Abstract
Background:
Femoral suspensory fixation for anterior cruciate ligament (ACL) reconstruction has evolved from fixed- to adjustable-loop devices. However, there are still controversies regarding undesired lengthening of adjustable-loop devices.
Hypothesis:
Adjustable-loop fixation will achieve similar elongation to that of fixed-loop devices, and intraoperative preconditioning will reduce initial elongation for adjustable-loop constructs.
Study Design:
Controlled laboratory study.
Methods:
Three adjustable-loop devices (GraftMax, TightRope, and Ultrabutton) and 2 fixed-loop devices (Endobutton and RetroButton) were used in an intraoperative surgical technique workflow according to an in vitro model with porcine bone and bovine tendons (8 specimens per device; N = 40 constructs tested). Each construct underwent 1000 cycles of position- and force-controlled dynamic loading, whereby a total elongation threshold of 3 mm was defined as clinical failure. Constructs were finally pulled to failure at 50 mm/min.
Results:
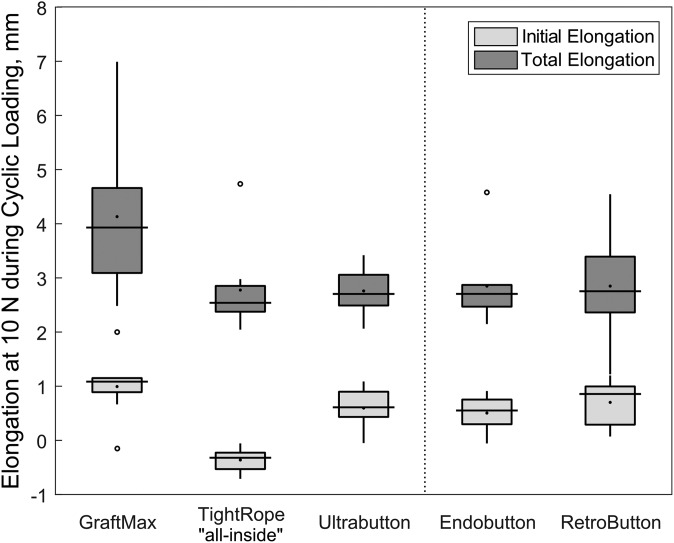
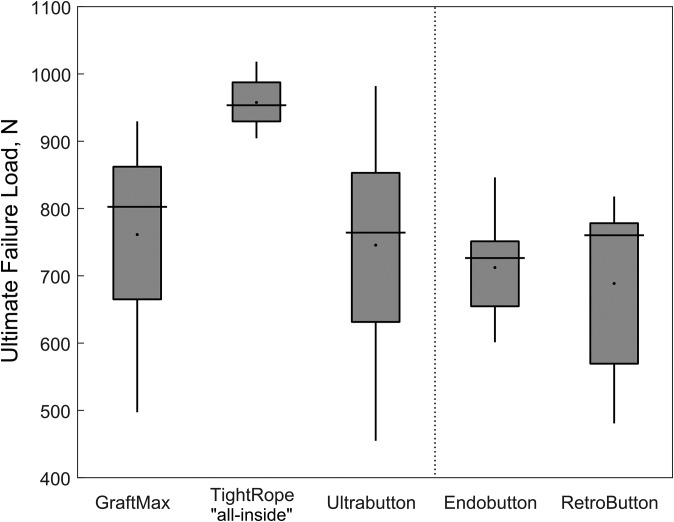
There were no statistically significant differences among the devices for total or dynamic elongation. Total elongation (mean ± SD) for adjustable-loop constructs was 4.13 ± 1.46 mm for GraftMax, 2.78 ± 0.85 mm for TightRope, and 2.76 ± 0.45 mm for Ultrabutton; for the fixed-loop devices, total elongation was 2.85 ± 0.74 mm for Endobutton and 2.85 ± 1.03 mm for RetroButton. The GraftMax had a significantly lower initial force (95.5 ± 58.0 N) after retensioning, with the highest initial elongation (0.99 ± 0.60 mm). The Ultrabutton showed the greatest force loss (–105.9 ± 13.5 N) during position control cycling, which was significantly different from the GraftMax (–22.3 ± 28.2 N), with the smallest force loss (P < .001). The TightRope construct had a significantly smaller initial elongation (–0.36 ± 0.22 mm) and the greatest pull-to-failure load (958 ± 40 N) as compared with all of the other devices.
