Abstract
There is a complex interaction between cancer and the immune system. Tumor-associated macrophages (TAMs) can be subverted by the cancer to adopt a pro-tumor phenotype to aid tumor growth. These anti-inflammatory, pro-tumor TAMs have been shown to contribute to a worsened outcome in several different types of cancer. Various strategies aimed at combating the pro-tumor TAMs have been developed. Several therapies, such as oncolytic viral therapy and high-intensity focused ultrasound, have been shown to stimulate TAMs and suppress tumor growth. Targeting TAMs is a promising way to combat cancer, but sensitive imaging methods that are capable of detecting these therapeutic responses are needed. A promising idea is to use imaging contrast agents to label TAMs to determine their relative number and location within, and around the tumor. This can provide information about the efficacy of TAM depletion therapies, as well as macrophage-stimulating therapies. In this review, we describe various in vivo MRI methods capable of tracking TAMs, and conclude with a short section on tracking TAMs in patients.
Keywords: Tumor-associated macrophage, cancer, imaging, cell tracking, magnetic resonance imaging
Introduction
The interaction of cancer and the immune system plays an intricate role in cancer progression and evasion from immune surveillance.1 It is known that there is extensive macrophage infiltration into the tumor in a wide variety of cancer types.2,3 In many cases, these tumor-associated macrophages (TAMs) are subverted by the cancer to adopt an anti-inflammatory phenotype and secrete factors to promote angiogenesis and tumor invasion.4
Although these anti-inflammatory TAMs contribute to worsened outcome in several types of cancer,5–7 there are promising new strategies that have been developed to target TAMs to either deplete them or polarize them into an anti-tumor phenotype to inhibit tumor growth.8–10
Unfortunately, conventional clinical magnetic resonance imaging (MRI) protocols do not appear to be a sensitive marker of treatment response.11 To aid immunotherapy research and translation into clinical use, new biomarkers are urgently needed to identify treatment response.
We believe that MRI is poised to play a major role in assessing the therapeutic response of TAM-directed immunotherapy. Tracking TAMs would be useful for assessing monocyte infiltration in immune stimulating therapies, and could also allow for detection of macrophage depletion in TAM suppressing therapies. Thus, imaging the number and phenotype of TAMs can provide valuable prognostic information. To facilitate this discussion, we review the current in vivo studies using MRI to track TAMs, and discuss the literature on tracking TAMs in clinical patients.
Tracking Tumor-Associated Macrophages: Concept and Evidence
Several studies have shown that TAMs can be tracked using MRI contrast agents: gadolinium (Gd), iron oxide nanoparticles, and fluorine 19 (19F). The mechanism to detect labeled TAMs is different, depending on the MRI contrast agents being investigated.
Gadolinium is the most commonly used clinical contrast agent that is used with standard 1H radiofrequency coils. It shortens T1, making the surrounding tissues brighter on T1w images. Gadolinium is not readily phagocytosed to macrophages, so they must be conjugated to antibodies that bind macrophage receptors to track TAMs. In addition, different tissues have different T1 (i.e. some appear bright, some appear dark on T1w images), so it is important to collect a pre-gadolinium image to serve as a baseline.
Gadolinium tagged with a fluorescent poly (l-glutamic acid) (PG-Gd) has been used to track TAMs in a C6 rat glioma model.12 MRI was collected using a Bruker 7.0T MRI. A pre-GD T1w spin echo MRI was collected initially. After intravenous administration of the fluorescent PG-Gd derivative, the tumor showed significant enhancement on T1w MRI. Depletion of monocytes and macrophages using clodronate liposomes significantly reduced the fluorescence intensity, suggesting that phagocytes play a role in the tumor enhancement process. Immunohistochemistry showed that the PG-Gd compound was co-localized with macrophages. Although this technique is promising for labeling of TAMs, it is known that Gd has a relatively low sensitivity, meaning that a high concentration of Gd is needed to cause a detectable change on MRI.13
Iron oxide nanoparticles are used with conventional 1H radiofrequency coils. They are T2* contrast agents that reduces T2* and cause darkening on T2* weighted images. Iron oxide nanoparticles are predominantly engulfed by phagocytic cells such as macrophages and Kupffer cells, so they do not need to be conjugated to antibodies and can be directly injected intravenously. A pre-contrast image is required to image TAMs, since necrosis and deoxyhemoglobin can all produce signal loss similar to iron oxide nanoparticles. Iron oxide nanoparticles produce a “blooming effect” on T2*w MRI and produces signal loss in a large area surrounding the iron. This makes it difficult to interpret the spatial distribution of TAMs, and do not allow for quantification based on changes in signal intensity. This problem can be overcome by either collecting a T2* map (and thus calculating R2*) with a multi-gradient echo (GE) sequence, or by quantifying the change in susceptibility caused by the contrast agent via quantitative susceptibility mapping (QSM). Both R2* and absolute susceptibility are linearly related to the concentration of iron oxide nanoparticles, thus providing spatial information and allowing for quantification of iron concentration.
