Abstract
Biomolecules exist and function in cellular microenvironments that control their spatial organization, local concentration, and biochemical reactivity. Due to the complexity of native cytoplasm, the development of artificial bioreactors and cellular mimics to compartmentalize, concentrate, and control the local physico-chemical properties is of great interest. Here, we employ self-assembling polypeptide coacervates to explore the partitioning of the ubiquitous cytoskeletal protein actin into liquid polymer-rich droplets. We find that actin spontaneously partitions into coacervate droplets and is enriched by up to ∼30-fold. Actin polymerizes into micrometer-long filaments and, in contrast to the globular protein BSA, these filaments localize predominately to the droplet periphery. We observe up to a 50-fold enhancement in the actin filament assembly rate inside coacervate droplets, consistent with the enrichment of actin within the coacervate phase. Together these results suggest that coacervates can serve as a versatile platform in which to localize and enrich biomolecules to study their reactivity in physiological environments.
Introduction
The biological functions of intracellular organelles are defined by the composition and properties of the compartments, which often differ significantly from that of bulk cytoplasm. Well-known examples include the acidic pH of lysosomes and the mitochondrial redox potential (1). Although the compartmentalization of these organelles requires a lipid bilayer as a physical barrier, recent work has shown that organelles can also form as phase-separated droplets that do not require such a membrane (2, 3). The physicochemical properties of membraneless organelles likely regulate partitioning and reactivity of biomolecules, thereby serving an important role in their physiological function. The compositional complexity of individual cellular bodies, granules, and organelles poses a major challenge in discerning general mechanisms for partitioning and reaction regulation. One useful strategy has been to reduce compositional complexity by in vitro reconstitution of cellular bodies (4, 5). However, the sequence and structural complexity of natural biopolymers make systematic variation of microenvironment properties difficult.
A complementary approach is to selectively tune the physical and chemical properties of phase-separated microenvironments through the rational design of synthetic polymers that spontaneously phase-separate via known mechanisms, and then use these materials as a platform to study biomolecule partitioning and reactivity. For instance, charged homopolymers (polyelectrolytes) form polymer-dense liquid phases via complex coacervation (6, 7) and localize charged proteins (8, 9, 10, 11) and small molecules (12, 13). Precise chemical control of polypeptide-based polyelectrolytes allows for fine-tuning of several physio-chemical properties of the coacervate phase (7, 14), including functional groups, water content, viscosity, and surface tension, thereby enabling systematic investigations of protein interactions and activities in controlled microenvironments (15, 16). Knowledge of the general mechanisms by which microenvironment properties tune protein partitioning and activity could provide needed insight into the function of membraneless organelles as well as design principles for synthetic biology and engineering applications.
Here, we report the spontaneous partitioning and polymerization of the cytoskeletal protein actin inside model polypeptide coacervates (17, 18) as a proof-of-concept demonstration of coacervates as bioreactors for studying biomolecular reactions in cell-like physical environments. Our results establish polyelectrolyte complex coacervates as a viable platform to study mechanisms of partitioning and biochemical regulation by controlled perturbation of a condensed-phase microenvironment.
Materials and Methods
Solutions containing pLK/pRE coacervates and fluorescently labeled proteins were imaged on a spinning disk confocal microscope. We quantify the partitioning of the proteins BSA and actin to pLK/pRE coacervates by measuring the relative accumulated mass (RAM) of protein localized to coacervates. RAM values are calculated from thresholded fluorescence images acquired near droplet midplanes ∼20 min after the final mixing reaction. With the exception of Fig. 4, the RAM values reported represent the average fluorescence intensity within thresholded droplets normalized by the average fluorescence intensity exterior to droplets, and thus include contributions from both the droplet interior and periphery. For the analysis in Fig. 4, thresholded droplets were further subdivided into interior and peripheral regions, and auxiliary RAM values for each of these regions are reported.
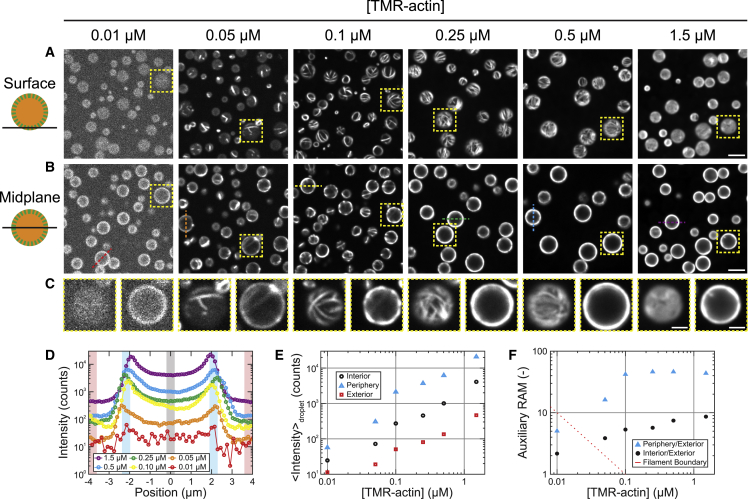
Figure 4.
Partitioning increases the local protein concentration in coacervates. (A and B) Confocal fluorescence micrographs are given of polypeptide coacervates containing variable concentrations of TMR-actin on nonadherent substrates, focused at the interface of the coacervates and the substrate (surface, A), or near the droplet midplane (B). Contrast is adjusted individually for each concentration of TMR-actin, but is consistent between confocal slices for each condition. (C) Magnified images are given of the yellow boxed regions outlined in (A) and (B). Scale bars, 5 μm (A and B) and 2 μm (C). (D) Fluorescence intensity is given along the colored dashed lines in (B). (E) Mean fluorescence intensity is given of interior (circle, black), periphery (triangle, cyan), and exterior (square, red) of coacervates droplets, obtained from the black-, cyan-, and red-shaded regions in (D). (F) Auxiliary RAM values, showing ratios of peripheral to exterior fluorescence (triangles, cyan) and interior to exterior fluorescence (circles, black), are given for the data in (D) and (E). Red line indicates where product of the auxiliary RAM value and the total actin concentration equals the critical concentration for actin assembly of ∼0.1 μM. Filaments are expected to the right of the red line, but not to the left. Conditions are 47% TMR-labeled Mg-ATP-actin at a range of concentrations, incubated with 5 mM pLK either alone (1.5- and 0.5-μM actin) or with 0.25 μM 91% Alexa-647-labeled BSA (0.25-, 0.1-, 0.05-, 0.01-μM actin) before addition of 5 mM pRE in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72 μM ATP (all concentrations final). To see this figure in color, go online.