Conclusion:
Adjustable- and fixed-loop configurations achieved statistically comparable fixation strength for total elongation. However, the GraftMax construct exceeded the total elongation threshold of clinical failure. The Ultrabutton produced the greatest loss of force during position control cycling, and the GraftMax button design prevented proper retensioning. The TightRope had a significant greater ultimate strength when compared with all other devices.
Clinical Relevance:
Biomechanical testing according to a surgical technique workflow suggests that adjustable-loop devices can be considered a safe alternative to fixed-loop devices in ACL reconstruction.
Keywords: ACL reconstruction, cortical button, adjustable loop, fixed loop, biomechanics, cyclic loading, suspensory fixation
Anterior cruciate ligament (ACL) ruptures are one of the most common injuries. There are more than 150,000 ACL reconstructions (ACLRs) performed every year.19,23 Suspensory femoral cortical fixation for ACLR has evolved from a fixed-loop device (FLD) to an adjustable-loop device (ALD). Advantages of FLDs include strong fixation that limits initial elongation while the graft is being incorporated. However, FLDs require precise mathematical calculations to ensure proper bone tunnel measurements for graft insertion. Some advantages of ALDs include ease of insertion without intraoperative calculations,8 a single loop size for all patients,9 the possibility of a longer amount of tendon within the femoral tunnel that may optimize graft incorporation, and the capability of retensioning the graft after initial fixation.8,22 Despite these advantages, a clinical ACLR with a femoral FLD and tibial screw fixation remains the benchmark for biomechanical performance evaluation for configurations utilizing adjustable-loop fixation.
There is controversy surrounding the increased elongations observed with ALDs as compared with FLDs.8,15,18,30 Some researchers have questioned the testing methods as an explanation for the large elongation values observed with ALDs.14 Conversely, ALDs have been shown to possess similar elongation values to FLDs27 and have been implanted in surgery with successful clinical outcomes.9 Specifically, the influence of retensioning ALDs during testing is often discussed. Noonan et al29 demonstrated that retensioning ALDs minimized the total cyclic displacement values, while Johnson et al18 found that retensioning did not have a statistically significant difference on elongation for ALDs. However, both these studies were single-device testing as opposed to full-construct testing (with bone and soft tissue) at zero load.
There have been newly released ALDs, but reported testing of these devices was not available in the literature at the time of writing.
The purpose of this study was to comparatively test previously and newly released ALDs in a full-construct surgical technique–based manner for stability evaluation in relation to the benchmark FLD configuration. The FLD benchmark metrics were defined as force maintenance throughout position-controlled cycling; initial, dynamic, and total elongation throughout force-controlled cycling; and ultimate load and stiffness during pull to failure. The hypotheses were that ACLR with ALD fixation would behave comparably in terms of biomechanics with FLD and that intraoperative preconditioning with graft precycling and retensioning would significantly reduce initial elongation for ALD constructs.
Methods
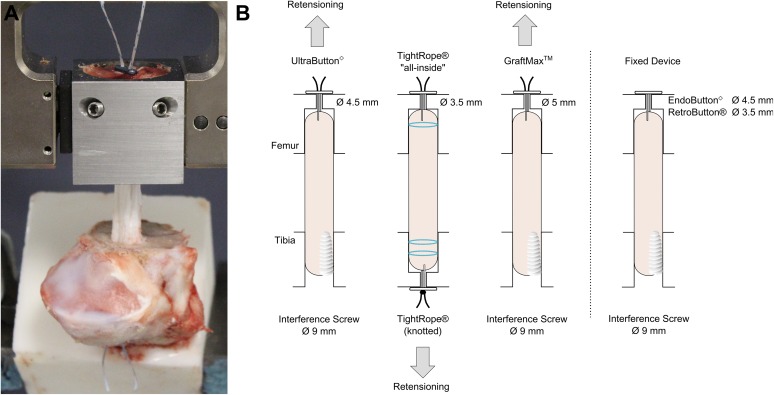
Five femoral cortical suspension devices were selected to be biomechanically tested in an in vitro model with porcine bone and bovine tendons (8 specimens per device; N = 40 constructs tested). The 3 ALDs included were the GraftMax (GM; ConMed Linvatec), TightRope (TR; Arthrex), and Ultrabutton (UB; Smith & Nephew). The 2 FLDs, which served as the positive control, were the Endobutton (EB; Smith & Nephew) and RetroButton (RB; Arthrex), and both consisted of a 20-mm continuous loop (Figure 1).
