Short abstract
Background
The natural history of multiple sclerosis (MS) typically presents with the clinically isolated syndrome (CIS), an episode of neurological symptoms caused by central nervous system inflammation or demyelination that does not fulfil the diagnostic criteria for MS.
Objective
As preclinical studies have suggested that exposure to ultraviolet radiation (UVR) could regulate the development of MS, the Phototherapy for CIS (PhoCIS trial) was established to examine the effects of narrowband UVB phototherapy on patients with CIS, and their conversion to MS.
Methods
Of the 20 participants, half received 24 sessions of narrowband UVB exposure over eight weeks; participants in both arms were followed for 12 months. All participants were supplemented to 25-hydroxyvitamin D3 levels of >80 nmol/l.
Results
By 12 months, 100% of those in the no phototherapy arm and 70% in the phototherapy arm had converted to MS, although this difference was not statistically significant.
Conclusion
This study provides a basis for further studies to determine if there are any benefits of the therapeutic effects of narrowband UVB radiation on MS progression.
Keywords: Narrowband UVB phototherapy, clinically isolated syndrome, UV-induced immunosuppression, trial, vitamin D supplementation
Introduction
Increased multiple sclerosis (MS) onset and relapses during periods of low ultraviolet radiation (UVR) exposure suggest that environmental UVR contributes to MS pathogenesis in predisposed individuals. Cutaneous UVR exposure causes systemic immune suppression in humans1 but is also a major source of vitamin D. Reduced vitamin D levels may increase MS risk.2,3 However, several studies3–6 suggest that immunoregulation by UVR is by both vitamin D-dependent and vitamin D-independent pathways. Vitamin D and sun exposure are additive, independent risk factors for MS development.7,8 A protective association of sun exposure on magnetic resonance imaging (MRI) measures of neurodegeneration in MS, independently of 25-hydroxyvitamin D3 (25(OH)D) levels, has also been reported.9 In the trial reported here, the modulatory effect of narrowband ultraviolet B (UVB) irradiation (311–312 nm) on individuals at high risk of developing MS was investigated. Thus, the trial participants were given a treatment that would stimulate vitamin D production, as well as all the other molecules induced in UV-irradiated skin that may have immunomodulatory effects.5 Importantly, there was a control group who did not receive narrowband UVB phototherapy, and all participants in both the control and intervention arms were supplemented to more than sufficiency with vitamin D.5
The clinically isolated syndrome (CIS) is the first clinical presentation of a disease that shows characteristics of inflammatory demyelination consistent with MS, but has yet to fulfil the criteria of dissemination in time and space. The time immediately following CIS may be an opportunity for intervention to curtail the risk of MS onset. T-cell activation is a fundamental feature of MS development.10 As induced T regulatory (Treg) cell and reduced memory T-cell responses have been implicated in UVR-induced immunomodulation, the plausibility for positive outcomes in this trial was supported.5,11
For this trial of narrowband UVB phototherapy for CIS patients (PhoCIS), the primary outcome was MRI changes or new symptoms to satisfy the 2010 McDonald criteria for diagnosis of MS.12 Additional outcomes were levels of fatigue and well-being. It was hypothesised that narrowband UVB phototherapy would reduce, or delay, the conversion of high-risk CIS participants to MS.
Materials and methods
Trial design
The PhoCIS trial was a 12-month, non-blinded randomised trial of the effects of narrowband UVB therapy on radiological markers of disease in individuals recruited within 120 days of their first demyelinating event (diagnosis of CIS). The study was performed in Perth, Western Australia (32 degrees South); participants were enrolled from November 2014 and the trial ceased in March 2017 due to low recruitment. The trial design has been previously published.5 The study was approved by the Bellberry Human Research Ethics Committee (2014-02-083) and endorsed by the Human Research Ethics Office of the University of Western Australia (RA/4/1/6796). The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN 12614000185662).
Participants
To be eligible for the PhoCIS trial, potential participants were aged 18–65; were within 120 days of a well-defined, uni- or multi-focal first demyelinating event; had an MRI brain scan that was supportive of demyelinating disease with high risk of conversion to MS (Paty A or Paty B criteria, i.e. the presence of at least four T2 lesions greater than 3 mm, or at least three such T2 lesions, one of which was periventricular); had an Expanded Disability Status Scale score <6.5 and could give written informed consent. Participants needed to be able to stand in the phototherapy cubicle for up to five minutes at a time. Exclusion criteria included autoimmune disease or illness that might be exacerbated by phototherapy; a history of skin cancers; previous use of UVB phototherapy, photochemotherapy or sunbeds; pregnancy; very fair skin that burns with minimal sun exposure; on disease-modifying treatment (DMT) or having contraindications to MRI scanning or intravenous gadolinium administration. Participants who had a short three- to five-day course of intravenous corticosteroids for their recent symptoms were required to have a one-month wash-out period before recruitment. It was not possible to blind the groups receiving phototherapy or not; however, the investigators of MRI outcomes were blind to the treatment status. The randomisation list was made by SG using a random number generator in Excel; the recruiting neurologist was not involved. The seven participants who did not have serum 25(OH)D3 levels >80 nmol/l at enrolment (two and five in the phototherapy and control groups, respectively) were prescribed oral vitamin D (up to 5000 IU/day) to reach this level within two months.