The RAM is related to a more commonly used quantity known as the equilibrium partition coefficient, which is defined as the ratio of the concentration of the molecule inside the partitioning medium to that of the molecule outside the partitioning medium (4). At equilibrium, the RAM of a molecule in a partitioning medium is equal to its partition coefficient in that medium. The RAM values reported for BSA are thus equivalent to equilibrium partition coefficients.
In contrast, interpretation of the RAM of actin in coacervates measured by fluorescence in terms of equilibrium partition coefficients is fundamentally more challenging for two reasons. First, because actin filaments of different lengths are distinct chemical species, each with their own chemical potential, a partition coefficient for all species of actin together is ill-defined. Rather, a separate partition coefficient is required for each polymer species present (19). Second, the average TMR-fluorescence value we measure is insensitive to the distribution of actin filament lengths present, and thus the individual concentrations of each species present are unknown. Instead, the average TMR-fluorescence is directly proportional to the total mass of actin monomers present. For these reasons, we report the mass of actin accumulated in the coacervate, relative to that in solution outside of coacervates, instead of a partition coefficient. We expect that these two challenges are not specific to actin, but rather would hold for any instance in which partitioning is linked to a reaction in which the reactants and products cannot be readily distinguished and are therefore all included in the accumulated mass.
An additional practical complication is that the timescales for the equilibration of both the reaction and reactant partitioning can be quite long, particularly for equilibration of the actin filament length distribution by diffusive length fluctuations (20). Additional details of all experimental methods and analysis can be found in the Supporting Material.
Results
We use a model coacervate system (18) composed of the polycation poly-L-lysine (pLK) and the polyanion poly-(L,D)-glutamic acid (pRE), typically with ∼100 amino acids per polypeptide (Table S1). Phase separation at room temperature is rapid; initially clear aqueous solutions become visibly turbid in seconds upon mixing of pLK- and pRE-containing solutions at total polypeptide concentrations of 10 μM or more (Movie S1), and this is driven primarily by the release of condensed counterions (21). The presence of a polydisperse size distribution of polypeptide-rich coacervate droplets in solution, ranging in size from ∼0.4 < R < 4 μm, is confirmed directly by differential interference contrast (DIC) microscopy (Fig. 1, A–C; Movie S1). The round, droplet-like appearance of the condensed pLK/pRE coacervate phase is suggestive of a fluid phase (18). Under similar conditions, the surface tension has been measured to be γ ∼ 1 mN/m (22). Consistent with liquid-like properties on the timescale of seconds and longer, merging pLK/pRE droplets rapidly coalescence into a single, larger droplet (Fig. S1). From coalescence observations, we estimate the inverse capillary velocity = 1.6 ms/μm (Fig. S1 (5, 23)). This yields a viscosity of η = 1.6 Pa·s, ∼1000-fold higher than water. Thus, this simple model system is sufficient to create viscous phase-separated droplets with picoliter volumes.
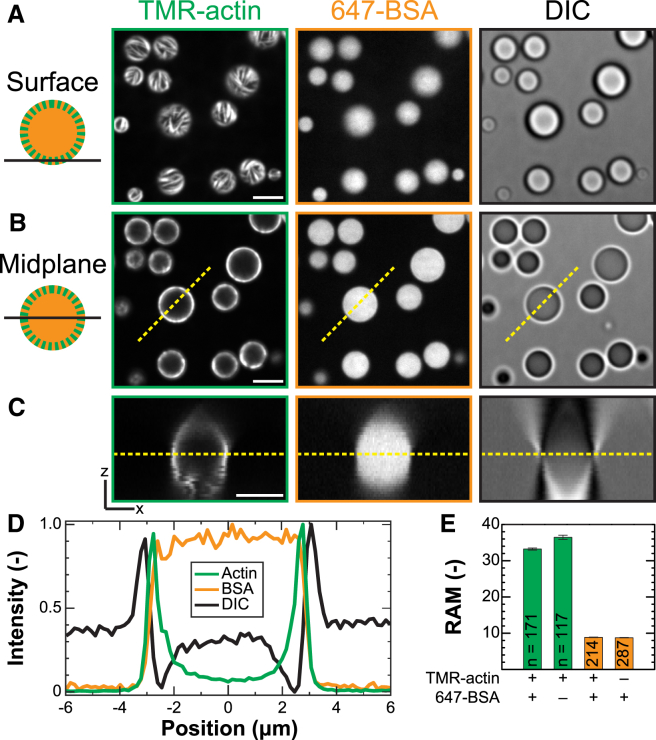
Figure 1.
F-actin localizes to the periphery of polypeptide coacervates. (A and B) Confocal fluorescence (left and middle) and DIC (right) micrographs are shown of polypeptide coacervates containing both TMR-actin (green) and 647-BSA (orange) on nonadherent substrates. (A and B) Focal plane is at the interface of the coacervates and the substrate (surface (A)), or near the midplane of the largest droplet (B), indicated by the dashed yellow line in (C). (C) An x-z cross section is formed from the intensity values along the dashed lines in (B) evaluated in all planes of a confocal z-stack. Scale bar, 5 μm in (A–C). (D) Normalized intensity line scans are given along the dashed yellow lines as indicated in (B) and (C). (E) Average RAM is given for coacervates in samples containing 0.5 μM actin alone, 0.5 μM BSA alone, or 0.25 μM actin and 0.25 μM BSA together (A–D). Error bars denote standard error of the mean. The number of droplets included in each condition is listed on the bar. Conditions are 0.5 μM total protein (0.25 μM Mg-ATP-actin (47% TMR-labeled) and 0.25 μM BSA (91% Alexa-647-labeled)) incubated with 5 mM pLK before addition of 5 mM pRE in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72 μM ATP (all concentrations final). To see this figure in color, go online.
Charged proteins spontaneously partition into coacervate droplets
Using a previously published protocol, proteins are mixed with the cationic pLK before initiation of phase separation by the addition of anionic pRE (8). It was previously found that the negatively charged protein BSA localizes preferentially to pLK/pRE coacervates, and is uniformly distributed within them (Fig. 1, A–D; Fig. S2). This preference for the coacervate phase is described quantitatively by a partition coefficient, defined here as the ratio of fluorescence intensity inside to outside the coacervates. We find an average partition coefficient of PCavg 8, regardless of whether BSA is added to solution before or after phase separation (Fig. 1 E; Fig. S3), which is indicative of spontaneous partitioning.
