Abstract
Pulmonary exacerbations are common events in cystic fibrosis and have a profound impact on quality of life, morbidity, and mortality. Pulmonary exacerbation outcomes remain poor and a significant proportion of patients fail to recover their baseline lung function despite receiving aggressive treatment with intravenous antibiotics. This focused review provides an update on some of the recent advances that have taken place in our understanding of the epidemiology, pathophysiology, diagnosis, and management of pulmonary exacerbations in cystic fibrosis as well as direction for future study.
Keywords: cystic fibrosis, exacerbations, treatment
Introduction
Despite improvements in lung function and nutritional outcomes for individuals with cystic fibrosis (CF) over the past decade, pulmonary exacerbation (PEx) remains common. In 2016, according to US Cystic Fibrosis Foundation Patient Registry data, one in three patients required at least one course of intravenous (IV) antibiotics to treat a PEx 1. Exacerbations have a profound impact on the morbidity and quality of life of individuals with CF, and unfortunately PEx outcomes remain suboptimal with poor recovery of baseline lung function following PEx treatment 2. As a result, efforts are under way within the CF research community to improve the management of these clinically impactful events. The focus of this review is to summarize some of the recent advances that have taken place in our understanding of the epidemiology, pathophysiology, diagnosis, and management of PEx in CF and to provide direction for future study.
Pulmonary exacerbation definition and diagnosis
Although there is general agreement on the importance of PEx, there is no consensus definition of what constitutes a PEx by CF clinicians, researchers, and the broader CF community. However, an ideal or consensus PEx definition (or scoring system) is likely to remain elusive without a gold standard to compare it against. Varying PEx definitions employed in the CF literature have been a major impediment to research progress and have inherently confounded many of the studies included in this review. This caveat must be kept in mind while reading this review because of the potential lack of specificity about what is being studied and discussed.
In an attempt to move toward a consensus PEx definition, the EuroCFCare Working group has recommended the use of a modified Fuchs criteria to define a PEx which includes the need for additional antibiotic treatment (oral or IV) and a recent change of at least two of the following six criteria: change in sputum volume or color; increased cough; increased fatigue, malaise, or lethargy; anorexia or weight loss; decrease in pulmonary function by 10% or more or radiographic changes; and increased dyspnea 3. However, this definition has been criticized by some experts in the field because the diagnosis of PEx should be independent of the physician’s decision to treat and pulmonary function testing limits the age range of patients assessed. Some recent pediatric trials have used the PEx definition employed in the Early Pseudomonas Infection Control (EPIC) trial, which consists of one major criterion (decrease in forced expiratory volume in one second [FEV 1] at least 10% from baseline with the previous 6 months; oxygen saturation less than 90% on room air or at least 5% decline from baseline; new lobar infiltrates or atelectasis on chest X-ray; hemoptysis) or two minor symptoms/signs (increased respiratory rate; new or increased adventitial sounds on lung exam; weight loss of at least 5% in the previous 6 months; increased cough; decreased exercise tolerance; increased chest congestion or change in sputum) for at least 5 days or with significant symptom severity 4.
Despite the lack of a consensus PEx definition, recent studies have focused on strategies to diagnose CF PEx earlier given the risk of poor outcomes. In the Standardized Treatment of Pulmonary Exacerbations (STOP) study, a multi-site observational study of patients with CF treated with IV antibiotics in hospital, most patients (85%) described symptoms more than 7 days before admission and nearly one-third (32%) had symptoms more than 21 days beforehand 5. Less than half (48%) of these individuals received oral antibiotics prior to admission, suggesting that certain patients may have prolonged symptoms in keeping with PEx but, due to delays in diagnosis, are not started on treatment. For these reasons, there has been a recent focus on home monitoring of symptoms and lung function to promote earlier PEx detection and treatment to prevent irreversible lung damage.
A multi-center study from the Netherlands examining electronic home monitoring of symptoms and lung function for early PEx detection confirmed a change in symptoms at least 4 weeks prior to PEx diagnosis in most patients, and further symptom deterioration in the 2 weeks prior to PEx diagnosis 6. However, a large multi-center randomized trial recently conducted in the US demonstrated that although electronic home monitoring of symptoms and spirometry is feasible and leads to earlier and more frequent diagnoses of PEx compared with usual care, this strategy does not lead to less lung function lost over 1 year 7. An earlier diagnosis in the home-monitoring arm led to more frequent use of oral (versus IV) antibiotics compared with the usual-care arm (67% versus 43%), and this might have resulted in a higher rate of non-recovery of FEV 1 % predicted to within 5% of baseline (47% versus 21%) due to a less robust response to oral antibiotic treatment 7. The authors concluded that identifying PEx earlier may not be sufficient and that future studies must also find better approaches to treatment of exacerbations once they are detected. However, there may still be utility for home monitoring for early PEx detection, particularly for individuals who are poor perceivers of their symptoms, have frequent exacerbations, or reside in rural areas which may delay access to care.