Using a breast tumor mouse model, it was shown that ferumoxytol, a Food and Drug Association (FDA)-approved ultra-small iron oxide nanoparticle (USPIO) used to treat anemia, is capable of labeling TAMs in vivo.14 The authors used a 2T Bruker MRI and collected a T2* weighted GE image before and after ferumoxytol and found significant darkening that corresponds to the presence of iron on the GE 24 hours after ferumoxytol administration. Immunohistochemistry showed that the predominant source of ferumoxytol uptake was due to TAMs, not cancer cells, indicating high specificity of labeling within the tumor. Ferumoxytol-related MRI changes were reversed after depletion of macrophage/monocytes with anti-CSF1 monoclonal antibody.14 This indicates that macrophages are needed for the T2* enhancement post ferumoxytol.
19F is a nucleus that can undergo nuclear magnetic resonance. A standard 1H MRI coil cannot be used to image 19F, as this nucleus is on a different frequency compared to 1H. Fluorine 19 can be incorporated into perfluorocarbon compounds (PFCs), which are engulfed by macrophages and Kupffer cells. PFCs do not need to be conjugated to antibodies to label TAMs, and can be injected intravenously. Since 19F is found in very low levels in animals and humans, a pre-contrast baseline is not required. Because of its low background in vivo, 19F can provide excellent visualization of macrophages. In addition, as 19F has a different frequency than 1H, it does not interfere with conventional 1H MRI sequences such as diffusion weighted imaging or spectroscopy. This is a major advantage of using 19F to label TAMs. However, one would need to use a coil capable of switching the frequency of its radio frequency (RF) pulse between 1H and 19F to provide anatomical information, as well as information about the fluorine atom.
A study comparing the ability of iron oxide nanoparticles and 19F at detecting TAMs was done by Makela et al. The authors utilized a breast tumor model where 4T1 breast tumor cells are injected into the 4th mammary pad of mice. TAMs were imaged at either 4 days or 3 weeks post implantation with both ferumoxytol and PFCs. For ferumoxytol imaging, a 3T General Electric MRI was used to acquire a balanced steady state free precession (bSSFP) scan before and 24 hours after ferumoxytol injection. For 19F imaging, a 9.4T MRI was used to acquire a bSSFP scan 24 hours after PFC injection. They showed that PFC accumulated in similar areas of the tumor compared to ferumoxytol, which was validated via immunohistochemitry. At 3 weeks where the tumor was large, ferumoxytol overestimates the TAM density on T2*w GE images due to the blooming effect.15
These results were corroborated by a proof of principle study by another group examining TAMs in tongue squamous carcinomas.16 Two different carcinoma cell lines were implanted in the bilateral flank of mice. Using an 11.7T Bruker MRI and a T2w rapid acquisition with refocusing echoes (RARE) sequence, they showed that there was significant PFC accumulation in the tumor after PFC injection. Immunohistochemistry showed a significant correlation between the number of 19F atoms and macrophage count 10 days after PFC injection. Furthermore, their study provides evidence showing that different tumor cell lines have different immunogenicity, as there was a significant difference in macrophage and 19F accumulation between the two cell lines. 19F is a promising MRI technique to track and quantify TAMs.