Here, we study the partitioning of actin, a cytoskeletal protein that self-assembles to form linear filaments (F-actin). Actin monomers and the chemically inert BSA are globular proteins of similar size (42 and 66 kDa, respectively) and carry comparable negative charge (isoelectric points of 5.23 and 5.60) (24). We find that actin also partitions to pLK/pRE coacervates and immediately observe linear structures localized preferentially to the coacervate periphery (Fig. 1, A–D).
Due to the polymerization of actin into filaments, which results in a monomer-polymer equilibrium of actin within the coacervate that is coupled to the exterior monomer pool, interpretation of these intensities as an equilibrium partition coefficient is difficult (see Materials and Methods). However, by integrating the total actin intensity localized to a droplet at a given timepoint after reaction initiation, we can calculate the RAM at that time. In the case of equilibrium partitioning of an inert molecule, such as BSA, the RAM measured at equilibrium is equivalent to the partition coefficient. This equivalence no longer holds when partitioning is linked to a reaction in which the reactants and products are both included in the accumulated mass, as is the case for actin monomer and filaments; rather, the RAM of actin reflects the partitioning of actin monomer and filament species of all lengths, which may or may not be equilibrated at the time of measurement. We find that the RAM of actin 20 min after assembly initiation is four-fold higher than that for BSA (Fig. 1 E). Interestingly, the RAM of BSA and actin are the same whether one or both proteins are present in solution (Fig. 1 E; Fig. S2). This suggests that, under the conditions explored here, BSA and actin do not compete directly for space in the coacervate. Both partitioning and peripheral localization of actin are robust to the order of addition (Fig. S4).
Self-assembled F-actin of canonical structure localizes to the coacervate periphery
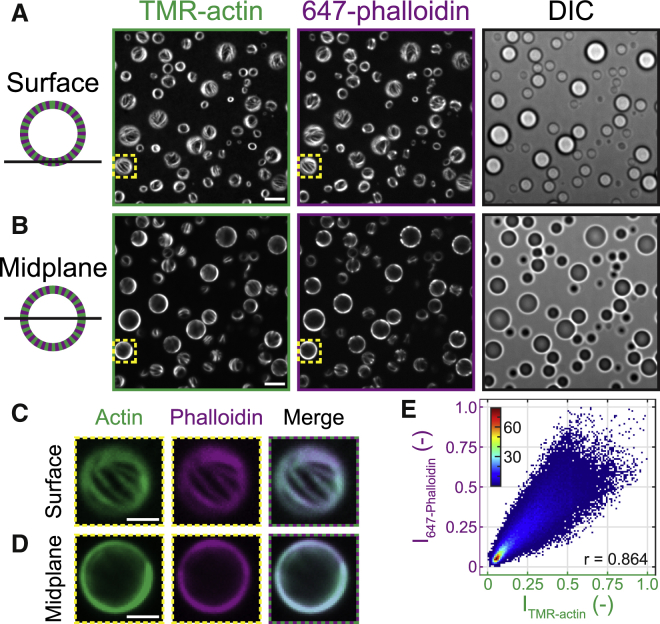
To test whether the linear actin structures are bona fide F-actin, we stained with fluorescently labeled phalloidin (647-phalloidin). Phalloidin is a small, uncharged toxin recognized for its ability to specifically bind to F-actin (25). 647-phalloidin was introduced into the solution after the coacervate formation and actin assembly, and found to localize along the linear actin structures. Confocal fluorescence micrographs at both the coverslip surface (Fig. 2, A and C) and droplet midplane (Fig. 2, B and D) reveal strong colocalization of phalloidin fluorescence to the linear actin structures with a Pearson’s correlation coefficient of 0.86 (Fig. 2 E; Supporting Material). This provides strong evidence that these linear actin-rich structures are composed of F-actin of canonical structure. Given the brightness of the F-actin structures, and previous work demonstrating that concentrations of polycations (and pLK in particular) lower than those in the coacervate phase are sufficient to bundle F-actin (26), we presume that the structures visible in Figs. 1 and 2 are F-actin bundles, rather than individual filaments.
Figure 2.
Linear actin filaments maintain canonical F-actin structure. (A and B) Confocal fluorescence (left and middle) and DIC (right) micrographs are given of polypeptide coacervates containing TMR-actin (green) after the addition of Alexa-647-Phalloidin (purple) on nonadherent substrates. (A and B) Focal plane is at the interface of the coacervates and the substrate (surface (A)) or near the midplane of the largest droplet in the field of view (B). Scale bars, 5 μm. Conditions are 0.5 μM Mg-ATP-actin (47% TMR-labeled) incubated with 5 mM pLK before addition of 5 mM pRE in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72 μM ATP (all concentrations final). 0.25 μM Alexa-647-Phalloidin was flown into the chamber in the same buffer after droplets had sedimented. (C and D) False-colored fluorescence images are given of the regions outlined in yellow boxes in (A) and (B) from the surface (C) and midplane (D). Right column shows a merge. Scale bars, 2 μm. (E) Correlation between normalized TMR-actin and 647-phalloidin fluorescence intensity values is shown for all coacervate-positive pixels in (A). Colors represent count density. Pearson’s correlation coefficient is r = 0.864. To see this figure in color, go online.
Actin assembly is enhanced in coacervates
Having demonstrated that actin polymerization proceeds in pLK/pRE coacervates, we next ask to what extent the coacervate microenvironment impacts the reaction rate. Actin is a convenient model protein for this purpose, owing to the existence of established spectroscopic tools for quantitatively monitoring assembly kinetics (27). In particular, the fluorescence intensity of the pyrene fluorophore increases ∼20-fold when pyrene-labeled monomers are incorporated into filaments and is a well-established method to track actin assembly (27), as depicted in the schematic in Fig. 3 A. In solution, the polymerization time course of 1.5 μM actin shows a characteristic lag phase, indicative of the kinetically slow filament nucleation step (28), followed by a phase of rapid growth and then saturation once a steady state is reached (Fig. 3 A) (27). At this actin concentration, the initial lag phase is typically ∼10 min and steady state is reached in ∼120 min (Fig. 3 B, black). The presence of pLK/pRE coacervates eliminates the lag phase and steady state is achieved within 10 min (Fig. 3 B, red). Thus, actin filament assembly is stimulated significantly by pLK/pRE coacervates.
Figure 3.