Pulmonary exacerbation epidemiology
Pulmonary exacerbation prevalence
The proportion of patients requiring at least one course of IV antibiotics per year has not decreased significantly over the past decade 1. Although this might seem concerning in light of the overall improvements in lung function observed over the same time period, this appears to be driven by a lower threshold among clinicians to diagnose and treat PEx 8. Some important studies have highlighted the wide variability in the recognition and treatment of PEx between CF clinics and individual CF physicians in both the US and the UK 9, 10. A multi-center study in the UK demonstrated higher IV antibiotic use among centers with higher baseline FEV 1 % predicted 10, whereas an older multi-center US study found variability among centers with respect to care (those with a higher median FEV 1 % predicted had more frequent monitoring and antibiotics) 9. These findings have led to quality improvement initiatives to reduce variability 11, 12.
Pulmonary exacerbation risk factors
In order to prevent PEx and their associated sequelae, there has been a great deal of interest in identifying the risk factors for future PEx. VanDevanter et al. investigated factors associated with increased risk of PEx requiring IV antibiotics 13. The study found that, out of numerous clinical variables, including sputum microbiology and treatment characteristics, the strongest risk factor for a PEx requiring IV antibiotic therapy was the occurrence of a PEx requiring IV antibiotics in the preceding year 13. Not surprisingly, individuals with three or more exacerbations had the highest risk of future PEx compared with those with one or two exacerbations 13.
Pulmonary exacerbation outcomes
With regard to PEx treatment outcomes, exacerbations treated with oral antibiotics are often considered to be milder events, whereas those treated with IV antibiotics are considered to represent more severe events. However, labeling a PEx on the basis of route of antibiotic treatment (oral versus IV) is limited and potentially biased, since factors other than PEx severity (based on symptoms, inflammatory markers, or lung function decline or a combination of these) may influence the decision of antibiotic route. For instance, IV antibiotics may be chosen on the basis of medication allergies/intolerances, bacterial resistance to oral antibiotics, or other non-disease severity-related factors (such as psychosocial issues or insurance coverage).
In support of the concept that exacerbations treated with oral antibiotics may not represent mild events, based on a recent retrospective study using the Toronto CF database from 2000 to 2014, nearly 20% of exacerbations treated with oral antibiotics did not recover to within 90% of baseline FEV 1 % predicted within 3 months of treatment 14. Furthermore, the greater the number of cumulative oral antibiotic-treated events over the study period, the steeper the rate of lung function decline 14. Consequently, close follow-up post-treatment is warranted to ensure recovery, and patients with repeated exacerbations treated with oral antibiotics may warrant more intensive treatment with IV antibiotics.
Even among patients who receive aggressive treatment with IV antibiotics, PEx outcomes remain suboptimal. Based on CF registry data from both the US and Canada, 25% of individuals who experience exacerbations fail to recover baseline lung function as defined by 90% of baseline within 3 months of treatment 2, 15. Factors independently associated with non-response included female sex; malnourishment; pancreatic insufficiency; persistent infection with Pseudomonas aeruginosa, Burkholderia cepacia complex, or methicillin-resistant Staphylococcus aureus; allergic bronchopulmonary aspergillosis; larger drop in FEV 1 % predicted at the time of PEx; and longer time from baseline spirometric assessment 2.
It is important to recognize that the proportion of non-responders can vary substantially depending on the definition of “response” used. For example, when a definition of FEV 1 improvement to 90% of baseline is employed, 25% of exacerbations are classified as non-responders, whereas an improvement to 100% of baseline yields a non-response rate as high as 60% 16. Furthermore, it should be noted that up to 25% of patients in the STOP trial had their best lung function at the time of PEx diagnosis and therefore a sizeable proportion of patients will be defined as responders even before treatment has started, leading to underestimation of treatment non-response. Most studies have used the best FEV 1 in the 3 months following the end of IV antibiotic treatment as the follow-up FEV 1, since lung function improvement can continue following the completion of IV antibiotics and therefore end-of-treatment values can underestimate rates of response.
Pulmonary exacerbation triggers
Viral respiratory tract infections have been estimated to be associated with about 50% of exacerbations and this might explain why exacerbations are more frequent during the winter months 17– 19. The most common viral pathogens include rhinovirus, respiratory syncytial virus (RSV) (in children), parainfluenza, influenza, adenovirus, coronavirus, and coxsackie/echovirus 17, 18, 20, 21. It was previously hypothesized that viruses could increase bacterial density of chronic colonizing organisms; however, a recent study has refuted this, demonstrating no change in P. aeruginosa density between viral- versus non-viral-associated PEx 17. Interestingly, anti-viral interferon signaling in response to RSV infection is capable of inducing P. aeruginosa biofilm formation through dysregulated iron homeostasis and this could represent a putative mechanism for viral-triggered PEx but warrants further study 22. It is also important to note that although there appears to be a strong association between viruses and PEx, this does not necessarily imply a causal relationship, as studies have demonstrated the presence of virus even when patients are well (suggesting asymptomatic nasopharyngeal carriage) 23, 24.