Narrowband UVB phototherapy intervention
Participants randomised to the phototherapy group received phototherapy three times per week for eight weeks to their full body (face and genitals covered) under the care of the study dermatologist (JMC). For one participant, 24 exposures were delivered over 10 weeks. A Wayne Electronics, Series M.S.1 phototherapy cabinet (Pompton Plains, NJ, USA) with output between 311 and 312 nm of 0.6 mW/cm2 was used. Phototherapy was delivered according to the Dundee protocol, based on assessment of patient skin type. The starting dose was 20 mJ/cm2 (approximately 0.7 of a minimum erythemal dose for skin type II). Doses increased by 40% increments of their initial dose, which reduced to 20% increments after six exposures, and in the absence of skin erythema increased incrementally until the final visit. Skin assessment was conducted both before and after phototherapy by the study dermatologist (JMC). Narrowband UVB phototherapy is a routine therapy for patients with psoriasis.13
MRI scans
MRI scans and medical review were performed after 3, 6 and 12 months. Images were acquired with a 3 Tesla magnet. Three-dimensional fluid-attenuated inversion recovery (1 mm), T1-, T2-weighted and axial T1-weighted post-gadolinium-diethylenetriaminepentaacetic acid administration sequences were acquired.
Assessment of human leucocyte antigen (HLA) genes, anti-Epstein–Barr virus (EBV) antibodies and 25(OH)D3
Blood was obtained at enrolment and after 1, 2, 3, 6 and 12 months. HLA genes were measured by standard procedures at PathWest Laboratories (Perth, Australia) on buffy coat cells from ethylenediaminetetraacetic acid tubes. The concentration of anti-EBV antibodies (immunoglobulin (Ig)M and IgG to viral capsid antigen (VCA), IgG to EBV nuclear antigen-1 (EBNA-1)) in serum obtained at enrolment was measured by standardised assays at the PathWest Laboratories. Serum 25(OH)D3 levels were measured on all samples by liquid chromatography mass spectrometry/mass spectrometry as previously reported.14
Measurement of past environmental UVR exposure and during first 60 days of trial
Silicon rubber imprints of the skin at the back of the hand were taken to measure past environmental UVR exposure.15 Microtopography is one of the most common and reliable methods for examining cumulative sun exposure; casts were prepared according to standard procedures using kits from Affinis (C6601, Coltène, OH, USA) and graded from 1 to 6 by the Beagley-Gibson system.15 Day-to-day environmental UVR exposure for all participants during the first 60 days of the study was measured using a wrist dosimeter (Scienterra Limited, Otago, New Zealand) that was set to capture UVR exposure between 7 a.m. and 5 p.m. The dosimeter was removed during phototherapy sessions. The average daily exposure (uW/cm2) was calculated by dividing the total exposure by the number of days the device was worn.
Assessment of fatigue and well-being
At recruitment and after 6 and 12 months, the participants completed a validated questionnaire assessing fatigue severity16 and the health-related quality of life Medical Outcomes Study 36-item Short Form (SF-36).17,18 The SF-36 is a relatively brief and simple questionnaire containing 36 items covering eight health concepts chosen for reliability, validity and frequency of measurement in health surveys.
Sample size and statistical analyses
The PhoCIS trial aimed to recruit 30 participants with CIS to receive narrowband UVB phototherapy and 30 to not receive phototherapy (controls). Assuming that new MRI lesions can be expected in 90% of individuals with high-risk CIS over 18–24 months,19 this would allow detection of a large effect size of 30%.
The biological and clinical characteristics at enrolment are presented, and questionnaire scores for the participants in the phototherapy and control groups were compared at each follow-up using Fisher’s exact test for categorical outcomes, and a Mann–Whitney U test for continuous outcomes. The primary outcome, proportion of patients satisfying the MS criteria at each of 3, 6 and 12 months was compared between groups using Fisher’s exact test and logistic regression adjusting for 25(OH)D levels. Secondary analysis involved comparison of time from diagnostic MRI (when CIS was confirmed) to post-enrolment assessment at 3, 6 and 12 months between groups using a log-rank (Mantel–Cox) test and SPSS Statistics, v25. Using Stata 15 for analysis, a linear-mixed model was used to examine the relationship between phototherapy and serum 25(OH)D3 at different time points, adjusting for the baseline values and correlation of outcomes within individuals and season (winter, spring, summer, autumn).