Coacervates and poly-L-lysine enhance actin assembly via different mechanisms. (A) Cartoon depicts the time course for spontaneous actin assembly monitored by changes in pyrene-actin fluorescence. (B) Spontaneous assembly is shown of 1.5 μM Mg-ATP-actin (12% pyrene-labeled) alone (black), with 3 μM pLK (cyan), or with 3 μM pLK and 3 μM pRE (red). (C) The assembly rate (1/t1/2) is shown for 1.5 μM actin samples with pLK alone (cyan) or equal concentrations of pLK and pRE (red) as a function of the concentration of pLK. Dashed lines denote the assembly rate of 1.5 μM actin alone measured in parallel with pLK-containing (cyan dashed) or pLK- and pRE-containing (red dashed) samples. (D) Timecourse of pyrene excimer fluorescence during spontaneous assembly is given of 1.5 μM Mg-ATP-actin (12% pyrene-labeled) alone (black), with 3 μM pLK alone (cyan), or with 3 μM pLK and 3 μM pRE together (red). In all experiments with polypeptides, Mg-ATP-actin is incubated with variable pLK in low salt before addition of pRE (red) or a buffer blank (cyan) in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72–150 μM ATP (all concentrations final). To see this figure in color, go online.
To assess reaction kinetics quantitatively, we estimate the assembly rate 1/t1/2, defined as the inverse of the time at which the pyrene fluorescence intensity reaches half of its relative change during the course of actin polymerization (Fig. 3 A; Supporting Material). The actin assembly rate 1/t1/2 increases from 0.03 to >1 min−1 as the total pLK concentration increases from 0.3 to 30 μM, while maintaining a pLK/pRE ratio of 1 (Fig. 3 C). Above 30 μM, the assembly rate saturates. Thus, the actin assembly rate is enhanced by nearly two orders of magnitude in the presence of coacervates (Figs. 1, 2, and 4).
Polylysine and coacervates stimulate actin assembly via distinct mechanisms
One possible explanation for the enhanced assembly rate is polycation-mediated F-actin nucleation. Polylysine has been shown to promote formation of antiparallel actin dimers (29) that nucleate F-actin (30, 31). Spontaneous assembly of pyrene-labeled actin in the presence of pLK shows a concentration-dependent increase in the rate of actin assembly (Fig. 3, B and C, blue data). It is tempting to compare the filament formation rate in solution directly to the rates observed within coacervates. However, the local pLK concentration within the coacervate phase is actually much higher, on the order of 1–3 M (see Supporting Material), such that the pLK concentrations reported in Fig. 3 C should not be directly compared. Furthermore, pLK/pRE interactions within the coacervate could limit pLK-mediated antiparallel dimer formation.
To test whether pLK-stabilized antiparallel actin dimers contribute to the assembly of F-actin in pLK/pRE coacervates, we monitored pyrene excimer fluorescence (29). In the absence of pLK, 1.5 μM actin displays no change in pyrene excimer fluorescence during the nucleation-dominated early phase of assembly (Fig. 3 D, black). In the presence of pLK, excimer fluorescence is highest during the initial nucleation phase, and decays rapidly as assembly proceeds (Fig. 3 D, blue). This excimer fluorescence time course is the hallmark of actin assembly mediated by pLK-stabilized antiparallel actin dimers (29). Importantly, in the presence of pLK/pRE coacervates, excimer fluorescence does not have these features characteristic of antiparallel dimer-mediated nucleation events (Fig. 3 D, red). These data strongly suggest that pLK-mediated nucleation is not the dominant mechanism by which actin assembly is enhanced in pLK/pRE coacervates.
Partitioning increases the local protein concentration in coacervates
A direct consequence of partitioning is that the local actin concentration in the coacervate phase is higher than that in the polymer-dilute phase. Thus, an alternate mechanism underlying enhanced assembly rates is an increased local actin concentration, clocal, within coacervates.
We tested this possibility by varying the global actin concentration, cglobal, from 0.01 to 1.5 μM. The threshold monomer concentration, or critical concentration c∗, required for polymerization of Mg-ATP-actin, is ∼0.1 μM (32, 33). If actin is concentrated in coacervate droplets ∼30-fold via partitioning, we would expect actin assembly within coacervates at global actin concentrations of ∼0.003 μM. Importantly, we observe coacervate-associated F-actin at global actin concentrations as low as 0.05 μM (Fig. 4, A–C). Interestingly, we observe peripherally biased partitioning of actin to pLK/pRE coacervates at all actin concentrations examined, even at the lowest concentration (0.01 μM) for which no filaments are clearly discernible. We note that the density of peripherally localized F-actin changes as a function of the global actin concentration. Whereas isolated filaments or bundles are visible at 0.05 μM, an F-actin shell too dense to resolve individual structures forms at 1.5 μM (Fig. 4, A–C).
To systematically characterize the localization of actin fluorescence, we examined fluorescence intensity line scans through the midplane, depicted in Fig. 4 B and shown in Fig. 4 D. These curves may be divided into three regions: the droplet exterior (red), periphery (blue), and interior (black). Under each experimental condition, fluorescence is highest at the droplet periphery, followed by the droplet interior. The lowest fluorescence intensities are consistently observed exterior to droplets (Fig. 4 E). The fluorescence increases nearly linearly with actin concentration both inside and outside the coacervate droplets (Fig. 4 E).
In addition to the average RAM of actin, we report two auxiliary RAM values derived from these intensity profiles: one for the ratio of droplet periphery (maximum observed intensity) to average intensity in the exterior of the droplet (RAMPeriph) (Fig. 4 F, blue/red), and a second for the ratio of droplet interior intensity (near the center of the droplet) to the average intensity in the exterior (RAMInt) (Fig. 4 F, black/red). Both of these auxiliary RAM values are greater than unity, indicative of partitioning of actin to the polymer-dense coacervate phase from the polymer-dilute phase. RAMPeriph and RAMInt both tend to reach plateaus for actin concentrations >0.1 μM; RAMPeriph values grow almost 10-fold before stabilizing at ∼45 once the global actin concentration reaches 0.1 μM, whereas RAMInt values increase over the range of actin concentrations investigated in this study, and appear to approach a plateau value of ∼10. The saturation of the RAMPeriph with global actin concentration suggests that exchange of protein between the polymer-dense and -dilute phases occurs readily, as has been reported in other liquid phase-separated systems (4).