Figure 1.
The 5 suspensory femoral cortical devices tested from left to right: GraftMax, TightRope, Ultrabutton, Endobutton (fixed-loop device), and RetroButton (fixed-loop device). The adjustable-loop devices can modify loop length by pulling on the shortening sutures, and the fixed-loop devices have a loop length of 20 mm.
Specimen Preparation
Full-construct testing was set up with the porcine bone and bovine tendons. Fresh bovine tendons (2 years of age) were obtained from the local slaughterhouse. Extensor digitorum tendons were harvested in our laboratory from the hind legs, which have been shown to possess similar viscoelastic properties to human hamstring tendons.17 The tendons were stored at –20°C and thawed several hours before testing. Porcine bone (6 months of age) was chosen owing to its previous use in ACL studies2,30,35 and similarity to human bone.1,16,28 The bones were stored at –20°C and thawed overnight at room temperature before testing.
The porcine tibias and femurs were initially prepared by removing all the soft tissue from the bone. The tibias were embedded with RenCast mixture (Huntsman Advanced Materials) in a custom rectangular steel fixture about 2 cm distal to the tibial tunnel on the medial side. The lateral plateau of the tibia was sawed off to create a constant 40-mm bone tunnel.
The porcine femurs were prepared by measuring 35 mm from the lateral condyle of the femur and sawing off the medial condyle at that point. A 35-mm cylinder drill was used to saw the lateral condyle of the femur to create a bony block. The cylindrical bony block was docked in a custom-made steel fixture.
Graft Preparation
The bovine tendon grafts for the GM, UB, and 2 FLDs were measured to 325 mm and trimmed along the fiber orientation to a diameter of 9 mm when the graft was quadrupled. A graft size of 9 mm was chosen because it represents a standard graft diameter for human ACLR and smaller grafts (<8 mm) were shown to have increased failure after 2 years.24 The last 20 mm of the ends of the tendon were whipstitched with No. 2 suture to create a doubled graft,7 which was then folded over the loop to create a quadruple-stranded graft.
Biocomposite interference screws were chosen for tibial fixation of the GM and FLDs according to the corresponding surgical technique guides.6,12,36,38 To add consistency to the study, a biocomposite interference screw was also chosen for the UB since the surgical technique guide stated that tibial fixation was determined by the surgeon’s preference.36 The GraftLink all-inside technique for the TR, which consisted of an ALD and button for tibial fixation, was chosen because it was the method promoted by the manufacturer5,27 (Figure 2).
Figure 2.
(A) Test setup with femoral bony block in the steel fixture, embedded porcine tibia, and bovine tendon graft. The tibia and femur are 30 mm apart. The graft is aligned to create worst-case scenario testing. (B) Graft setup for all the devices. The arrow indicates the direction of retensioning for the adjustable-loop devices.
The bovine tendon grafts for the TR with the GraftLink technique were measured to 280 mm, and the diameter was sized to 9 mm when quadrupled. The graft was prepared by threading each end through an adjustable TR loop. Last, the 2 free ends each went through the adjustable loop again, which created a quadruple-stranded loop. This loop was then U-stitched with a No. 0 suture with 4 to 5 stitches and ends overlapping for about 5 mm to create a closed continuous loop.5,35 The femoral end of the graft included a full TR construct with the button incorporated around the loop, while the tibial end of the graft included only the TR loop with sutures. A button was subsequently attached during graft insertion. Next, the 4 tendon strands on each end of the construct ends were link stitched with a No. 2 suture incorporating each tendon strand. There were 2 link stitches on the tibial end and 1 link on the femoral side, as altered from the surgical technique guide describing 2 link stitches on both the tibial and femoral sides. The final graft preparation for the TR included burying the knots from the sutures and having the sutures on the inside of the graft.35
All grafts were pretensioned for conditioning reasons in a graft preparation board and a 9-mm compression tube with 80 N for 5 minutes prior to insertion.21,26 The grafts were kept moist during testing with a physiologic saline solution.
Tunnel Preparation
The guide wire in the tibia represented the native ACL footprint and was used to drill the bone tunnels in the tibias. The 40-mm full bone tunnel for the GM, UB, and FLDs were created with a 9-mm cannulated drill. To create a bone socket, the porcine tibias for the TR were prepared by using a 9-mm cannulated drill for 30 mm and then a 3.5-mm cannulated drill for the remaining 10 mm toward the cortex, which differed from the surgical technique guide describing the usage of a flip cutter drill.