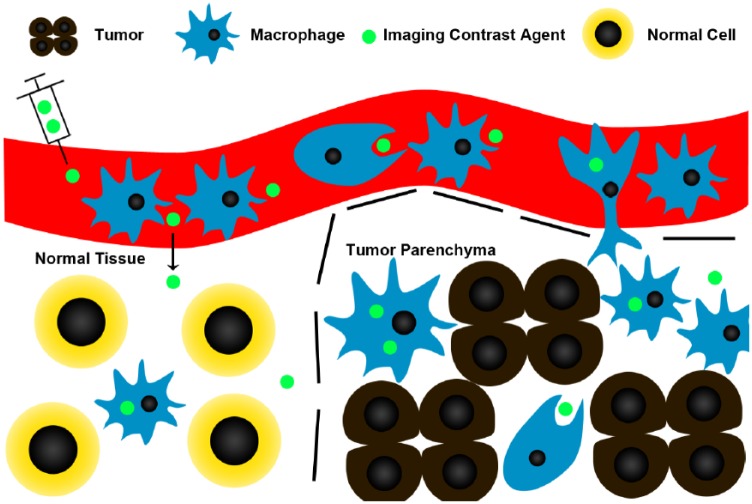
Figure 1 illustrates the process by which TAMs can be imaged in vivo using contrast agents. The post contrast scan is usually acquired 24 hours after contrast administration to allow time for phagocytosis and monocyte infiltration into the tumor, while giving time for un-engulfed contrast agents to be cleared from the tumor. Since some of these contrast agents are already clinically approved for other purposes, there is high potential for clinical translation to aid trials targeting TAMs.
Figure 1.
Contrast agents, such as iron oxide nanoparticles, are preferentially phagocytosed by monocytes and macrophages, and can be used to label TAMs. Dotted lines represent the tumor boundaries. Contrast agents are injected intravenously where they are phagocytosed by circulating monocytes. Some of these monocytes that have phagocytosed the contrast agent migrate into the tumor and differentiate into macrophages. As tumors often have increased vascular permeability, contrast agents could also leak into the tumor and be picked up by macrophages. Post-contrast images are usually acquired at least 24 hours after administration of contrast, to allow time for phagocytosis/cell migration, and wash out of non-phagocytosed contrast agent. Although macrophages in normal tissue may still take up contrast agents, there will be fewer of them, and the normal vascular permeability may decrease contrast leakage into normal tissue.
MRI is not the only method that can track TAMs. Two other methods capable of tracking TAMs that are worth mentioning are positron emission tomography (PET) and intra-vital fluorescence microscopy.
Proof of principle studies have been carried out to show that various PET tracers are capable of labeling TAMs.17,18 High-density lipoproteins (HDL) are phagocytosed by macrophages. HDLs tagged with 89Zr have been used to target and label TAMs in 4T1 breast cancer model.18
Mannose receptors, which are upregulated on TAMs, have been labeled with a 64Cu liposome.17 Accumulation of 64Cu liposomes were detected in the tumor in a mouse lung cancer model. These liposomes were co-localized with macrophages, indicating target specificity.
PET is a promising candidate for imaging TAMs. Compared to other imaging modalities, it produces low background noise, allows for high target specificity and has relatively high sensitivity.
TAMs of superficially located tumors can also be tracked using fluorescence microscopy in knock in mice whose macrophages express fluorophores. It was shown that myeloid derived cells in the tumor–stroma border were much more motile compared to the ones found inside the tumor.19 These myeloid derived cells can be classified into three populations based on their motility, phagocytic capacity, and cell surface marker expression. These distinct populations of myeloid derived cells within different regions of the tumor presumably have different functions, highlighting the benefits of using fluorescence live imaging to study TAMs in vivo.
A novel fluorescence microscopy method has been developed to image TAMs using endogenous fluorescence.20 Nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) are fluorescent metabolites involved in glycolysis and cellular respiration.20 Szulczewski et al used Fluorescence Lifetime Imaging Microscopy (FLIM) to construct a fluorescence decay curve, which allows the concentration of NADH and FAD to be quantified. Using a mammary carcinoma mouse model, they observed that over 75% of cells with high FAD within the tumor are macrophages (F4/80 positive). This is a promising technique to study TAMs without the need for transgenic animals.
Fluorescence microscopy is an excellent tool for in vivo tracking of TAMs in animal models with high target specificity. The fact that FAD/NADH has the potential to provide non-invasive contrast for imaging TAMs in patients is interesting.
MRI Tracking of Tumor-Associated Macrophages: Applications
TAM imaging has been used to provide valuable prognostic information in preclinical animal models, such as detecting of treatment response of monocyte/macrophage stimulating therapies, and magnetic guidance of labeled macrophages toward the cancer. Table 1 summarizes the studies that use MRI to track TAMs in various tumor models with various contrast agents.
Table 1.
