang Z., Yan J., et al. (2013). SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. 65 667–679. 10.1016/j.freeradbiomed.2013.07.007 [DOI] [PubMed] [Google Scholar]
- Ye G., Metreveli N. S., Donthi R. V., Xia S., Xu M., Carlson E. C., et al. (2004). Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. 53 1336–1343. 10.2337/diabetes.53.5.1336 [DOI] [PubMed] [Google Scholar]
- Yekollu S. K., Thomas R., O’sullivan B. (2011). Targeting curcusomes to inflammatory dendritic cells inhibits NF-κB and improves insulin resistance in obese mice. 60 2928–2938. 10.2337/db11-0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You S., Qian J., Sun C., Zhang H., Ye S., Chen T., et al. (2018). An Aza resveratrol-chalcone derivative 6b protects mice against diabetic cardiomyopathy by alleviating inflammation and oxidative stress. 22 1931–1943. 10.1111/jcmm.13477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Wu J., Cai F., Xiang J., Zha W., Fan D., et al. (2012). Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. 7:e52013. 10.1371/journal.pone.0052013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Zha W., Ke Z., Min Q., Li C., Sun H., et al. (2016). Curcumin protects neonatal rat cardiomyocytes against high glucose-induced apoptosis via PI3K/Akt signalling pathway. 2016:4158591. 10.1155/2016/4158591 [DOI] [PMC free article] [PubMed] [Google Scholar]