The femurs for the ALDs were initially prepared with a 9-mm cannulated drill for a distance of 20 mm. Then, for the last 15 mm, a bone bridge was drilled until the femoral cortex was reached with a 5-mm, 3.5-mm, and 4.5-mm cannulated drill for the GM, TR, and UB, respectively. The femurs for the FLDs were prepared with a 9-mm cannulated drill for 23 mm into the bone. For the remaining last 12 mm, a 4.5-mm hole for the EB and a 3.5-mm hole for the RB were drilled.
Device Insertion Techniques
The steel fixture with the femoral bony block was clamped into the tensile testing machine (Instron ElectroPuls 10000). The tibia was inserted into the tensile testing machine at an angle such that the graft was in line with the femoral insertion, creating “worst-case scenario” testing owing to aligned load axes, and was clamped tightly (Figure 2). A consistent joint space of 29 mm was measured between the femur and tibia for ACLR primary fixation, which corresponded to a knee in simulated 30° of flexion and served as a reference for elongation analysis. The graft was inserted into the femur and tibia with a passing suture. Countertension was applied on the graft as it was inserted into the femur. For constructs with the GM or UB, a space of 2 to 3 mm was left in the femoral socket, allowing retensioning.
For the GM, UB, and the FLDs, a 50-N weight was suspended on the graft while a 9 × 28–mm biocomposite interference screw was inserted until it reached the superior portion of the tibial bone. For the TR tests, the femoral-sided graft was fully inserted and tensioned until graft tunnel docking, because retensioning was performed on the tibial side. Next, a button (Adjustable Button System; Arthrex) was applied to the tibial-sided TR suture loop and manually tensioned to 50 N by pulling on the shortening strands. During pulling, the applied force was monitored on the tensile testing machine.
Intraoperative Preconditioning
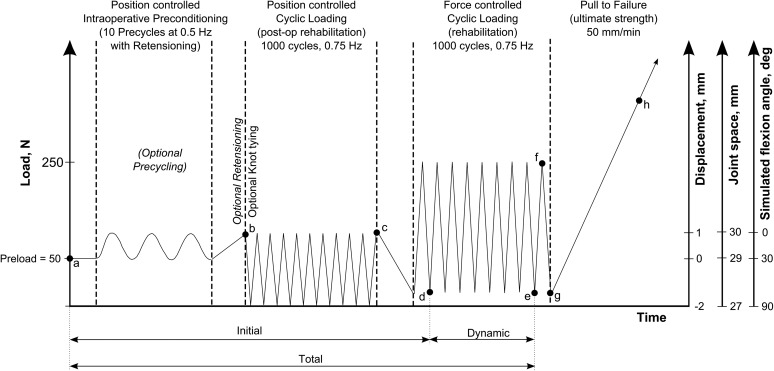
The testing protocol (Figure 3) included an “ACL length over flexion angle” relationship to simulate knee flexion activity. In vivo kinematic data have shown that the ACL experiences a consistent length decrease from 30 mm to 27 mm when the knee is moved between full extension and 90° of flexion.20 In reference to the primary fixation position with a graft length of 29 mm, which corresponds to 30° of knee flexion, a knee in full extension and 90° of angled flexion is simulated by a graft length increase of 1 mm (30-mm joint space) and a decrease of 2 mm (27-mm joint space), respectively. Therefore, the testing protocol (Figure 3) for the ALDs started with 10 precycles in position control between +1 mm and –2 mm at 0.5 Hz to simulate knee movement between full extension and 90° of flexion at the knee.
Figure 3.
Test protocol for the devices. Measurements included initial elongation (Δad), dynamic elongation (Δde), total elongation (Δae), force decrease (Δbc), and stiffness during pull to failure (Δgh). The fixed-loop device did not undergo preconditioning and retensioning; the TightRope was the only device knotted on the tibial side.
An advantage of ALDs is the retensioning, which implies the ability to increase the graft force after primary fixation.22 All surgical technique guides recommended retensioning; however, there was no defined force for the device to be retensioned. It was shown that the FLD graft force was about 130 N after the screw was inserted and the space between the tibia and femur was increased 1 mm, representing full extension. Therefore, the force for retensioning of the ALDs after precycling was chosen to be 200 N, which was reproducible. If reaching 200 N was not achievable with the device being tested, the maximal tensioning possible was applied.