Summary of various MRI studies that used MRI contrast agents to track TAMs in vivo.
| Citation | Tumor Type | Site of Tumor | Contrast Agent | Hardware | Sequence |
|---|---|---|---|---|---|
| Melancon | C6 Glioma | Intracranial | Gd-PG | Bruker 7T | T1w SE |
| Shin | 4T1 Mammary | Subcutaneous | PFC | Bruker 7T | 19F T2*w (FLASH) |
| Khurana | SCC4/Cal27 Carcinoma | Bilateral Lower Flank | PFC | Bruker 11.7T | T2w RARE SE |
| Makela | 4T1/168FARN/67NR | Mammary | PFC | 9.4T | bSSFP |
| Weibel | Melanoma | Subcutaneous | PFC | Bruker 7T | 19F Turbo Spin Echo |
| Makela | 4T1 | Mammary | PFC/ferumoxytol | GE 3T/9.4T | bSSFP/bSSFP |
| Yang | Glioma | Intracranial | Ferumoxytol | Bruker 9.4T | T2* mapping (multi-GE) |
| Zanganeh | Mammary | Mammary, Lung, Liver | Ferumoxytol | GE 7T | T2* mapping (multi-GE) |
| Neulwelt | Intracranial Tumor | Brain - Patient | Ferumoxtran | Phillips 1.5T | T1w and T2w SE |
| Dosa | Intracranial Tumor | Brain - Patient | Ferumoxytol | Siemens 3T | T1w and T2w SE |
| Alsaid | CHL-1 Melanoma | Subcutaneous | Ferumoxytol | Bruker 9.4T | T2*w GE |
| Daldrup-link | Transgenic | Mammary | Ferumoxytol | Bruker 1-2T | T2*w GE |
| Shih | Fragment Transplant | Subcutaneous | Feridex | Bruker 7T | T2*w GE (FLASH) |
The density of TAMs in metastatic breast tumor models can be quantified using 19F MRI,21 which can provide useful prognostic information and aid therapies targeted at TAMs. In this study, the authors used three breast tumor cell lines and implanted them into the 4th mammary pad of mice. Imaging was carried out with a 9.4T small animal MRI and a custom built dual channel coil capable of 1H and 19F imaging. PFC was injected into the tail vein, and then imaged using a bSSFP sequence. Signal intensity was used to quantify the amount of PFC accumulation. The authors found the three breast tumor cell lines had varying degrees of PFC (and thus TAM) accumulation, with the most aggressive cell line (4T1) having the highest TAM density. They confirmed that PFCs are labelling macrophages with immunohistochemistry, showing co-localization of PFC with F4/80, a marker of macrophages. Quantifying TAMs are important, as they can provide important prognostic and therapeutic information.
MRI imaging of TAMs using 19F has also been utilized to assess the efficacy of strategies designed to stimulate the innate immune system.22 19F MRI was used to study the effects of oncolytic virotherapy on inflammation using mouse models of carcinoma.22 Animals were treated with a Vaccina virus strain GLV-1h68 and were injected with PFC 4 or 6 days after treatment. MRI was done using a 7T Bruker system 8 days after treatment to determine tumor location and size, and PFC accumulation in tumors (using a Turbo SE sequence). The authors found significant 19F accumulation in the tumor of the virotherapy treated animals. The pattern of 19F enhancement was consistent with the histological assessment of macrophage distribution. This suggests that the degree of PFC enhancement correlated with virotherapy-induced pro-inflammatory macrophages infiltration.
19F PFC has also been used in conjunction with 1H measurement of apparent diffusion constant (ADC) to detect the therapeutic efficacy of high-intensity focused ultrasound (HIFU) induced inflammation.23 Using a subcutaneously implanted 4T1 breast carcinoma model and a 7T Bruker MRI to acquire a GE scan for 19F detection, it was shown that treatment with HIFU significantly increased 19F accumulation in the rim of the tumors compared to controls. The regions with increased 19F accumulation had significantly increased ADC and reduced tumor growth. These findings were corroborated with increased F4/80 macrophages in the HIFU-treated tumors, suggesting that 19F is able to detect the effects of HIFU-induced pro-inflammatory macrophage infiltration into the tumor.
USPIO TAM imaging has been shown to provide valuable prognostic information. The authors used a fragment transplantation of subcutaneously implanted oncogene inserted cells as their animal model. Imaging was carried out using a 7T Bruker MRI. They showed that there was significant tumor enhancement on the T2*w GE 24 hours after Feridex injection, which was not present on the baseline scan, indicating the presence of TAMs. Furthermore, there was iron enhancement (and thus TAM accumulation) in areas adjacent to the tumor. There was eventual tumor invasion into those same areas days later.24 These results are in agreement with our understanding of the role of TAMs in tumor invasion in surrounding tissue. Angiogenesis and extracellular matrix (ECM) remodeling are critical regulators of tumor growth, as without sufficient blood supply and the remodeling of surrounding ECM, it will be difficult for the tumor to invade surrounding tissue.25,26 TAMs facilitate this process by secreting cytokines that induce angiogenesis, inhibit apoptosis, and breaks down the ECM to help tumor cells invade.25,26 Therefore, by investigating the spatial location of TAMs, it may be possible to predict the areas of tumor expansion, which opens up new possibilities for treatment options.