Discussion
We present proof-of-concept experiments demonstrating that a polypeptide-based complex coacervate can be used as a model bioreactor to control the localization and activity of the self-assembling cytoskeletal protein actin. We find that actin partitions spontaneously to the coacervate phase, and that its partitioning is not influenced by BSA. Strong partitioning of actin to pLK/pRE coacervates increases the local actin concentration, contributing substantially to a >50-fold increase in the actin assembly rate at the highest concentrations of actin and coacervate. Actin filaments of canonical structure localize preferentially to the coacervate periphery, effectively forming core-shell particles, with the actin shell density controlled by the actin concentration.
Partitioning versus encapsulation of client proteins
Previous work interpreted the preferential localization of the client protein to coacervate phases as “encapsulation” (8, 34, 35). The implication of this language is that exchange of client molecules between the coacervate and dilute phases is either nonexistent or so small as to be negligible, as with encapsulation within lipid vesicles or emulsion droplets (36, 37). Indeed, Black et al. (8) argued that entry of the large (66 kDa) client BSA into pLK/pRE coacervates requires the formation of an intermediate electrostatic complex between the client and an oppositely charged polyelectrolyte in solution before phase separation, and that client release is triggered by pH-induced dissolution of the coacervate phase (8).
Our present results are more consistent with a molecular view termed “partitioning” (4), where the partition coefficient reflects the equilibration of steady fluxes of client molecules into and out of the coacervate phase. For instance, we observe partitioning of BSA and actin within ∼30 s upon addition of either to preformed pLK/pRE coacervates (Fig. S3). Given the very low polypeptide concentration in the dilute phase (<30 nM pLK), this suggests that recruitment of the client to the coacervate does not require the formation of an intermediate complex with a polyelectrolyte. Further, the recovery of BSA fluorescence after photobleaching of an entire coacervate droplet directly demonstrates exchange of partitioned proteins with the exterior pool (Fig. S5). Although we expect pLK/pRE coacervates to retain zero net electrical charge (38), both in the presence and absence of partitioned client proteins, we anticipate that the nonzero charge on both actin and BSA plays a direct role in determining the magnitude of the RAM measured here, as has been shown for super-charged GFP-variants in another model coacervate system (39).
Equilibrium partitioning in synthetic polypeptide coacervates is reminiscent of other recent in vitro work wherein client proteins of low-valency spontaneously partition into liquid phase-separated structures composed of high-valency scaffold proteins (4), as well as in coacervates formed from natural biopolymers (39). This is particularly interesting in that binding is mediated by specific low-affinity protein-protein interactions in the former case, in contrast to the nonspecific electrostatic interactions presumed in the case of coacervates. This suggests that the capacity to selectively partition client molecules may be a general property of condensed liquid-like phases, independent of the interactions driving partitioning. Here, the coupling of actin partitioning to polymerization within coacervates significantly delays the equilibration of either process, as monomer consumption by the polymerization reaction maintains a chemical potential difference for monomer between the dilute and condensed phases, resulting in the net partitioning of additional monomer. It will be interesting for future studies to explore how the relative timescales of client partitioning and reactions direct compositional maturation of condensed phases.
Origin of peripheral F-actin localization
Below, we examine three nonmutually exclusive physical mechanisms for the peripheral localization of F-actin in coacervates droplets: filament buckling, macromolecular depletion, and interfacial adsorption.
F-actin does not appear to protrude from micron-sized coacervate droplets, suggesting that coacervate surface tension may play a role in confining F-actin. One mechanism for peripheral filament localization is that surface tension causes filaments to buckle once the contour length exceeds the droplet diameter. A comparison of the energy required to increase the coacervate surface area to accommodate a protruding filament of length L and diameter d with a cylindrical cap, , with the energy required to bend the filament into a circular arc with radius R equal to that of the droplet, , yields the shortest length greater than 2R for which bending is energetically favorable:
| (1) |
where kB is the Boltzmann constant, T is temperature, and lp = 10 μm is the persistence length of F-actin (40). In a 1-μm-diameter coacervate droplet with the surface tension γ = 1 mN/m (22) at room temperature, bending is preferable for filaments longer than ∼1 μm. Coacervate surface tension is thus sufficient to bend F-actin with contour lengths larger than the droplet diameter. However, the observation that filaments and bundles even shorter than the droplet diameter are peripherally localized (0.05 μM; Fig. 4, A–C) indicates that surface tension-induced buckling cannot be the sole cause.
The peripheral localization of F-actin is reminiscent of the well-known crowding of F-actin to interfaces observed in the presence of macromolecular crowding agents (41, 42), which arises from depletion interactions (43). To assess this, we estimate the osmotic pressure needed to crowd F-actin to an interface to be Π∗ 450 Pa (Supporting Material). We estimate the osmotic pressure of the coacervate interior as that arising from a solution of flexible polymers characterized by a mesh size (44), ξ, as . This suggests that a coacervate with mesh size ξ ≤ 20 nm would generate sufficient osmotic pressure to drive peripheral localization of F-actin. We estimate the mesh size of our pLK/pRE coacervates to be 2–3 nm (Supporting Material), which supports the plausibility of a depletion-based mechanism. Noting the empirical observation that long filaments crowd more readily than short ones, macromolecular depletion could preferentially crowd long, high-aspect ratio filaments and bundles, leaving short filaments, actin monomer, and BSA uniformly distributed. A discrepancy with this model, however, is the observation that, unlike BSA, actin filaments are not mobile within the interior (Fig. S5). This, suggests that, although osmotic pressure may be sufficient for peripheral localization, the limited F-actin mobility within coacervates would preclude this from being the dominant mechanism for long filaments.
An additional, nonmutually exclusive mechanism for peripheral localization of F-actin is filament adsorption to the coacervate/bulk interface. For example, electrostatic interactions between F-actin and the coacervate could drive adsorption in a process akin to that of polyelectrolyte-mediated emulsion stabilization (45). Alternately, a difference in the interfacial tensions between F-actin and the solution and the coacervate, respectively, could drive localization of filaments to the coacervate/solution interface, such as seen in Pickering emulsions (46). In support of an adhesion-based mechanism, we note that filaments occasionally wrap around coacervate droplets when assembling in solution (Fig. S6; Movie S2), indicative of an attractive interaction between F-actin and the coacervate/solution interface. Our current experiments cannot conclude whether the protein is localized at the outside or inside surface of the coacervate, and future experiments with higher resolution are required.