Retensioning was performed in full extension (30-mm joint space) for the TR and UB, whereas the GM was tensioned in simulated 30° of knee flexion (29-mm joint space) to enable the button-locking mechanism. For the TR, the graft was retensioned on the tibial side to 200 N and then knotted with a surgeon’s knot and 3 half-hitch knots with an arthroscopic knot pusher. The GM and UB were retensioned at the femoral side to 200 N or the maximal force possible. All femoral ALDs were kept knotless, which represents clinical practice by reducing possible postoperative knot irritation or knot tying through soft tissue layers. The test protocol for the FLDs did not include precycling and retensioning, as it was not technically possible to compensate tension loss owing to screw insertion.
Position Control Loading
After the preconditioning protocol, the position control loading block started and consisted of 1000 cycles at 0.75 Hz between +1 mm (30-mm joint space) and –2 mm (27-mm joint space). The included slack on the graft enabled a complete unloading (0 N) to occur, which may have constituted an unfavorable loading situation for the ALDs. Position-controlled cycling simulated the in vivo kinematics of the ACL during weightbearing knee flexion, as also established by Monaco et al.26 The initial peak and final force loss were measured within the position control block.
Force Control Loading
After position control loading, there were 1000 cycles of load control between 10 N and 250 N at 0.75 Hz. The 250-N load was chosen because peak ACL forces while walking and early rehabilitation were estimated to be 303 N33 and as 250 N is a commonly used load level in ACL testing.8,15,29,30 The initial elongation was measured as the valley elongation from the start of testing until the first cycle of the force control block was completed. The dynamic elongation represents a relative valley elongation during force-controlled cyclic loading. The total elongation is the sum of initial and dynamic elongation.
Pull to Failure
For all constructs, a pull to failure at a rate of 50 mm/min was performed with ultimate failure load and stiffness calculated and mechanism of failure noted. The ultimate stiffness was determined with the linear portion of the load-elongation curve within the load range of 200 N and 450 N. Cyclic loading and load-to-failure data were recorded with Wavematrix software (Instron) with a sampling rate of 500 Hz.
Statistical Analysis
The programming software MATLAB (v R2015b; MathWorks) was used for data analysis. Statistical analysis was performed with Sigma Plot Statistics for Windows (v 13.0; Systat Software). The primary statistical analysis included a 1-way analysis of variance (ANOVA) for each dependent variable. For ANOVAs that were deemed significant, a Tukey post hoc test was performed to further analyze which groups were different. Statistical significance was defined as P ≤ .05, and the desired power level was set at 0.8.
The Shapiro-Wilk test was used to confirm that each data set followed a normal distribution. A nonparametric test, the Kruskal-Wallis, was used for data sets that failed this test. For Kruskal-Wallis tests that found significance, a Tukey post hoc test was conducted to further analyze the differences.
The mean power value of all 1-way ANOVAs was 0.845, which was higher than the desired power level of 0.8, leading us to conclude that our sample size was sufficient.
Results
The results (mean ± SD) for each device are presented in Table 1. The P values for all the tests among devices are reported in Table 2.
TABLE 1.
Results for Each Device Testeda
| Adjustable-Loop Devices | Fixed-Loop Devices | ||||
|---|---|---|---|---|---|
| GraftMax | TightRope | Ultrabutton | Endobutton | RetroButton | |
| Initial force, N | 95.5 ± 58.0 | 206.3 ± 9.1 | 193.6 ± 16.6 | 124.3 ± 27.8 | 124.5 ± 34.1 |
| Force loss, N | –22.3 ± 28.2 | –89.5 ± 18.6 | –105.9 ± 13.5 | –44.7 ± 14.6 | –38.0 ± 8.6 |
| Elongation, mm | |||||
| Initial | 0.99 ± 0.60 | –0.36 ± 0.22 | 0.60 ± 0.39 | 0.50 ± 0.36 | 0.70 ± 0.44 |
| Dynamic | 3.10 ± 1.22 | 3.14 ± 0.88 | 2.16 ± 0.59 | 2.35 ± 0.59 | 2.15 ± 0.65 |
| Total | 4.13 ± 1.46 | 2.78 ± 0.85 | 2.76 ± 0.45 | 2.85 ± 0.74 | 2.85 ± 1.03 |
| Specimens with >3 mm of total elongation, n | 6 | 1 | 2 | 1 | 3 |
| Ultimate failure load, N | 761 ± 150 | 958 ± 40 | 746 ± 180 | 712 ± 78 | 689 ± 134 |
| Stiffness, N/mm | 193.3 ± 17.4 | 169.9 ± 13.9 | 215.2 ± 30.0 | 186.9 ± 19.4 | 207.1 ± 18.8 |
| Method of failure, % | Suture slippage (62.5) Graft slippage (25) Combination (12.5) | TR suture rupture (75) Bone breakage (25) | Bone breakage (37.5) Button breakage (12.5) Combination (12.5) Graft slippage (37.5) | Bone breakage (50) Graft slippage (25) Combination (25) | Bone breakage (50) Graft slippage (25) Suture rupture (25) |
aResults are presented as mean ± SD unless noted otherwise. TR, TightRope.