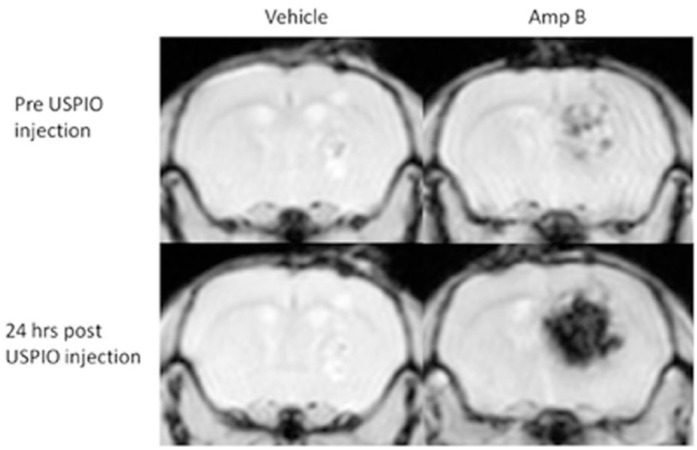
Our group has shown that innate immune stimulation in an animal model of glioblastoma can be tracked using ferumoxytol.27 The innate immune system can be stimulated with amphotericin B (Amp B) to reduce tumor growth in vivo.10 As few as 7 days of treatment with Amp B can cause an increase in tumor iron signal (measured with a 9.4T Bruker MRI and a multi-GE sequence to map T2*), indicating an increase in ferumoxytol and thus monocyte infiltration into the tumor. In contrast, there was no significant change in iron signal in the vehicle treated animals (Figure 2). The results were validated with immunohistochemistry, showing that there was a significantly higher number of macrophage/microglia in the tumor of Amp B-treated animals compared to vehicle, and that macrophage/microglia were co-localized with iron staining. Non-specific leakage of particles of the same size as ferumoxytol was ruled out by assessing leakage of fluorescein isothiocyanate (FITC)-dextran, indicating that the ferumoxytol is likely carried into the tumor via monocytes.
Figure 2.
Pre and 24 hours post ferumoxytol T2*w FLASH scans of brain tumor implanted animals treated with Amp B or vehicle. The entire dataset was published previously,27 and this animal showed the highest contrast enhancement after ferumoxytol.
Similar studies using ferumoxytol to study melanomas have shown similar results.28 GSK2849330 is a monoclonal antibody against HER3, an important target in breast tumors. Aside from inhibiting HER3 signaling, GSK2849330 is also designed to increase cell-mediated cytotoxicity by stimulating macrophages. The authors showed that GSK2849330 treatment significantly reduced the tumor size of subcutaneously implanted HER3 cells and resulted in decreased signal intensity in the tumor post ferumoxytol injection, indicating the increased presence of macrophages. These MRI data were collected using a 9.4T Bruker MRI, and ferumoxytol was imaged with a T2*w GE before and 24 hours after ferumoxytol injection. MRI findings were corroborated with immunohistochemistry, showing increased levels of F4/80 positive macrophages. In addition, the intensity of the Prussian blue staining for iron had a strong correlation with changes in signal intensity on MRI (r = -082, p < 0.01). This indicates that macrophages can be accurately labeled with ferumoxytol, and that the use of USPIOs can detect the effects of therapies aimed at reprogramming innate immunity to suppress cancer.
Interestingly, metabolism of ferumoxytol by macrophages (and not monocytes) can polarize them into an anti-tumor phenotype.29 Intravenous injection of ferumoxytol can suppress tumor growth in several mouse tumor models, and ferumoxytol allowed its own therapeutic effects to be detected by monitoring its metabolism by using a multi-GE to quantify T2* with a 7T MRI.29 These results were validated with histopathology and flow cytometry, showing that ferumoxytol treatment caused an increase in pro-inflammatory macrophage markers (CD80), and a decrease in anti-inflammatory marker (CD206) within the tumor. Ferumoxytol can preferentially recruit and polarize macrophages into an anti-tumor phenotype, cementing the notion that ferumoxytol is a promising agent in the treatment and monitoring of immune stimulating therapies in cancer.