While non-viral-associated exacerbations were also believed to be the result of increased bacterial density of the chronic primary pathogen, recent studies have challenged this dogma. Multiple studies have demonstrated no significant changes in bacterial density from stable to PEx state 25– 28. Although decreases in bacterial density are observed following anti-microbial treatment, these effects are transient and are poorly predictive of clinical response 28– 30. Research has shown that CF airway infections are polymicrobial and that interactions between these microbes may increase or decrease pathogenicity 31. It is believed by some experts that organisms (such as anaerobes) can interact with the primary pathogen to enhance virulence without a change in density 31. For example, a recent study evaluating airway bacterial communities with 16S rRNA sequencing found no significant differences in bacterial community diversity or density between paired stable and PEx samples, but there was a change in community structure for a subgroup of patients 26. Furthermore, the absolute and relative abundance of Gemella spp. increased in the majority of samples from stable to PEx state, and this was most discriminative of health status (stable versus PEx) 26.
Air pollution is also an important trigger of PEx, and a seminal study linking the US CF National Registry to the US Environmental Protection Agency Aerometric Information Retrieval System demonstrated a significant association between annual average exposure to particulate matter and risk of PEx 32. A recent case-crossover analysis confirmed this finding and found that increased exposure to particulate matter less than 10 mm in diameter (PM 10), nitrogen dioxide, and ozone was associated with increased need for oral or IV antibiotics for PEx on the day of exposure 33.
Pulmonary exacerbation treatment
Treatment endpoints
In general, the goal of PEx treatment is to improve symptoms and recover lost lung function 34. Based on the STOP study, physicians identified recovery of lung function as the primary objective of treatment in 53% of exacerbations compared with improvement of symptoms in 43% of exacerbations 5. There is considerable variability as to what constitutes an acceptable threshold for FEV 1 improvement before antibiotics can be stopped. Based on the STOP study, the mean FEV 1 improvement was 9% (standard deviation [SD] 10%) predicted at the end of IV antibiotic treatment and 7% (SD 11%) predicted at day 28. Patients with baseline FEV 1 of more than 50% predicted had a greater increase in FEV 1 % predicted from admission to day 28 than patients with baseline FEV 1 less than 50% predicted (10% versus 3%). Interestingly, there was discordance between physician treatment targets in terms of lung function improvement and evaluation of treatment success. Based on the STOP study, 84% of clinicians deemed PEx treatment successful, although only 61% of patients achieved at least 90% of their target FEV 1 by the end of IV antibiotic therapy 35.
The treatment endpoint for an individual patient is likely to depend on the primary motivating factor for treatment. In the STOP study, a significant proportion of patients (20%) were admitted for IV antibiotics despite presenting with their best recorded FEV 1 % predicted in the prior 6 months 5. In these cases, symptoms were the primary driver for treatment and symptom resolution would be the most appropriate endpoint of treatment response. In general, exacerbations are more likely to be defined based on symptoms alone in children compared with adults (in whom drop in lung function may be more likely to occur) 36. The most widely used daily scoring system for monitoring of respiratory/infectious symptoms during PEx treatment is the Cystic Fibrosis Respiratory Symptom Diary – Chronic Respiratory Infection Symptom Score (CFRSD-CRISS) 37. Total scores range from 0 to 100, and an 11-point decrease is considered clinically significant 38. Based on STOP, CFRSD-CRISS decreased by 26.1 (95% confidence interval 23.8–28.3) and 83% of patients achieved a clinically significant improvement 35. However, it should be noted that the CFRSD-CRISS has limitations in that it has been used and evaluated in a research setting only and has not been accepted by the US Food and Drug Administration as a validated endpoint.
Whereas short-term goals of PEx treatment are to recover lost lung function and improve symptoms, long-term treatment goals generally are to prevent recurrent events and reduce the rate of lung function decline. Interestingly, a recent study found that symptom improvement (that is, CFRSD-CRISS score) in response to PEx treatment is poorly predictive of long-term response in terms of recovery of baseline lung function at 3 months or time to next IV antibiotics; however, immediate FEV 1 response (>10% relative improvement) was predictive of recovery of baseline FEV 1 by 3 months 39. Synthesizing all of this evidence suggests that a composite outcome that includes symptom and lung function improvement might be the most appropriate endpoint in prospective studies examining PEx therapies.