Importantly, not all actin fluorescence is peripherally localized. Actin fluorescence intensity in the center of pLK/pRE coacervates is diffuse and enriched by as much as 10-fold compared to the surrounding solution (Fig. 4 F). Interior fluorescence increases with global actin concentration, which is inconsistent with the peripheral localization of all filaments. In that case, the interior fluorescence would correspond to solely actin monomers, which we would predict to have a constant local concentration of clocal = c∗ = 0.1 μM at steady state. This suggests that the coacervate interior contains a mixture of monomers and filaments. Given that a 250-nm filament (∼90 actin subunits) cannot be resolved with conventional light microscopy, an interior including both monomers and short filaments is consistent with the diffuse fluorescence signal we observe. The aforementioned mechanisms of peripheral localization all depend on F-actin length. As such, the presence of short filaments in the interior does not qualitatively distinguish between them. Understanding how peripheral localization is regulated will be an exciting avenue for future studies.
Mechanism of actin assembly enhancement
The assembly of actin within coacervates for global actin concentrations below the critical concentration is largely predicted by the 30-fold increase in the local actin concentration via localization into the coacervate phase. Assuming that the measured RAMInt corresponds primarily to monomer, we calculate the concentrations at which filament assembly within coacervates is expected and find that partitioning is sufficient to explain filament assembly down to 0.05 μM (red dashed line; Fig. 4 F). However, our measured partitioning is not quite sufficient to polymerize actin within coacervates at the lowest concentration (0.01 μM), yet we observe strong peripheral intensity (and interpret this to be polymerized actin). It is possible that the coacervate environment may alter the reaction rate kinetics of actin assembly (47, 48), as has been seen for transcription in cell-lysate coacervates (49). Indeed, because the coacervate-phase volume fraction (40%) and viscosity (2 Pa-s) are similar to those of the cytoplasm, this system may serve as a useful platform to study biochemical reactions in a more physiological environment.
Implications for biochemical reaction regulation
The high local concentrations generated by partitioning provide for an elegant means to both spatially localize and enhance the rates of biochemical reactions. Spontaneous partitioning to a condensed liquid-like phase substantially reduces the quantities of protein needed to study reactions under more physiological conditions. Of particular interest is the possibility of having direct control over partition coefficients and other local physicochemical properties of the coacervate phase as a means to control biochemical reactivity.
In summary, we have illustrated spontaneous partitioning of proteins inside coacervate droplets, leading to markedly increased actin assembly rates and spatial confinement of filaments. Assembly rate enhancements reported here are qualitatively consistent with a model in which these enhancements were contributed to by an increase in the local effective concentration of actin monomers in the coacervate phase. Our work introduces exciting avenues for the use of synthetic polymers to control physical and biological properties of bioreactors, and address questions in biology about the biochemistry of molecules in cell-like microenvironments.
Author Contributions
S.L.P. and M.L.G. conceived the project. P.M.M., S.S., S.L.P., D.R.K., M.L.G., and M.V.T. designed experiments. P.M.M., S.S., and S.L.P. performed experiments. D.R.K., M.L.G., and M.V.T. contributed essential reagents. P.M.M. analyzed image data and contributed analytical tools. P.M.M. and S.S. analyzed kinetic data. P.M.M. and S.S. performed physical estimates and calculations. P.M.M. prepared figures. P.M.M., S.S., and M.L.G. wrote the article with input from all authors.
Acknowledgments
The authors thank C. Suarez, K. Weirich, T. Witten, J. Vieregg, K. Ishihara, and J. Löber, as well as the Gardel, Kovar, and Tirrell labs, for helpful discussion and suggestions. We thank L. Li for assistance with gel permeation chromatography measurements.
This work was primarily supported by the University of Chicago Materials Research Science and Engineering Center, which is funded by the National Science Foundation under award DMR-1420709. P.M.M. thanks the University of Chicago MRSEC for a graduate fellowship.
Editor: Enrique De La Cruz.
Footnotes
Patrick M. McCall and Samanvaya Srivastava contributed equally to this work.
Margaret L. Gardel and Matthew V. Tirrell contributed equally to this work.
Patrick M. McCall’s present address is Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany.
Samanvaya Srivastava’s present address is Department of Chemical and Biomolecular Engineering, University of California, Los Angeles, Los Angeles, California.
Sarah L. Perry’s present address is Department of Chemical Engineering, University of Massachusetts Amherst, Amherst, Massachusetts.
Supporting Materials and Methods, six figures, one table, and two movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(18)30251-0.
Contributor Information
Margaret L. Gardel, Email: gardel@uchicago.edu.
Matthew V. Tirrell, Email: mtirrell@uchicago.edu.
Supporting Citations
References (50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63) appear in the Supporting Material.
Supporting Material
Scale bar, 10 μm. Playback is 30 frames per s. Time stamp format is mm:ss. DIC time-lapse imaging is given of pLK/pRE coacervate droplets in solution sedimenting onto a passivated glass coverslip due to gravity. Many droplets coalescence, merging into larger droplets. Conditions are 5 mM pLK, 5 mM pRE in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72 μM ATP (all concentrations final).
Scale bar, 4 μm. Playback is 30 frames per s. Time stamp format is mm:ss. Fluorescence time-lapse imaging is given of growing actin filaments winding around presumed coacervate droplets in the presence of methylcellulose. An image montage of the droplet in the lower center of the field of view is presented in Fig. S5 A. Conditions are 0.3 μM Mg-ATP-actin (unlabeled) added to a solution of 6 mM pLK/pRE coacervates and Alexa488-Phalloidin in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), 72 μM ATP, 0.5 % (w/v) 14-kDa methylcellulose, and an oxygen scavenging system (all concentrations final).