Currently, it is difficult to use only MRI to differentiate pro-tumor macrophages from those that acquired an anti-tumor phenotype. Although there is evidence suggesting that pro-inflammatory macrophages have a higher capacity to phagocytose USPIO compared to anti-inflammatory macrophages, the difference is small and cannot be used to distinguish the two populations.30 The most common way to differentiate pro-tumor (anti-inflammatory) and anti-tumor (pro-inflammatory) macrophages are by examining different enzymes and cell surface receptors levels. Pro-inflammatory macrophages typically have high levels of inducible nitric oxide synthase (iNOS) and express cell surface receptors such as CD16/32 or CD86. On the other hand, anti-inflammatory macrophages usually have high levels of arginase and express cell surface receptors such as CD163 or CD206. The levels of enzymes and cell surface receptors can be quantified using antibody-based approaches such as immunohistochemistry or flow cytometry. Therefore, TAM tracking techniques could be combined with a technique to determine monocyte/macrophage polarization, such as flow cytometry. The MRI would be able to provide information about the relative number of cells entering the tumor, while flow cytometry would be able to provide clues about the phenotype of the TAMs.
Tracking Tumor-Associated Macrophages: Therapeutic Potential
A promising, emerging field is the use of USPIOs to label macrophages or microvesicles, guide the labeled targets to a specific location within the body using external magnetic fields, and then confirm guidance with MRI. This technique can greatly enhance the precision of cancer therapies and minimize side effects.
It has been shown that spatial guidance of macrophages can be achieved using gradient magnetic fields,31 hardware that is fundamental to all clinical and research MRIs. Macrophages were incubated with an oncolytic virus along with iron oxide nanoparticles, and then intravenously injected into animals with pulmonary metastatic tumors at a dose of 3 million cells/mice. Animals that underwent magnetic guidance had significantly more USPIO loaded macrophage accumulation in the tumor, which was observed with MRI and confirmed using immunohistochemistry. These animals showed significantly less pulmonary metastasis and smaller tumors compared to ones without magnetic guidance, indicating that magnetic guidance can greatly enhance therapeutic efficacy.
Magnetic guidance has a high potential to be combined with other methods of drug delivery. Instead of injecting ex-vivo labeled macrophages, it is possible to intravenously inject liposomes loaded with iron oxide nanoparticles and cancer therapeutics. Circulating monocytes have high capacities to phagocytosed liposomes, so phagocytosis of the iron filled liposomes by monocytes will allow them to be subjected to magnetic guidance. Magnetic guidance could also be used together with innate immune stimulating agents, since monocytes/macrophages are able to phagocytose ferumoxytol.32 Magnetic guidance could further enhance the effects of monocyte stimulating drugs in such therapies in combination with ferumoxytol, by guiding more pro-inflammatory monocytes/macrophages into the tumor. One major advantage of MRI cell guidance is that successful delivery of therapeutics can be monitored real time. As more and more cancer therapies directed at the TAMs are being developed, this can become an invaluable technique.
Tracking Tumor-Associated Macrophages: Clinical Implication
Tracking TAMs with MRI can have profound impacts on the field of cancer immunotherapy. Many of these iron oxide contrast agents have been approved for other use in patients, so translating such a tool into clinical use will be significantly easier.