Treatment controversies
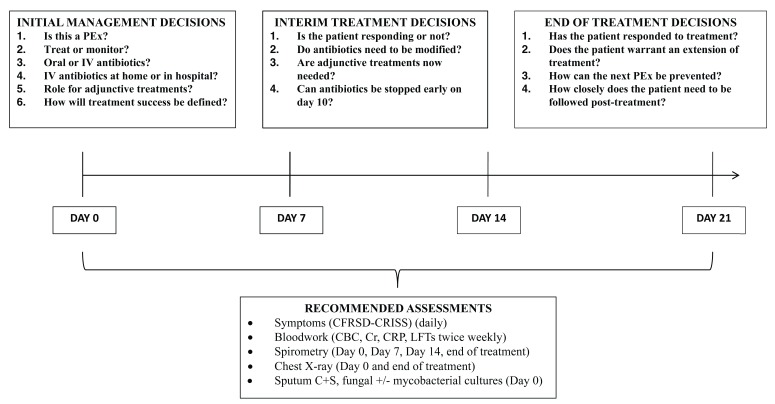
Based on the 2009 Cystic Fibrosis Foundation PEx treatment guidelines, there was insufficient evidence to provide recommendations on a number of decisions related to PEx management, including site of treatment (inpatient versus outpatient), antibiotic duration, and the use of adjunctive therapies (such as systemic steroids) ( Figure 1) 40. Since this publication, observational studies have provided additional insights into these treatment controversies and randomized controlled trials (RCTs) are ongoing or have been recently completed.
Figure 1. Outline of pulmonary exacerbation treatment decisions.
CBC, complete blood count; CFRSD-CRISS, Cystic Fibrosis Respiratory Symptom Diary-Chronic Respiratory Infection Symptom Score; Cr, creatinine; CRP, C-reactive protein; C+S, culture and sensitivity; IV, intravenous; LFT, liver function test; PEx, pulmonary exacerbation.
Site of treatment
There are some circumstances in which admission to hospital for optimal PEx treatment is clearly indicated (such as hypoxia, complications such as pneumothorax, or co-morbidities such as renal failure), but in many situations outpatient treatment with IV antibiotics appears to be a reasonable option. However, in deciding on the optimal treatment setting, an important factor is not just safety and feasibility but also whether one treatment setting is more efficacious than the other. One small randomized, two-factor, mixed-design comparative study involving 17 adults with CF has been performed and showed similar results for most outcome measures for home and hospital treatment 41. Owing to selection bias, the results of a larger RCT of the two treatment settings may not be generalizable and will be challenging to interpret because of lack of blinding, attrition bias, high rates of crossover, and lack of standardized resources for home care at various care centers 40.
A small, single-center retrospective study examined 143 PEx events from 50 patients and compared hospital with home IV therapy 42. The baseline characteristics between the two groups were similar; however, the hospitalized group had a greater improvement in lung function with a shorter duration of therapy 42. In a recent observational study of pediatric CF patients from the Epidemiologic Study of CF (ESCF), those with an acute decline in lung function were more likely to recover to within 90% of baseline FEV 1 when receiving treatment as an inpatient versus outpatient 43. However, observational studies involving treatment are often confounded by indication bias. In other words, patients are not randomly assigned and therefore the intensity of treatments is not standardized between comparisons; ultimately, patients in hospital might receive more intensive treatments. Another recent study that also used the ESCF employed statistical approaches to control for indication bias and found that PEx with a greater proportion of days treated as an inpatient (versus outpatient) with IV antibiotics was more likely to lead to return of FEV 1 % predicted to at least 90% of baseline 44. Although this observational study is not definitive (as there was likely residual confounding), the benefits of inpatient PEx treatment are likely due to multiple factors other than the IV antibiotics alone, including improved medication adherence, better nutrition, real-time adjustment of adjunct therapies, increased airway clearance treatments, and increased rest.
Ultimately, the decision regarding treatment site should be made based on careful consideration of patient factors (with a low threshold for inpatient treatment if there are concerns about patient reliability and adherence to airway clearance therapies, sufficient home supports, or more complex IV antibiotic regimens) 40.
Duration of antibiotics
For historical reasons, CF exacerbations are typically treated for 14 days. Based on the STOP study, the mean duration of treatment was 15.9 (SD 6.0) days; however, 11% of patients received treatment for 10 days or less and 60% received treatment for more than 14 days with no significant differences in duration for individuals younger than 18 years old versus those older than 18 years old 35. There have been no randomized studies examining the duration of IV antibiotics to provide evidence-based recommendations. An observational study that used the US CF Foundation Patient Registry found that FEV 1 % predicted plateaus by day 10 of treatment and duration of treatment did not influence time until next PEx 45. More recent retrospective data on the benefits of prolonging antibiotic treatment are conflicting, as a study using the Toronto CF Database found improved outcomes with longer treatment duration (>14 days) whereas a larger study using data from the ESCF did not find a significant association between treatment duration and rate of recovery of FEV 1 % predicted to within 90% of baseline 16, 44. However, as in observational studies of treatment setting, interpretation is limited by indication bias, since patient factors influence treatment duration (patients with lower lung function received longer treatment courses) 45. A large multi-center study (STOP2) is under way in the US examining IV antibiotic duration since it was identified as the most important research question by CF physicians and patients/caregivers. A divergent trial design is being used and randomly assigns patients to 10 versus 14 days or 14 versus 21 days of IV antibiotics depending on initial symptom and lung function response by day 7 46.