Results
Participants
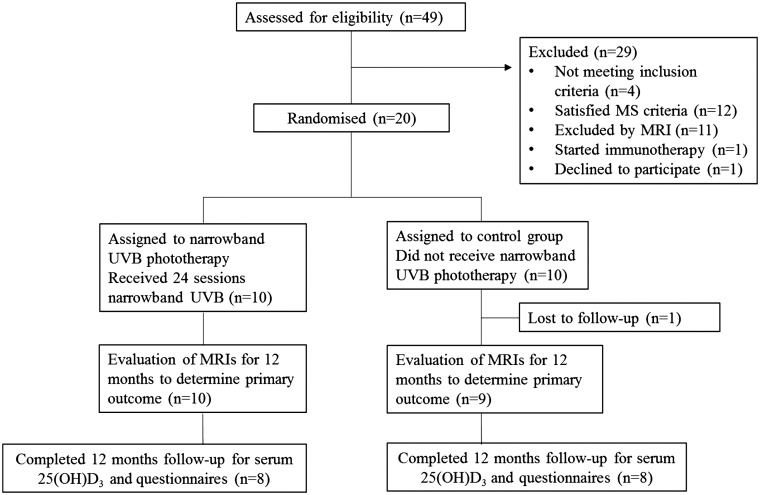
Of the 49 individuals assessed for eligibility by the study neurologist (AGK), 20 satisfied the inclusion criteria, agreed to participate and were randomly assigned to receive, or not receive, phototherapy (Figure 1). All had Fitzpatrick skin types II and III. For the primary outcome of conversion to MS11 after 3, 6 or 12 months, results were recorded for 19 participants. One participant assigned to the control arm was lost to follow-up one month after enrolment. Three participants did not participate in the trial schedule for obtaining serum and questionnaire data for the full 12 months (due to anxiety or relocation) although their MRI schedules were maintained and the primary outcome determined.
Figure 1.
Flow diagram of involvement of participants in the PhoCIS trial.
CIS: clinically isolated syndrome; MRI: magnetic resonance imaging; MS: multiple sclerosis; PhoCIS: trial of narrowband UVB phototherapy for CIS patients; UVB: ultraviolet B.
The participants appeared similar on their biological characteristics and MS risk factors (Table 1(a)) except there appeared to be more males in the phototherapy group. Clinical symptoms and MRI outcomes on enrolment were also similar for the two groups (Tables 1(b) (c)), and gadolinium-enhancing lesions in the cerebrum or spinal cord were not observed in any of the participants. There appeared no differences between the groups in environmental UVR exposure during the first 60 days of their participation (data not shown).
Table 1.
Biological (a) and clinical (b) characteristics of the participants at enrolment. (c) MRI of the participants at enrolment. Values given are either median (minimum to maximum values) or n (%).
| Phototherapy (n = 10) | Controls (n = 10) | |
|---|---|---|
|
(a)
| ||
| Age | 40.0 (27.3 to 48.5) | 34.8 (23.4 to 54.3) |
| Female sex | 4 (40%) | 8 (80%) |
| CIS duration (days since diagnostic MRI) | 34.0 (26 to 119) | 37.5 (–1 to 91) |
| EDSS | 1.75 (0 to 3) | 1.0 (0 to 2) |
| BMI | 25.8 (22.3 to 31.2) n = 7 |
27.4 (23.1 to 37.8) n = 6 |
| Smoking status | ||
| Never | 7 (70%) | 8 (80%) |
| Past | 2 (20%) | 1 (10%) |
| Current | 1 (10%) | 1 (10%) |
| HLA status: | ||
| HLA-DRB1*15:01 | 4 (40%) | 6 (60%) |
| HLA-DRB1*03:01 | 2 (20%) | 2 (20%) |
| HLA-DRB1*08:01 | 2 (20%) | 0 (0%) |
| Season at enrolment: | ||
| Summer | 1 (10%) | 3 (30%) |
| Autumn | 5 (50%) | 2 (20%) |
| Winter | 3 (30%) | 4 (40%) |
| Spring | 1 (10%) | 1 (10%) |
| Serum 25(OH)D3 level (nmol/l) | 92.5 (43.7 to 135.6) | 75.8 (57.6 to 133.6) |
| Steroid treatment for CIS before enrolment: | ||
| Yes | 2 (20%) | 4 (40%) |
| No | 8 (80%) | 6 (60%) |
| EBV serology: | ||
| EBNA IgG | 588 (3a to 600a) | 526 (3a to 600a) |
| VCA IgG | 289 (73.8 to 750b) | 310 (178 to 750b) |
| VCA IgM | 10c (10 to 18.4) | 10.25 (10c to 25.4) |
| Skin cast measuring actinic damage | 2 (1 to 3) | 2 (1 to 5) |
| Fatigue Severity Score at enrolment (Scale 1–7) | 3.67 (2.11 to 6.00), n = 8 with data available |
3.23 (1.78 to 5.44), n = 8 with data available |
| SF-36v2 mental composite score at enrolment | 49.9 (36.5 to 60.0), n = 8 | 55.6 (31.3 to 58.6), n = 9 |
| SF-36v2 physical composite score at enrolment | 57.3 (46.9 to 62.6), n = 8 | 56.6 (49.9 to 61.1), n = 9 |
| (b) Clinical symptoms | ||
| Optic neuritis | 6 | 7 |
| Hemispheric | 0 | 2 |
| Transverse myelitis | 3 | 1 |
| Brainstem (transient diplopia) | 1 | 0 |
| (c) MRI of participants at enrolment | ||
| Number of T2 lesionsCerebral MRI | ||
| 4–9 | 2 | 2 |
| >9 | 8 | 8 |
| Spinal cord MRI | Phototherapy (n = 7)d | Controls (n = 8)d |
| ≥1 | 5 | 5 |
aDetection limits of assay 3 − 600.
bDetection limits of assay 10 − 750.
cLower limit of assay 10.