Several iron oxide nanoparticles have been studied clinically as contrast agents for brain tumor imaging with the goal of labeling TAMs. Using an USPIO called Ferumoxtran-10, it was shown that there was iron-related tumor contrast after USPIO administration in patients with primary brain tumors. This was confirmed with histology after the tumor was surgically resected, showing abundant iron staining in the periphery of the tumor that appears to be located in cells with fibrillary processes, which are likely microglia or reactive astrocytes.33
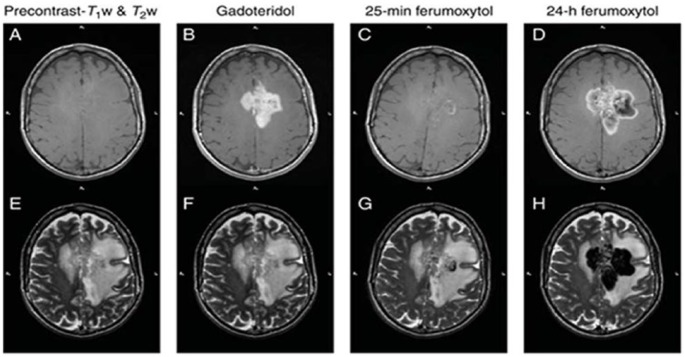
The same group later expanded on their results and showed that the enhancement is likely due to monocytes infiltrating into the tumor.34 They imaged malignant brain tumor patients with both ferumoxytol as well as Gd at ~30 minutes and 24 hours post contrast injection. They found that almost all patients had increased tumor iron signal 30 minutes after Gd. In contrast, the majority of patients did not show any changes in tumor iron signal 30 minutes after ferumoxytol (Figure 3). Only 4 out of 23 patients had an acute increase in tumor iron signal after ferumoxytol, all of which were minor. However, at the 24 hour time point, all patients showed significant signal increase consistent with ferumoxytol entry. This suggests that ferumoxytol predominantly enters the central nervous system (CNS) via phagocytic cells. Combined with time-matched blood plasma analysis of the phenotype of monocytes (ie, through flow cytometry), cell tracking with MRI could provide valuable prognostic information on effects of therapies that impact the innate immune system.
Figure 3.
Patient with glioblastoma multiforme. (A) non-enhanced and (B) Gd-enhanced T1w images. (C) Twenty-five minutes after ferumoxytol administration, the T1w image demonstrates some faint enhancement within the mass. (D) Twenty-four hours after ferumoxytol injection, mixed SI changes are seen in the approximate region where Gd enhancement is noted. (E-H) T2w images obtained before and after Gd and ferumoxytol injection.
Source: Adapted from Dosa et al34 with permission for use.
19F and iron-based cell tracking are both promising methods to eventually track TAMs in the clinical setting. 19F has been used in adenocarcinoma patients to study the effects of dendritic cell therapy.35 Ferumoxytol is an FDA-approved compound for treatment of anemia in patients with chronic kidney disease.36 While ferumoxytol is used clinically, it should be mentioned that current use in imaging are all off-label, and FDA approval for specific imaging has yet to be granted.
Conclusion
Significant advances of in vivo TAM imaging have been made in various preclinical animal models of cancer. These studies can provide information on the interaction between cancer cells and TAMs. Combined with a marker of myeloid cell polarization, imaging TAMs can provide information on the potential prognosis after innate immune altering therapy. TAM imaging with MRI has high potential to be translated into clinical use. It can be a promising imaging biomarker to detect immunotherapy treatment response in the clinical setting.
Footnotes
Funding:This work is supported by a Brain Canada Platform Grant (J.F.D.), Alberta Innovates Health Solutions—Alberta Cancer Foundation Collaborative Research and Innovative Opportunities grant (R.Y., S.S., V.W.Y., J.F.D.), Alberta Innovates Health Solutions MD-PhD Studentship (R.Y.), and Branch Out Neurological Foundations Graduate Studentship (R.Y.)
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RY and JFD developed the concept of this review paper. RY conducted the literature search, prepared the figures and wrote the first draft of the manuscript. SS, VWY and JFD provided input and edits on the structure and content of the review paper.
References
- 1. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35:S185–S198. [DOI] [PubMed] [Google Scholar]
- 2. de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. [DOI] [PubMed] [Google Scholar]
- 3. DeNardo DG, Barreto JB, Andreu P, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poon CC, Sarkar S, Yong VW, Kelly JJ. Glioblastoma-associated microglia and macrophages: targets for therapies to improve prognosis. Brain. 2017;140:1548–1560. [DOI] [PubMed] [Google Scholar]
- 5. Dannenmann SR, Thielicke J, Stockli M, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Petrillo M, Zannoni GF, Martinelli E, et al. Polarisation of tumor-associated macrophages toward M2 phenotype correlates with poor response to chemoradiation and reduced survival in patients with locally advanced cervical cancer. PLoS ONE. 2015;10:e0136654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manthey CL, Johnson DL, Illig CR, et al. JNJ-28312141, a novel orally active colony-stimulating factor-1 receptor/FMS-related receptor tyrosine kinase-3 receptor tyrosine kinase inhibitor with potential utility in solid tumors, bone metastases, and acute myeloid leukemia. Mol Cancer Ther. 2009;8:3151–3161. [DOI] [PubMed] [Google Scholar]