Adjunctive therapies
A recent study evaluating inpatient PEx treatment practices for pediatric CF patients across the US demonstrated wide variability in the use of adjunctive treatments, including hypertonic saline, azithromycin, and systemic corticosteroids 47. Several adjunct therapies for the treatment of CF PEx have recently been evaluated or are currently under investigation. These adjunct therapies function by either optimizing airway clearance or reducing airway inflammation.
Although nebulized hypertonic saline is well established as a strategy for PEx prevention 48, it has only recently been studied as an adjunct during PEx treatment. In an RCT, Dentice et al. compared PEx outcomes in individuals randomly assigned to nebulized 7% hypertonic saline versus taste-masked control thrice daily 49. The majority of patients in both groups had either never or only intermittently used hypertonic saline prior to enrollment. Although this study did not meet its primary endpoint in terms of reduced length of hospital stay, there was greater improvement in symptoms and higher rates of FEV 1 recovery in the hypertonic saline group 49. Furthermore, the study provided reassurance that nebulized hypertonic saline was safe to start (or increase) in the context of acute PEx 49.
Another recent study examined doxycycline as an adjunct therapy for CF PEx, acting as a small-molecule inhibitor of matrix metalloproteinase-9 (MMP-9), which has been implicated in CF airway pathophysiology, particularly during PEx 50, 51. This single-center RCT randomly assigned 39 CF patients with PEx requiring inpatient care to either doxycycline 100 mg orally twice daily or placebo for 8 days in addition to standard patient care (IV antibiotics and increased airway clearance) 52. Compared with the placebo group, the doxycycline group had a significant decrease in total and active sputum MMP-9 levels, improved protease-antiprotease imbalance in the airways, greater improvement in FEV 1 % predicted from admission, and longer time to next PEx 52. Whether doxycycline has direct effects on dysregulated protease activity versus indirect effects due to changes in the microbiome remains unclear, but this single-center study has set the stage for a larger multi-center placebo-controlled RCT 52.
Although systemic corticosteroids are used in up to 20% of CF exacerbations 35, 39, there is limited evidence to support their use 40. Just one small pilot placebo-controlled study involving 24 patients (≥10 years old) examined oral prednisone (2 mg/kg per day up to a maximum of 60 mg divided twice daily) versus placebo for the first 5 days as an adjunct to standard-of-care PEx treatment 53. Although there was no significant effect on lung function, symptom improvement, or sputum inflammatory markers compared with placebo, the study was underpowered to evaluate treatment effects 53. A multi-center, randomized, placebo-controlled trial evaluating prednisone—referred to as the Prednisone in CF Pulmonary Exacerbation (PIPE) study—is under way and involves six pediatric and adult CF clinics across Canada. This study will provide more definitive evidence regarding the role of systemic corticosteroids during PEx. Patients receiving IV antibiotic treatment and who have not responded to standard of care alone by day 7 (that is, not recovered at least 90% of their baseline FEV 1 % predicted) will be randomly assigned to prednisone (2 mg/kg per day up to a maximum of 60 mg divided twice daily) or placebo for 7 days.
Pulmonary exacerbation biomarkers
In the field of CF, there is tremendous interest in identifying a biomarker that could aid in the earlier diagnosis of a CF PEx or assist in tracking the response to PEx treatment or do both. Earlier diagnosis can allow for the timelier initiation of treatment, which might result in better PEx outcomes. A reliable biomarker of response to treatment could also identify non-responders earlier during the course of treatment so that therapies can be modified or extended accordingly.
The majority of biomarkers in relation to PEx have focused on inflammation in the sputum and blood 54, 55. Although sputum is an attractive option because it most closely reflects airway inflammation, its evaluation has been limited to research studies because it can be challenging to collect and process in clinical laboratories. Several sputum biomarkers of inflammation, including interleukin-8 (IL-8), neutrophil elastase (NE), calprotectin, club cell secretory protein (CCSP), and MMP-9, have been investigated during PEx 56– 59. Although most of these sputum biomarkers change significantly from stable to PEx state or following PEx treatment, the results have been variable between studies. Sputum NE appears to be promising, as a recent study by Waters et al. found that a decrease in sputum NE was independently associated with response to IV antibiotic treatment at day 14 and higher NE levels at day 14 were associated with greater risk of subsequent PEx 57.
Among blood biomarkers, numerous markers of inflammation have been evaluated as recently reviewed 60. Serum C-reactive protein (CRP) and calprotectin have been the most extensively studied in the context of PEx and offer the most promise for clinical use 54. All studies evaluating CRP and calprotectin in CF have demonstrated significant reductions in levels from beginning to end of PEx treatment 54, 56, 58. However, a recent study demonstrated that CRP increases in 25% of patients during the first 5 days of IV antibiotic treatment prior to decreasing, thus making it a challenging biomarker to monitor early response to treatment 61. Individuals with persistently elevated CRP and calprotectin levels following IV antibiotics also experience a shorter time to re-exacerbation 57, 62.
Although several candidate biomarkers that correlate with clinical outcomes during PEx treatment have been identified, it remains unclear whether their prospective use can influence treatment decisions to improve PEx outcomes and whether they add incremental utility to monitoring of symptoms and lung function alone.