25(OH)D3: 25-hydroxyvitamin D3; BMI: body mass index; CIS: clinically isolated syndrome; EBNA: Epstein–Barr virus nuclear antigen-1; EBV: Epstein–Barr virus; EDSS: Expanded Disability Status Scale; HLA: human leucocyte antigen; Ig: immunoglobulin; MRI: magnetic resonance imaging; MS: multiple sclerosis; SF-36v2: Medical Outcomes Study Short Form 36, version 2; UVB: ultraviolet B; VCA: viral capsid antigen.
dSpinal cord magnetic resonance imaging (MRI) not performed in three Phototherapy patients and two Controls.
Primary outcome of the effect of narrowband UVB phototherapy
All participants in the control group met the criteria for a diagnosis of MS by 12 months compared to seven (70%) of those who received phototherapy. The three who did not progress began phototherapy in winter (n = 2) and spring (n = 1) and one had steroid treatment before enrolment. Table 2 shows the number and percentage of participants with progression to MS at each time by intervention group. There was no statistically significant difference in time from day of diagnostic MRI to MS conversion (measured at 3, 6 or 12 months), p = 0.32 for log-rank test (Figure 2).
Table 2.
Primary outcome. The number and percentage of participants with progression to multiple sclerosis (MS) at 3, 6 and 12 months.
| Time of satisfyingMS criteria | Phototherapy | Controls | Fisher’sexact test (two-sided)p value |
|---|---|---|---|
| Three months | 5/10 (50%) | 5/9 (56%) | 1.00 |
| Six months | 5/10 (50%) | 7/9 (78%) | 0.35 |
| Twelve months | 7/10 (70%) | 9/9 (100%) | 0.21 |
Figure 2.
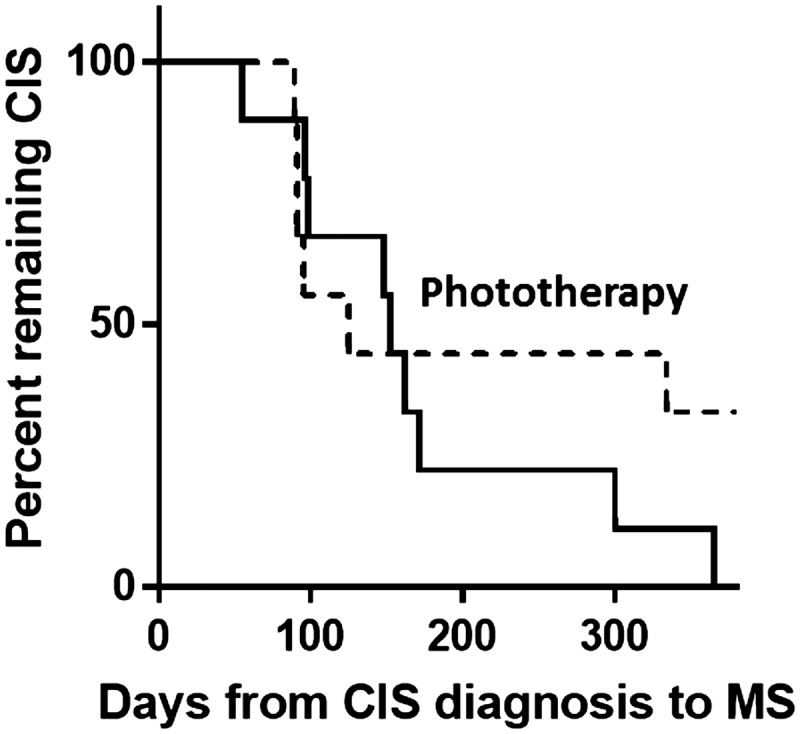
Days from date of diagnostic MRI to date of CIS to MS conversion. The result for the 10 participants receiving narrowband UVB phototherapy is shown as a broken line, and the nine controls (no phototherapy) by the solid line; p = 0.32 by log-rank test. CIS: clinically isolated syndrome; MRI: magnetic resonance imaging; MS: multiple sclerosis; UVB: ultraviolet B.
Changes in 25(OH)D3 in sera of trial participants
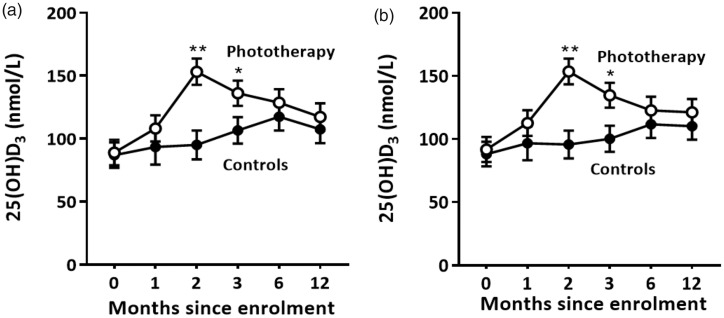
As phototherapy-induced 25(OH)D3 may have influenced the primary outcome of the trial, serum 25(OH)D3 levels were investigated. Using a linear-regression model adjusting for the time since enrolment, 25(OH)D3 levels were higher for the phototherapy group relative to the control group after two and three months (Table 3(a), Figure 3(a)). Levels of 25(OH)D3 were lower in winter (Table 3(b)). When levels of serum 25(OH)D3 were deseasonalised by adjusting for season of blood draw, 25(OH)D3 levels were again higher after two and three months for the phototherapy group relative to the control group data and confirmed that phototherapy increased 25(OH)D3 levels independently of season (Table 3(b), Figure 3(b)). There were no significant differences in 25(OH)D3 at 12 months. Adjustment for baseline 25(OH)D3 level had minimal impact on the primary outcome (conversion to MS). The hazard ratio for the phototherapy group, relative to the control group, saw little attenuation (0.56 to 0.58) following adjustment for 25(OH)D3 level, with the adjusted effect not statistically significant (p = 0.29) irrespective of the time scale used.