References
- 1.Alberts B., Johnson A., Walter P. 5th Ed. Garland Science; New York: 2007. Molecular Biology of the Cell. [Google Scholar]
- 2.Brangwynne C.P., Eckmann C.R., Hyman A.A. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science. 2009;324:1729–1732. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 3.Mitrea D.M., Kriwacki R.W. Phase separation in biology; functional organization of a higher order. Cell Commun. Signal. 2016;14:1. doi: 10.1186/s12964-015-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banani S.F., Rice A.M., Rosen M.K. Compositional control of phase-separated cellular bodies. Cell. 2016;166:651–663. doi: 10.1016/j.cell.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feric M., Vaidya N., Brangwynne C.P. Coexisting liquid phases underlie nucleolar subcompartments. Cell. 2016;165:1686–1697. doi: 10.1016/j.cell.2016.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Gucht J., Spruijt E., Cohen Stuart M.A. Polyelectrolyte complexes: bulk phases and colloidal systems. J. Colloid Interface Sci. 2011;361:407–422. doi: 10.1016/j.jcis.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava S., Tirrell M.V. Polyelectrolyte complexation. In: Rice S.A., Dinner A.R., editors. Advances in Chemical Physics. John Wiley & Sons; New York: 2016. pp. 499–544. [Google Scholar]
- 8.Black K.A., Priftis D., Tirrell M. Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 2014;3:1088–1091. doi: 10.1021/mz500529v. [DOI] [PubMed] [Google Scholar]
- 9.de Kruif C.G., Weinbreck F., de Vries R. Complex coacervation of proteins and anionic polysaccharides. Curr. Opin. Colloid Interface Sci. 2004;9:340–349. [Google Scholar]
- 10.Lindhoud S., Claessens M.M. Accumulation of small protein molecules in a macroscopic complex coacervate. Soft Matter. 2016;12:408–413. doi: 10.1039/c5sm02386f. [DOI] [PubMed] [Google Scholar]
- 11.Martin N., Li M., Mann S. Selective uptake and refolding of globular proteins in coacervate microdroplets. Langmuir. 2016;32:5881–5889. doi: 10.1021/acs.langmuir.6b01271. [DOI] [PubMed] [Google Scholar]
- 12.Kim M., Yeo S.J., Lee D. One-step generation of multifunctional polyelectrolyte microcapsules via nanoscale interfacial complexation in emulsion (NICE) ACS Nano. 2015;9:8269–8278. doi: 10.1021/acsnano.5b02702. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M., Eghtesadi S.A., Zacharia N.S. Partitioning of small molecules in hydrogen-bonding complex coacervates of poly(acrylic acid) and poly(ethylene glycol) or pluronic block copolymer. Macromolecules. 2017;50:3818–3830. [Google Scholar]
- 14.Liu Y., Winter H.H., Perry S.L. Linear viscoelasticity of complex coacervates. Adv. Colloid Interface Sci. 2017;239:46–60. doi: 10.1016/j.cis.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Aumiller W.M., Jr., Keating C.D. Phosphorylation-mediated RNA/peptide complex coacervation as a model for intracellular liquid organelles. Nat. Chem. 2016;8:129–137. doi: 10.1038/nchem.2414. [DOI] [PubMed] [Google Scholar]
- 16.Koga S., Williams D.S., Mann S. Peptide-nucleotide microdroplets as a step towards a membrane-free protocell model. Nat. Chem. 2011;3:720–724. doi: 10.1038/nchem.1110. [DOI] [PubMed] [Google Scholar]
- 17.Pacalin N.M., Leon L., Tirrell M. Directing the phase behavior of polyelectrolyte complexes using chiral patterned peptides. Eur. Phys. J. Spec. Top. 2016;225:1805–1815. [Google Scholar]
- 18.Perry S.L., Leon L., Tirrell M. Chirality-selected phase behaviour in ionic polypeptide complexes. Nat. Commun. 2015;6:6052. doi: 10.1038/ncomms7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka F. Cambridge University Press; Cambridge, United Kingdom: 2011. Polymer Physics: Applications to Molecular Association and Thermoreversible Gelation. [Google Scholar]
- 20.Mohapatra L., Lagny T.J., Kondev J. The limiting-pool mechanism fails to control the size of multiple organelles. Cell Syst. 2017;4:559–567.e14. doi: 10.1016/j.cels.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ou Z., Muthukumar M. Entropy and enthalpy of polyelectrolyte complexation: Langevin dynamics simulations. J. Chem. Phys. 2006;124:154902. doi: 10.1063/1.2178803. [DOI] [PubMed] [Google Scholar]
- 22.Priftis D., Farina R., Tirrell M. Interfacial energy of polypeptide complex coacervates measured via capillary adhesion. Langmuir. 2012;28:8721–8729. doi: 10.1021/la300769d. [DOI] [PubMed] [Google Scholar]
- 23.Brangwynne C.P., Mitchison T.J., Hyman A.A. Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proc. Natl. Acad. Sci. USA. 2011;108:4334–4339. doi: 10.1073/pnas.1017150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ProtParam. ExPASy - ProtParam tool. http://web.expasy.org/protparam/ [Accessed April 21, 2017].
- 25.Vandekerckhove J., Deboben A., Wieland T. The phalloidin binding site of F-actin. EMBO J. 1985;4:2815–2818. doi: 10.1002/j.1460-2075.1985.tb04008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang J.X., Janmey P.A. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J. Biol. Chem. 1996;271:8556–8563. doi: 10.1074/jbc.271.15.8556. [DOI] [PubMed] [Google Scholar]
- 27.Cooper J.A., Walker S.B., Pollard T.D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell Motil. 1983;4:253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- 28.Sept D., McCammon J.A. Thermodynamics and kinetics of actin filament nucleation. Biophys. J. 2001;81:667–674. doi: 10.1016/S0006-3495(01)75731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bubb M.R., Govindasamy L., McKenna R. Polylysine induces an antiparallel actin dimer that nucleates filament assembly: crystal structure at 3.5-Å resolution. J. Biol. Chem. 2002;277:20999–21006. doi: 10.1074/jbc.M201371200. [DOI] [PubMed] [Google Scholar]
- 30.Brown S.S., Spudich J.A. Nucleation of polar actin filament assembly by a positively charged surface. J. Cell Biol. 1979;80:499–504. doi: 10.1083/jcb.80.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oriol-Audit C. Polyamine-induced actin polymerization. Eur. J. Biochem. 1978;87:371–376. doi: 10.1111/j.1432-1033.1978.tb12386.x. [DOI] [PubMed] [Google Scholar]
- 32.Pollard T.D. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara I., Vavylonis D., Pollard T.D. Polymerization kinetics of ADP- and ADP-Pi-actin determined by fluorescence microscopy. Proc. Natl. Acad. Sci. USA. 2007;104:8827–8832. doi: 10.1073/pnas.0702510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nolles A., Westphal A.H., Borst J.W. Encapsulation of GFP in complex coacervate core micelles. Biomacromolecules. 2015;16:1542–1549. doi: 10.1021/acs.biomac.5b00092. [DOI] [PubMed] [Google Scholar]