- 9. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarkar S, Doring A, Zemp FJ, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2013;17:46–55. [DOI] [PubMed] [Google Scholar]
- 11. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Melancon MP, Lu W, Huang Q, et al. Targeted imaging of tumor-associated M2 macrophages using a macromolecular contrast agent PG-Gd-NIR813. Biomaterials. 2010;31:6567–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dekaban GA, Hamilton AM, Fink CA, et al. Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:469–483. [DOI] [PubMed] [Google Scholar]
- 14. Daldrup-Link HE, Golovko D, Ruffell B, et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17:5695–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makela AV, Gaudet JM, Foster PJ. Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Sci Rep. 2017;7:42109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khurana A, Chapelin F, Xu H, et al. Visualization of macrophage recruitment in head and neck carcinoma model using fluorine-19 magnetic resonance imaging. Magn Reson Med. 2018;79:1972–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated 64Cu liposomes. Biomaterials. 2012;33:7785–7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perez-Medina C, Tang J, Abdel-Atti D, et al. PET imaging of tumor-associated macrophages with 89Zr-labeled high-density lipoprotein nanoparticles. J Nucl Med. 2015;56:1272–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egeblad M, Ewald AJ, Askautrud HA, et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–167; discussion 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szulczewski JM, Inman DR, Entenberg D, et al. In vivo visualization of stromal macrophages via label-free FLIM-based metabolite imaging. Sci Rep. 2016;6:25086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Makela AV, Foster PJ. Imaging macrophage distribution and density in mammary tumors and lung metastases using fluorine-19 MRI cell tracking [published online ahead of print 12 January 2018]. Magn Reson Med. doi: 10.1002/mrm.27081. [DOI] [PubMed] [Google Scholar]
- 22. Weibel S, Basse-Luesebrink TC, Hess M, et al. Imaging of intratumoral inflammation during oncolytic virotherapy of tumors by 19F-magnetic resonance imaging (MRI). PLoS ONE. 2013;8:e56317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shin SH, Park SH, Kim SW, Kim M, Kim D. Fluorine MR imaging monitoring of tumor inflammation after high-intensity focused ultrasound ablation. Radiology. 2017:171603. [DOI] [PubMed] [Google Scholar]
- 24. Shih YY, Hsu YH, Duong TQ, Lin SS, Chow KP, Chang C. Longitudinal study of tumor-associated macrophages during tumor expansion using MRI. NMR Biomed. 2012;24:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chong YK, Sandanaraj E, Koh LW, et al. ST3GAL1-associated transcriptomic program in glioblastoma tumor growth, invasion, and prognosis. J Natl Cancer Inst. 2015;108:djv326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. [DOI] [PubMed] [Google Scholar]
- 27. Yang R, Sarkar S, Korchinski DJ, Wu Y, Yong VW, Dunn JF. MRI monitoring of monocytes to detect immune stimulating treatment response in brain tumor. Neuro Oncol. 2017;19:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alsaid H, Skedzielewski T, Rambo MV, et al. Non invasive imaging assessment of the biodistribution of GSK2849330, an ADCC and CDC optimized anti HER3 mAb, and its role in tumor macrophage recruitment in human tumor-bearing mice. PLoS ONE. 2017;12:e0176075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zanganeh S, Hutter G, Spitler R, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Settles M, Etzrodt M, Kosanke K, et al. Different capacity of monocyte subsets to phagocytose iron-oxide nanoparticles. PLoS ONE. 2011;6:e25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Muthana M, Kennerley AJ, Hughes R, et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat Commun. 2015;6:8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Metz S, Bonaterra G, Rudelius M, Settles M, Rummeny EJ, Daldrup-Link HE. Capacity of human monocytes to phagocytose approved iron oxide MR contrast agents in vitro. Eur Radiol. 2004;14:1851–1858. [DOI] [PubMed] [Google Scholar]
- 33. Neuwelt EA, Varallyay P, Bago AG, Muldoon LL, Nesbit G, Nixon R. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumours. Neuropathol Appl Neurobiol. 2004;30:456–471. [DOI] [PubMed] [Google Scholar]
- 34. Dosa E, Guillaume DJ, Haluska M, et al. Magnetic resonance imaging of intracranial tumors: intra-patient comparison of gadoteridol and ferumoxytol. Neuro Oncol. 2010;13:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72:1696–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Strauss WE, Dahl NV, Li Z, Lau G, Allen LF. Ferumoxytol versus iron sucrose treatment: a post-hoc analysis of randomized controlled trials in patients with varying renal function and iron deficiency anemia. BMC Hematol. 2016;16:20. [DOI] [PMC free article] [PubMed] [Google Scholar]