Table 3.
Differences in 25-hydroxyvitamin D3 (25(OH)D) levels for participants in the Phototherapy and Control groups at each time, adjusted for baseline values. (a) Results of the regression model. (b) Results of the regression model adjusted for season.
| Estimate | Standard error | 95% confidence interval | p value | ||
|---|---|---|---|---|---|
| (a) Not adjusted for season | |||||
| Phototherapy vs Control | |||||
| Baseline | 1.98 | 14.18 | –25.81 | 29.78 | |
| One month | 14.76 | 17.42 | –19.39 | 48.91 | 0.40 |
| Two months | 58.11 | 15.41 | 27.90 | 88.32 | <0.001 |
| Three months | 29.53 | 14.48 | 1.15 | 57.92 | 0.04 |
| Six months | 11.12 | 15.34 | –18.94 | 41.19 | 0.47 |
| Twelve months | 9.90 | 15.34 | –20.16 | 39.97 | 0.52 |
| (b) Adjusted for season | |||||
| Phototherapy vs Control | |||||
| Baseline | 3.66 | 13.92 | –23.63 | 30.95 | |
| One month | 16.00 | 16.89 | –17.10 | 49.09 | 0.34 |
| Two months | 57.82 | 15.00 | 28.42 | 87.22 | <0.001 |
| Three months | 34.40 | 14.12 | 6.73 | 62.07 | 0.02 |
| Six months | 10.91 | 14.81 | –18.12 | 39.94 | 0.46 |
| Twelve months | 11.10 | 14.82 | –17.95 | 40.15 | 0.45 |
| Effect of season on all 25(OH)D3 measures (reference = summer) | |||||
| Autumn | –11.30 | 8.79 | –28.52 | 5.91 | 0.20 |
| Winter | –17.14 | 7.72 | –32.27 | –2.00 | 0.03 |
| Spring | 4.79 | 8.03 | –10.95 | 20.53 | 0.55 |
Mixed model used to adjust for correlation within individuals over time, p value from Z test.
Figure 3.
The effect of narrowband UVB phototherapy on serum 25(OH)D3 levels. Levels of 25(OH)D3 were measured at enrolment (10, 10), one month (4, 9), two months (7, 9), three months (9, 10), six months (8, 8) and 12 months (8, 8). The numbers in brackets indicate the number of participants whose sera were analysed in the Control and Phototherapy groups, respectively. (a) Levels of 25(OH)D3. (b) Deseasonalised levels of 25(OH)D3. Mean ± SEM are shown; *p < 0.05; **p < 0.001. 25(OH)D3: 25-hydroxyvitamin D3; UVB: ultraviolet B.
Participant assessment of their well-being and disease characteristics
When the participants were asked to rate their fatigue at enrolment and after 6 and 12 months, there were no significant differences between groups at each time point (Table 4). When expressed as a mean percentage change of their fatigue score at enrolment in those participants with paired values available (n = 7 in both groups), there was a 15% decrease at six months for those who received phototherapy and a 18% increase for the controls (p = 0.10).
Table 4.
Participant assessment of their fatigue (Fatigue Severity Score). Scores are given using a scale range of 1 to 7, with lower scores indicating less fatigue. Median (minimum to maximum values) is shown.
| FatigueSeverity Score | Phototherapy | Controls | p |
|---|---|---|---|
| Enrolment(n = 8, 8) | 3.7 (2.1–6.0) | 3.2 (1.8–5.4) | |
| Six months(n = 8, 7) | 2.8 (1.6–5.3) | 3.3 (2.8–4.3) | 0.29 |
| Twelve months(n = 8, 8) | 3.4 (1.4–5.0) | 3.4 (2.1–5.0) | 0.86 |
Results from the SF-36 wellness questionnaire showed that the phototherapy and control groups were similar on most scales at most time points. However, on the Social Functioning scale, at six months, participants who had received phototherapy had significantly better scores than those in the control group (p = 0.03; Table 5). It is possible that this result was confounded by non-blinding of the participants to their group assignment.
Table 5.