- 35.Obermeyer A.C., Mills C.E., Olsen B.D. Complex coacervation of supercharged proteins with polyelectrolytes. Soft Matter. 2016;12:3570–3581. doi: 10.1039/c6sm00002a. [DOI] [PubMed] [Google Scholar]
- 36.Vieregg J.R., Tang T.-Y.D. Polynucleotides in cellular mimics: coacervates and lipid vesicles. Curr. Opin. Colloid Interface Sci. 2016;26:50–57. [Google Scholar]
- 37.Kakran M., Antipina M.N. Emulsion-based techniques for encapsulation in biomedicine, food and personal care. Curr. Opin. Pharmacol. 2014;18:47–55. doi: 10.1016/j.coph.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R., Shklovskii B.I. Phase diagram of solution of oppositely charged polyelectrolytes. Phys. Stat. Mech. Its Appl. 2005;352:216–238. [Google Scholar]
- 39.Pak C.W., Kosno M., Rosen M.K. Sequence determinants of intracellular phase separation by complex coacervation of a disordered protein. Mol. Cell. 2016;63:72–85. doi: 10.1016/j.molcel.2016.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough B.R., Grintsevich E.E., De La Cruz E.M. Cofilin-linked changes in actin filament flexibility promote severing. Biophys. J. 2011;101:151–159. doi: 10.1016/j.bpj.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhn J.R., Pollard T.D. Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 2005;88:1387–1402. doi: 10.1529/biophysj.104.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murrell M.P., Gardel M.L. F-actin buckling coordinates contractility and severing in a biomimetic actomyosin cortex. Proc. Natl. Acad. Sci. USA. 2012;109:20820–20825. doi: 10.1073/pnas.1214753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asakura S., Oosawa F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 1954;22:1255–1256. [Google Scholar]
- 44.de Gennes P.-G. 1st Ed. Cornell University Press; Ithaca, NY: 1979. Scaling Concepts in Polymer Physics. [Google Scholar]
- 45.Andelman D., Joanny J.-F. Polyelectrolyte adsorption. Comptes Rendus Acad. Sci. IV Phys. 2000;1:1153–1162. [Google Scholar]
- 46.Bon S.A.F. The phenomenon of Pickering stabilization: a basic introduction. In: Bon S.A.F., Ngai T., editors. Particle-Stabilized Emulsions and Colloids. Royal Society of Chemistry Publishing; Cambridge, United Kingdom: 2014. pp. 1–7. [Google Scholar]
- 47.Drenckhahn D., Pollard T.D. Elongation of actin filaments is a diffusion-limited reaction at the barbed end and is accelerated by inert macromolecules. J. Biol. Chem. 1986;261:12754–12758. [PubMed] [Google Scholar]
- 48.Frederick K.B., Sept D., De La Cruz E.M. Effects of solution crowding on actin polymerization reveal the energetic basis for nucleotide-dependent filament stability. J. Mol. Biol. 2008;378:540–550. doi: 10.1016/j.jmb.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sokolova E., Spruijt E., Huck W.T. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl. Acad. Sci. USA. 2013;110:11692–11697. doi: 10.1073/pnas.1222321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kramer J.R., Deming T.J. General method for purification of α-amino acid-n-carboxyanhydrides using flash chromatography. Biomacromolecules. 2010;11:3668–3672. doi: 10.1021/bm101123k. [DOI] [PubMed] [Google Scholar]
- 51.Spudich J.A., Watt S. The regulation of rabbit skeletal muscle contraction. I. biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J. Biol. Chem. 1971;246:4866–4871. [PubMed] [Google Scholar]
- 52.Kovar D.R., Kuhn J.R., Pollard T.D. The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 2003;161:875–887. doi: 10.1083/jcb.200211078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kudryashov D.S., Reisler E. Solution properties of tetramethylrhodamine-modified G-actin. Biophys. J. 2003;85:2466–2475. doi: 10.1016/s0006-3495(03)74669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hansen S., Zuchero J.B., Mullins R.D. Cytoplasmic actin: purification and single molecule assembly assays. In: Coutts A.S., editor. Adhesion Protein Protocols. Humana Press; New York, NY: 2013. pp. 145–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Molecular Probes. The Molecular Probes Handbook. https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook.html [Accessed April 21, 2017].
- 56.Winkelman J.D., Bilancia C.G., Kovar D.R. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. USA. 2014;111:4121–4126. doi: 10.1073/pnas.1322093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jönsson P., Jonsson M.P., Höök F. A method improving the accuracy of fluorescence recovery after photobleaching analysis. Biophys. J. 2008;95:5334–5348. doi: 10.1529/biophysj.108.134874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimozawa T., Yamagata K., Mimori-Kiyosue Y. Improving spinning disk confocal microscopy by preventing pinhole cross-talk for intravital imaging. Proc. Natl. Acad. Sci. USA. 2013;110:3399–3404. doi: 10.1073/pnas.1216696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pubchem. L-glutamic acid | C5H9NO4 - PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/L-glutamic_acid [Accessed May 8, 2017].
- 60.Spruijt E., Westphal A.H., van der Gucht J. Binodal compositions of polyelectrolyte complexes. Macromolecules. 2010;43:6476–6484. [Google Scholar]
- 61.Hanke F., Serr A., Netz R.R. Stretching single polypeptides: the effect of rotational constraints in the backbone. EPL Europhys. Lett. 2010;92:53001. [Google Scholar]
- 62.Dobrynin A.V. Electrostatic persistence length of semiflexible and flexible polyelectrolytes. Macromolecules. 2005;38:9304–9314. [Google Scholar]
- 63.Carrion-Vazquez M., Oberhauser A.F., Fernandez J.M. Mechanical and chemical unfolding of a single protein: a comparison. Proc. Natl. Acad. Sci. USA. 1999;96:3694–3699. doi: 10.1073/pnas.96.7.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scale bar, 10 μm. Playback is 30 frames per s. Time stamp format is mm:ss. DIC time-lapse imaging is given of pLK/pRE coacervate droplets in solution sedimenting onto a passivated glass coverslip due to gravity. Many droplets coalescence, merging into larger droplets. Conditions are 5 mM pLK, 5 mM pRE in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), and 72 μM ATP (all concentrations final).
Scale bar, 4 μm. Playback is 30 frames per s. Time stamp format is mm:ss. Fluorescence time-lapse imaging is given of growing actin filaments winding around presumed coacervate droplets in the presence of methylcellulose. An image montage of the droplet in the lower center of the field of view is presented in Fig. S5 A. Conditions are 0.3 μM Mg-ATP-actin (unlabeled) added to a solution of 6 mM pLK/pRE coacervates and Alexa488-Phalloidin in 50 mM KCl, 1 mM MgCl2, 1 mM EGTA, 10 mM imidazole (pH 7.0), 72 μM ATP, 0.5 % (w/v) 14-kDa methylcellulose, and an oxygen scavenging system (all concentrations final).