Future directions
Although small but incremental progress is being made in our understanding of PEx in CF on the basis of observational studies, there remain several gaps in knowledge and a need for more interventional studies to guide evidence-based practice. The CF community is eagerly awaiting the results of the aforementioned RCTs evaluating various IV antibiotic treatment durations and the adjunctive use of systemic corticosteroids and doxycycline, as these have the potential to improve PEx outcomes. In addition, a greater understanding of the sequence of events leading to a PEx at a molecular level is required to improve PEx phenotyping and to guide the development of more targeted treatments.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Patrick Flume, Department of Medicine and Pediatrics, Medical University of South Carolina, Charleston, SC, USA
Martin J Wildman, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
John J LiPuma, Department of Pediatrics and Communicable Diseases, University of Michigan Medical School, Ann Arbor, MI, USA
Funding Statement
BSQ receives salary support from the Michael Smith Foundation for Health Research and from Cystic Fibrosis Canada.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Cystic Fibrosis Foundation Patient Registry: 2015 Annual Report.Bethesda, Maryland.2016. Cystic Fibrosis Foundation. Reference Source [Google Scholar]
- 2. Sanders DB, Bittner RC, Rosenfeld M, et al. : Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. 2010;182(5):627–32. 10.1164/rccm.200909-1421OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilton D, Canny G, Conway S, et al. : Pulmonary exacerbation: towards a definition for use in clinical trials. Report from the EuroCareCF Working Group on outcome parameters in clinical trials. 2011;10(Suppl 2):S79–81. 10.1016/S1569-1993(11)60012-X [DOI] [PubMed] [Google Scholar]
- 4. Treggiari MM, Rosenfeld M, Mayer-Hamblett N, et al. : Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study'. 2009;30(3):256–68. 10.1016/j.cct.2009.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders DB, Solomon GM, Beckett VV, et al. : Standardized Treatment of Pulmonary Exacerbations (STOP) study: Observations at the initiation of intravenous antibiotics for cystic fibrosis pulmonary exacerbations. 2017;16(5):592–9. 10.1016/j.jcf.2017.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. van Horck M, Winkens B, Wesseling G, et al. : Early detection of pulmonary exacerbations in children with Cystic Fibrosis by electronic home monitoring of symptoms and lung function. 2017;7(1): 12350. 10.1038/s41598-017-10945-3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Lechtzin N, Mayer-Hamblett N, West NE, et al. : Home Monitoring of Patients with Cystic Fibrosis to Identify and Treat Acute Pulmonary Exacerbations. eICE Study Results. 2017;196(9):1144–51. 10.1164/rccm.201610-2172OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. VanDevanter DR, Elkin EP, Pasta DJ, et al. : Changing thresholds and incidence of antibiotic treatment of cystic fibrosis pulmonary exacerbations, 1995-2005. 2013;12(4):332–7. 10.1016/j.jcf.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 9. Johnson C, Butler SM, Konstan MW, et al. : Factors influencing outcomes in cystic fibrosis: a center-based analysis. 2003;123(1):20–7. 10.1378/chest.123.1.20 [DOI] [PubMed] [Google Scholar]
- 10. Hoo ZH, Campbell MJ, Curley R, et al. : Do cystic fibrosis centres with the lowest FEV 1 still use the least amount of intravenous antibiotics? A registry-based comparison of intravenous antibiotic use among adult CF centres in the UK. 2017; pii: S1569-1993(17)30916-5. 10.1016/j.jcf.2017.10.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Kraynack NC, Gothard MD, Falletta LM, et al. : Approach to treating cystic fibrosis pulmonary exacerbations varies widely across US CF care centers. 2011;46(9):870–81. 10.1002/ppul.21442 [DOI] [PubMed] [Google Scholar]
- 12. Kraynack NC, McBride JT: Improving care at cystic fibrosis centers through quality improvement. 2009;30(5):547–58. 10.1055/s-0029-1238913 [DOI] [PubMed] [Google Scholar]
- 13. VanDevanter DR, Morris NJ, Konstan MW: IV-treated pulmonary exacerbations in the prior year: An important independent risk factor for future pulmonary exacerbation in cystic fibrosis. 2016;15(3):372–9. 10.1016/j.jcf.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Stanojevic S, McDonald A, Waters V, et al. : Effect of pulmonary exacerbations treated with oral antibiotics on clinical outcomes in cystic fibrosis. 2017;72(4):327–32. 10.1136/thoraxjnl-2016-208450 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Waters V, Atenafu EG, Salazar JG, et al. : Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. 2012;11(1):8–13. 10.1016/j.jcf.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 16. Waters V, Stanojevic S, Klingel M, et al. : Prolongation of antibiotic treatment for cystic fibrosis pulmonary exacerbations. 2015;14(6):770–6. 10.1016/j.jcf.