Participant assessment of their wellness using the Medical Outcomes Study Short Form-36, version 2 (SF-36v2) questionnaire. Scores are given using a scale range of 0 to 100. Median (maximum to minimum values) is shown.
| SF-36v2 | Phototherapy | Controls | p |
|---|---|---|---|
| Physical Functioning | |||
| Enrolment (n = 8, 9) | 97.5 (70–100) | 100 (80–100) | |
| Six months (n = 9, 7) | 100 (80–100) | 100 (85–100) | 1.00 |
| Twelve months (n = 8, 8) | 97.5 (75–100) | 97.5 (75–100) | 0.93 |
| Role Physical | |||
| Enrolment (n = 9, 8) | 100 (75–100) | 93.75 (37.5–100) | |
| Six months (n = 9, 7) | 100 (81.25–100) | 93.75 (56.25–100) | 0.59 |
| Twelve months (n = 8, 8) | 100 (56.25–100) | 90.63 (68.75–100) | 0.50 |
| Bodily Pain | |||
| Enrolment (n = 8, 9) | 100 (74–100) | 100 (62–100) | |
| Six months (n = 9, 7) | 84 (51–100) | 84 (62–100) | 0.64 |
| Twelve months (n = 8, 8) | 84 (62–100) | 84 (62–100) | 0.92 |
| General Health | |||
| Enrolment (n = 8, 9) | 74.5 (25–92) | 77 (57–97) | |
| Six months (n = 9, 7) | 77 (37–100) | 72 (67–97) | 0.81 |
| Twelve months (n = 8, 8) | 77 (35–100) | 74.5 (52–100) | 1.00 |
| Vitality | |||
| Enrolment (n = 8, 9) | 62.5 (12.5–87.5) | 75 (18.75–87) | |
| Six months (n = 9, 7) | 75 (37.5–93.75) | 68.75 (31.25–75) | 0.42 |
| Twelve months (n = 8, 8) | 71.88 (37.5–87.5) | 56.13 (43.75–75) | 0.26 |
| Social Functioning | |||
| Enrolment (n = 8, 9) | 93.75 (75–100) | 100 (50–100) | |
| Six months (n = 9, 7) | 100 (75–100) | 87.5 (50–100) | 0.03 |
| Twelve months (n = 8, 8) | 93.75 (75–100) | 87.5 (75–100) | 1.00 |
| Role Emotional | |||
| Enrolment (n = 8, 9) | 100 (75–100) | 100 (33.3–100) | |
| Six months (n = 9, 7) | 91.67 (66.67–100) | 100 (33.3–100) | 0.65 |
| Twelve months (n = 8, 8) | 100 (50–100) | 100 (75–100) | 0.87 |
| Mental Health | |||
| Enrolment (n = 8, 9) | 75 (45–100) | 85 (55–90) | |
| Six months (n = 9, 7) | 80 (70–90) | 85 (60–95) | 0.99 |
| Twelve months (n = 8, 8) | 85 (65–100) | 82.5 (60–95) | 0.52 |
Discussion
In this world-first trial of narrowband UVB phototherapy in CIS, there was no statistically significant difference in conversion to a diagnosis of MS after 12 months. The trial did not reach its recruitment target; however, three of the 10 participants who received phototherapy had not converted to MS after 12 months, compared with 100% conversion in the control group. Of note, the 30% of CIS patients who had not converted to MS after 12 months was quantitatively similar to the 39% of psoriasis patients who remained disease free at 12 months after receiving a similar schedule of narrowband UVB phototherapy.13 A decrease of 30% in the conversion from CIS to MS is also similar to the conversion rate over 12 months in the Controlled High-Risk Avonex Multiple Sclerosis Study (CHAMPS) in which intramuscular interferon beta-1a was given every week for 12 months.19
The study had limitations, principally the small sample size. Participation required MRI to satisfy Paty A and Paty B criteria, which would predict a high risk of conversion to MS over 12 months.19–21 These criteria made recruitment difficult, and inclusion of only high-risk CIS patients explains the conversion to MS by approximately 50% of trial participants within three months. The design of the trial was to capture individuals early in their disease course. The data suggest that phototherapy may have helped a subgroup of CIS patients, particularly those who had not converted to MS within the first three months. If the study had more power with a larger sample size, particularly for those with CIS that are stable for three months after initial diagnosis, it is tempting to speculate that a significant effect of phototherapy may have been observed. Ongoing analyses of changes in the circulating cells of the participants may help validate hypotheses that the phototherapy has a modifying effect on disease course.
The participant characteristics were similar across the two groups at baseline although there were possibly more males in the phototherapy group. In a meta-analysis of 33 studies, there was a non-significant trend towards an increased risk of progression from CIS to MS in females (overall relative risk of females 1.2).22 Of the three CIS patients who did not convert in 12 months, one was male. The MRI characteristics at enrolment and past sun exposure levels for the phototherapy and control groups were similar. In addition, there were no remarkable differences in known risk factors for MS development (smoking, HLA genes).23 At enrolment, all participants in PhoCIS were free from the complex effects of DMTs that can regulate immune cell activity. In a previous study of patients with relapsing–remitting MS of median duration of 13 years, nine patients on multiple DMTs were given narrowband UVB phototherapy for six weeks.24 No neurological improvements were reported over the six-month trial; however, the DMTs may have swamped any effects of the phototherapy.24
Phototherapy-induced vitamin D may have contributed to the observed outcomes. Serum 25(OH)D3 levels were higher two and three months after beginning phototherapy. The median levels at baseline were above the vitamin D-insufficiency level of 75 nmol/l. The outcomes of trials of vitamin D supplementation for a multitude of non-skeletal disorders have generally returned disappointing results;25 it is possible that significant outcomes of supplementation may be more likely obtained in trials of participants very deficient in vitamin D (25(OH)D3 <50 nmol/l) at recruitment.25 We argue that 25(OH)D3 levels in the PhoCIS participants were above a threshold and they were not susceptible to biochemical effects of phototherapy-induced vitamin D3.