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 17. Chin M, De Zoysa M, Slinger R, et al. : Acute effects of viral respiratory tract infections on sputum bacterial density during CF pulmonary exacerbations. 2015;14(4):482–9. 10.1016/j.jcf.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Asner S, Waters V, Solomon M, et al. : Role of respiratory viruses in pulmonary exacerbations in children with cystic fibrosis. 2012;11(5):433–9. 10.1016/j.jcf.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wat D, Gelder C, Hibbitts S, et al. : The role of respiratory viruses in cystic fibrosis. 2008;7(4):320–8. 10.1016/j.jcf.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goffard A, Lambert V, Salleron J, et al. : Virus and cystic fibrosis: rhinoviruses are associated with exacerbations in adult patients. 2014;60(2):147–53. 10.1016/j.jcv.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Somayaji R, Goss CH, Khan U, et al. : Cystic Fibrosis Pulmonary Exacerbations Attributable to Respiratory Syncytial Virus and Influenza: A Population-Based Study. 2017;64(12):1760–7. 10.1093/cid/cix203 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Hendricks MR, Lashua LP, Fischer DK, et al. : Respiratory syncytial virus infection enhances Pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. 2016;113(6):1642–7. 10.1073/pnas.1516979113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Almeida MB, Zerbinati RM, Tateno AF, et al. : Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. 2010;16(6):996–9. 10.3201/eid1606.100063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stelzer-Braid S, Johal H, Skilbeck K, et al. : Detection of viral and bacterial respiratory pathogens in patients with cystic fibrosis. 2012;186(1–2):109–12. 10.1016/j.jviromet.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 25. Stressmann FA, Rogers GB, Marsh P, et al. : Does bacterial density in cystic fibrosis sputum increase prior to pulmonary exacerbation? 2011;10(5):357–65. 10.1016/j.jcf.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 26. Carmody LA, Zhao J, Schloss PD, et al. : Changes in cystic fibrosis airway microbiota at pulmonary exacerbation. 2013;10(3):179–87. 10.1513/AnnalsATS.201211-107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fothergill JL, Ledson MJ, Walshaw MJ, et al. : Comparison of real time diagnostic chemistries to detect Pseudomonas aeruginosa in respiratory samples from cystic fibrosis patients. 2013;12(6):675–81. 10.1016/j.jcf.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 28. Lam JC, Somayaji R, Surette MG, et al. : Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. 2015;15:145. 10.1186/s12879-015-0856-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Ordoñez CL, Henig NR, Mayer-Hamblett N, et al. : Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. 2003;168(12):1471–5. 10.1164/rccm.200306-731OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Tunney MM, Klem ER, Fodor AA, et al. : Use of culture and molecular analysis to determine the effect of antibiotic treatment on microbial community diversity and abundance during exacerbation in patients with cystic fibrosis. 2011;66(7):579–84. 10.1136/thx.2010.137281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicod LP, Kolls JK: Chair's Summary: Mechanisms of Exacerbation of Lung Diseases. 2015;12 Suppl 2:S112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goss CH, Newsom SA, Schildcrout JS, et al. : Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. 2004;169(7):816–21. 10.1164/rccm.200306-779OC [DOI] [PubMed] [Google Scholar]
- 33. Goeminne PC, Kiciński M, Vermeulen F, et al. : Impact of air pollution on cystic fibrosis pulmonary exacerbations: a case-crossover analysis. 2013;143(4):946–54. 10.1378/chest.12-1005 [DOI] [PubMed] [Google Scholar]
- 34. Stenbit AE, Flume PA: Pulmonary exacerbations in cystic fibrosis. 2011;17(6):442–7. [DOI] [PubMed] [Google Scholar]
- 35. West NE, Beckett VV, Jain R, et al. : Standardized Treatment of Pulmonary Exacerbations (STOP) study: Physician treatment practices and outcomes for individuals with cystic fibrosis with pulmonary Exacerbations. 2017;16(5):600–6. 10.1016/j.jcf.2017.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Waters V, Ratjen F: Pulmonary Exacerbations in Children with Cystic Fibrosis. 2015;12 Suppl 2:S200–6. [DOI] [PubMed] [Google Scholar]
- 37. Goss CH, Edwards TC, Ramsey BW, et al. : Patient-reported respiratory symptoms in cystic fibrosis. 2009;8(4):245–52. 10.1016/j.jcf.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 38. Goss CH, Caldwell E, Gries KNL, et al. : Validation of a novel patient-reported respiratory symptoms instrument in cystic fibrosis: CFRSD-CRISS. 2013:A251. [Google Scholar]
- 39. Heltshe SL, Goss CH, Thompson V, et al. : Short-term and long-term response to pulmonary exacerbation treatment in cystic fibrosis. 2016;71(3):223–9. 10.1136/thoraxjnl-2014-206750 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Flume PA, Mogayzel PJ, Jr, Robinson KA, et al. : Cystic fibrosis pulmonary guidelines: treatment of pulmonary exacerbations. 2009;180(9):802–8. 10.1164/rccm.200812-1845PP [DOI] [PubMed] [Google Scholar]