The mechanisms by which narrowband UVB phototherapy may have regulated MS development are currently unknown. When blood cells were isolated from vitamin D-deficient individuals (<50 nmol/l) given narrowband UVB phototherapy (two treatments/week for eight weeks) and their molecular signatures examined, there was a significant decrease in gene expression associated with interferon (IFN)alpha and IFNgamma responses.26 In response to narrowband UVB, increases in blood concentrations of Treg cells have also been reported.11,27 UVR-induced nitric oxide has also been implicated in increased Treg induction in vivo.28 Further studies will be required to determine if the circulating cells characterised in the blood of patients with MS29 were altered by the phototherapy intervention.
In this non-blinded trial, the participants who received phototherapy self-assessed that they felt better. After six months, there was a significant improvement in Social Functioning and although no significant effect was apparent for fatigue, a clinically relevant reduction was suggested. Although there may be many contributing factors, any change in fatigue may reflect increased production of anti-inflammatory cytokines after UVR exposure.30 UVR exposure may otherwise have reversed a deficiency of monoamines, particularly serotonin and noradrenalin, and normalised the hypothalamic-pituitary-adrenal-axis.31
To conclude, although this study was unable to confirm in humans the reported suppression by narrowband UVR of a mouse model of MS,32 there have been some tantalising observations. A delay, or even a halt, in progression to MS was observed in 30% of the participants that received phototherapy. The well-being of the participants may have improved although this result could be confounded by a lack of sham phototherapy for participants in the control group. Any possible benefit of phototherapy would be important for patients in Australia for whom no medication is approved for reimbursement until they fulfil diagnostic criteria for MS. In psoriasis, narrowband UVB phototherapy is safe for fortnightly maintenance therapy after clearance of disease, and for repeat treatment after a relapse. It is possible that additional UVB exposures in CIS patients who have not converted might have further benefits. The limitation of the study outcomes reflects our inability to recruit sufficient participants to the study within the first 120 days of their diagnosis. This study provides encouragement to other investigators to establish similar studies that investigate the potential effects of narrowband UVB phototherapy to modulate the development of MS.
Supplemental Material
Supplemental material for A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study by Prue H Hart, Anderson P Jones, Stephanie Trend, Lilian Cha, Marzena J Fabis-Pedrini, Matthew N Cooper, Catherine d’Este, Sian Geldenhuys, William M Carroll, Scott N Byrne, David R Booth, Judith M Cole, Robyn M Lucas and Allan G Kermode in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgement
We thank the patients who participated in our study.
Conflicts of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Prof AG Kermode has received speaker honoraria and scientific advisory board fees from Bayer, BioCSL, Biogen-Idec, Lgpharma, Merck, Novartis, Roche, Sanofi-Aventis, Sanofi-Genzyme, Teva, and NeuroScientific Biopharmaceuticals. The other authors have nothing to declare.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Health and Medical Research Council of Australia (ID 1067209). Drs AP Jones, S Trend and MJ Fabis-Pedrini were supported by MS Western Australia, and Prof RM Lucas by a National Health and Medical Research Council of Australia Senior Research Fellowship.
Supplementary material
Supplementary material is available for this article online
References
- 1.Kelly DA, Young AR, McGregor JM, et al. Sensitivity to sunburn is associated with susceptibility to ultraviolet radiation-induced suppression of cutaneous cell-mediated immunity. J Exp Med 2000; 191: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascherio A andMunger KL.. Epidemiology of multiple sclerosis: From risk factors to prevention – An update. Semin Neurol 2016; 36: 103–114. [DOI] [PubMed] [Google Scholar]
- 3.Hart PH Gorman S andFinlay-Jones JJ.. Modulation of the immune system by UV radiation: More than just the effects of vitamin D? Nat Rev Immunol 2011; 11: 584–596. [DOI] [PubMed] [Google Scholar]
- 4.Hart PH andGorman S.. Exposure to UV wavelengths in sunlight suppresses immunity. To what extent is UV-induced vitamin D3 the mediator responsible? Clin Biochem Rev 2013; 34: 3–13. [PMC free article] [PubMed] [Google Scholar]
- 5.Hart PH, Lucas RM, Booth DR, et al. Narrowband UVB phototherapy for clinically isolated syndrome: A trial to deliver the benefits of vitamin D and other UVB-induced molecules. Front Immunol 2017; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorman S andHart PH.. The current state of play of rodent models to study the role of vitamin D in UV-induced immunomodulation. Photochem Photobiol Sci 2012; 11: 1788–1796. [DOI] [PubMed] [Google Scholar]
- 7.Lucas RM, Ponsonby AL, Dear K, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology 2011; 76: 540–548. [DOI] [PubMed] [Google Scholar]
- 8.Bäärnhielm M, Hedström AK, Kockum I, et al. Sunlight is associated with decreased multiple sclerosis risk: No interaction with human leukocyte antigen-DRB1*15. Eur J Neurol 2012; 19: 955–962. [DOI] [PubMed] [Google Scholar]
- 9.Zivadinov R, Treu CN, Weinstock-Guttman B, et al. Interdependence and contributions of sun exposure and vitamin D to MRI measures in multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 10.International Multiple Sclerosis Genetics Consortium, Wellcome Trust Case Control Consortium 2, Sawcer S, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milliken SV, Wassall H, Lewis BJ, et al. Effects of ultraviolet light on human serum 25-hydroxyvitamin D and systemic immune function. J Allergy Clin Immunol 2012; 129: 1554–1561. [DOI] [PubMed] [Google Scholar]
- 12.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green C, Ferguson J, Lakshmipathi T, et al. 311 nm UVB phototherapy – An effective treatment for psoriasis. Br J Dermatol 1988; 119: 691–696. [DOI] [PubMed] [Google Scholar]
- 14.Clarke MW, Tuckey RC, Gorman S, et al. Optimized 25 hydroxyvitamin D analysis using liquid-liquid extraction with 2D separation with LC/MS/MS detection, provides superior precision compared to conventional assays. Metabolomics 2013; 9: 1031–1040. [Google Scholar]
- 15.Worswick SD Cockburn M andPeng D.. Measurement of ultraviolet exposure in epidemiological studies on skin and skin cancers. Photochem Photobiol 2008; 84: 1462–1472. [DOI] [PubMed] [Google Scholar]
- 16.Krupp LB, LaRocca NG, Muir-Nash J, et al. The Fatigue Severity Scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46: 1121–1123. [DOI] [PubMed] [Google Scholar]
- 17.Ware JE., Jr SF-36 Health Survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center, 1993. [Google Scholar]
- 18.Ware JE., Jr.The SF-36 Health Survey In: Spilker B. (ed) Quality of life and pharmaco-economics in clinical trials. 2nd ed Philadelphia: Lippincott-Raven Publishers, 1996: pp.337–345. [Google Scholar]
- 19.Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000; 343: 898–904. [DOI] [PubMed] [Google Scholar]
- 20.Brownlee WJ andMiller DH.. Clinically isolated syndromes and the relationship to multiple sclerosis. J Clin Neurosci 2014; 21: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 21.Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017; 389: 1336–1346. [DOI] [PubMed] [Google Scholar]
- 22.Dobson R Ramagopalan S andGiovannoni G.. The effect of gender in clinically isolated syndrome (CIS): A meta-analysis. Mult Scler 2012; 18: 600–604. [DOI] [PubMed] [Google Scholar]
- 23.van der Mei I, Lucas RM, Taylor BV, et al. Population attributable fractions and joint effects of key risk factors for multiple sclerosis. Mult Scler 2016; 22: 461–469. [DOI] [PubMed] [Google Scholar]
- 24.Breuer J, Schwab N, Schneider-Hohendorf T, et al. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol 2014; 75: 739–758. [DOI] [PubMed] [Google Scholar]
- 25.Autier P, Mullie P, Macacu A, et al. Effect of vitamin D supplementation on non-skeletal disorders: A systematic review of meta-analyses and randomised trials. Lancet Diabetes Endocrinol 2017; 5: 986–1004. [DOI] [PubMed] [Google Scholar]
- 26.Ponda MP, Liang Y, Kim J, et al. A randomized clinical trial in vitamin D-deficient adults comparing replenishment with oral vitamin D3 with narrow-band UV type B light: Effects on cholesterol and the transcriptional profiles of skin and blood. Am J Clin Nutr 2017; 105: 1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schweintzger N, Gruber-Wackernagel A, Reginato E, et al. Levels and function of regulatory T cells in patients with polymorphic light eruption: Relation to photohardening. Br J Dermatol 2015; 173: 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu C, Fitzpatrick A, Cong D, et al. Nitric oxide induces human CLA+CD25+Foxp3+ regulatory T cells with skin-homing potential. J Allergy Clin Immunol 2017; 140: 1441–1444. [DOI] [PubMed] [Google Scholar]
- 29.Jones AP, Kermode AG, Lucas RM, et al. Circulating immune cells in multiple sclerosis. Clin Exp Immunol 2017; 187: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knippenberg S, Damoiseaux J, Bol Y, et al. Higher levels of reported sun exposure, and not vitamin D status, are associated with less depressive symptoms and fatigue in multiple sclerosis. Acta Neurol Scand 2014; 129: 123–131. [DOI] [PubMed] [Google Scholar]
- 31.Rolf L, Muris AH, Bol Y, et al. Vitamin D3 supplementation in multiple sclerosis: Symptoms and biomarkers of depression. J Neurol Sci 2017; 378: 30–35. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Marling SJ, Beaver EF, et al. UV light selectively inhibits spinal cord inflammation and demyelination in experimental autoimmune encephalomyelitis. Arch Biochem Biophys 2015; 567: 75–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: The PhoCIS study by Prue H Hart, Anderson P Jones, Stephanie Trend, Lilian Cha, Marzena J Fabis-Pedrini, Matthew N Cooper, Catherine d’Este, Sian Geldenhuys, William M Carroll, Scott N Byrne, David R Booth, Judith M Cole, Robyn M Lucas and Allan G Kermode in Multiple Sclerosis Journal – Experimental, Translational and Clinical