- 41. Wolter JM, Bowler SD, Nolan PJ, et al. : Home intravenous therapy in cystic fibrosis: a prospective randomized trial examining clinical, quality of life and cost aspects. 1997;10(4):896–900. [PubMed] [Google Scholar]
- 42. Nazer D, Abdulhamid I, Thomas R, et al. : Home versus hospital intravenous antibiotic therapy for acute pulmonary exacerbations in children with cystic fibrosis. 2006;41(8):744–9. 10.1002/ppul.20433 [DOI] [PubMed] [Google Scholar]
- 43. Morgan WJ, Wagener JS, Pasta DJ, et al. : Relationship of Antibiotic Treatment to Recovery after Acute FEV 1 Decline in Children with Cystic Fibrosis. 2017;14(6):937–42. 10.1513/AnnalsATS.201608-615OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Schechter MS, VanDevanter DR, Pasta DJ, et al. : Treatment Setting and Outcomes of Cystic Fibrosis Pulmonary Exacerbations. 2018;15(2):225–33. 10.1513/AnnalsATS.201702-111OC [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Collaco JM, Green DM, Cutting GR, et al. : Location and duration of treatment of cystic fibrosis respiratory exacerbations do not affect outcomes. 2010;182(9):1137–43. 10.1164/rccm.201001-0057OC [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Heltshe SL, West NE, VanDevanter DR, et al. : Study design considerations for the Standardized Treatment of Pulmonary Exacerbations 2 (STOP 2): A trial to compare intravenous antibiotic treatment durations in CF. 2018;64:35–40. 10.1016/j.cct.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Cogen JD, Oron AP, Gibson RL, et al. : Characterization of Inpatient Cystic Fibrosis Pulmonary Exacerbations. 2017;139(2). pii: e20162642. 10.1542/peds.2016-2642 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Elkins MR, Robinson M, Rose BR, et al. : A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. 2006;354(3):229–40. 10.1056/NEJMoa043900 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Dentice RL, Elkins MR, Middleton PG, et al. : A randomised trial of hypertonic saline during hospitalisation for exacerbation of cystic fibrosis. 2016;71(2):141–7. 10.1136/thoraxjnl-2014-206716 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Gaggar A, Li Y, Weathington N, et al. : Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. 2007;293(1):L96–L104. 10.1152/ajplung.00492.2006 [DOI] [PubMed] [Google Scholar]
- 51. Hanemaaijer R, Visser H, Koolwijk P, et al. : Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. 1998;12(2):114–8. 10.1177/08959374980120010301 [DOI] [PubMed] [Google Scholar]
- 52. Xu X, Abdalla T, Bratcher PE, et al. : Doxycycline improves clinical outcomes during cystic fibrosis exacerbations. 2017;49(4): pii: 1601102. 10.1183/13993003.01102-2016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Dovey M, Aitken ML, Emerson J, et al. : Oral corticosteroid therapy in cystic fibrosis patients hospitalized for pulmonary exacerbation: a pilot study. 2007;132(4):1212–8. 10.1378/chest.07-0843 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Shoki AH, Mayer-Hamblett N, Wilcox PG, et al. : Systematic review of blood biomarkers in cystic fibrosis pulmonary exacerbations. 2013;144(5):1659–70. 10.1378/chest.13-0693 [DOI] [PubMed] [Google Scholar]
- 55. Sagel SD, Chmiel JF, Konstan MW: Sputum biomarkers of inflammation in cystic fibrosis lung disease. 2007;4(4):406–17. 10.1513/pats.200703-044BR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Horsley AR, Davies JC, Gray RD, et al. : Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. 2013;68(6):532–9. 10.1136/thoraxjnl-2012-202538 [DOI] [PubMed] [Google Scholar]
- 57. Waters VJ, Stanojevic S, Sonneveld N, et al. : Factors associated with response to treatment of pulmonary exacerbations in cystic fibrosis patients. 2015;14(6):755–62. 10.1016/j.jcf.2015.01.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Gray RD, Imrie M, Boyd AC, et al. : Sputum and serum calprotectin are useful biomarkers during CF exacerbation. 2010;9(3):193–8. 10.1016/j.jcf.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 59. Laguna TA, Williams CB, Brandy KR, et al. : Sputum club cell protein concentration is associated with pulmonary exacerbation in cystic fibrosis. 2015;14(3):334–40. 10.1016/j.jcf.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scott LK, Toner R: Clinically Promising Biomarkers in Cystic Fibrosis Pulmonary Exacerbations. 2017;195(4):397–401. 10.1007/s00408-017-0024-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Sharma A, Kirkpatrick G, Chen V, et al. : Clinical utility of C-reactive protein to predict treatment response during cystic fibrosis pulmonary exacerbations. 2017;12(2):e0171229. 10.1371/journal.pone.0171229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parkins MD, Rendall JC, Elborn JS: Incidence and risk factors for pulmonary exacerbation treatment failures in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. 2012;141(2):485–93. 10.1378/chest.11-0917 [DOI] [PubMed] [Google Scholar]