TABLE 2.
P Values for Each Tukey Post Hoc Analysisa
| Adjustable-Loop Devices | Fixed-Loop Devices | ||||
|---|---|---|---|---|---|
| GraftMax | TightRope | Ultrabutton | Endobutton | RetroButton | |
| Initial force, N | |||||
| GraftMax | — | <.001 (<.001) | <.001 (.008) | .440 (.933) | .431 (.945) |
| TightRope | <.001 (<.001) | — | .942 (.939) | <.001 (.008) | <.001 (.007) |
| Ultrabutton | <.001 (.008) | .942 (.939) | — | .002 (.081) | .002 (.073) |
| Endobutton | .440 (.933) | <.001 (.008) | .002 (.081) | — | ≥.999 (≥.999) |
| RetroButton | .431 (.945) | <.001 (.007) | .002 (.073) | ≥.999 (≥.999) | — |
| Force loss, N | |||||
| GraftMax | — | <.001 (.002) | <.001 (<.001) | .100 (.689) | .413 (.881) |
| TightRope | <.001 (.002) | — | .284 (.905) | <.001 (.123) | <.001 (.049) |
| Ultrabutton | <.001 (<.001) | .284 (.905) | — | <.001 (.010) | <.001 (.003) |
| Endobutton | .111 (.689) | <.001 (.123) | <.001 (.010) | — | .942 (.996) |
| RetroButton | .413 (.881) | <.001 (.049) | <.001 (.003) | .942 (.996) | — |
| Initial elongation, mm | |||||
| GraftMax | — | <.001 | .339 | .158 | .635 |
| TightRope | <.001 | — | <.001 | .002 | <.001 |
| Ultrabutton | .339 | <.001 | — | .992 | .987 |
| Endobutton | .158 | .002 | .992 | — | .879 |
| RetroButton | .635 | <.001 | .987 | .879 | — |
| Dynamic elongation, mm | |||||
| GraftMax | — | ≥.999 (.992) | .182 (.163) | .378 (.495) | .169 (.195) |
| TightRope | ≥.999 (.992) | — | .150 (.055) | .324 (.241) | .139 (.069) |
| Ultrabutton | .182 (.163) | .150 (.055) | — | .992 (.968) | ≥.999 (≥.999) |
| Endobutton | .378 (.495) | .324 (.241) | .992 (.968) | — | .989 (.981) |
| RetroButton | .169 (.195) | .139 (.069) | ≥.999 (≥.999) | .989 (.981) | — |
| Total elongation, mm | |||||
| GraftMax | — | .058 (.095) | .054 (.227) | .081 (.183) | .082 (.261) |
| TightRope | .058 (.095) | — | ≥.999 (.995) | ≥.999 (.998) | ≥.999 (.990) |
| Ultrabutton | .054 (.227) | ≥.999 (.995) | — | ≥.999 (≥.999) | ≥.999 (≥.999) |
| Endobutton | .081 (.183) | ≥.999 (.998) | ≥.999 (≥.999) | — | ≥.999 (≥.999) |
| RetroButton | .082 (.261) | ≥.999 (.990) | ≥.999 (≥.999) | ≥.999 (≥.999) | — |
| Ultimate failure load, N | |||||
| GraftMax | — | .030 | .999 | .937 | .782 |
| TightRope | .030 | — | .016 | .004 | .002 |
| Ultrabutton | .999 | .016 | — | .984 | .896 |
| Endobutton | .937 | .004 | .984 | — | .996 |
| RetroButton | .782 | .002 | .896 | .996 | — |
| Stiffness, N/mm | |||||
| GraftMax | — | .177 | .234 | .971 | .673 |
| TightRope | .177 | — | <.001 | .476 | .008 |
| Ultrabutton | .234 | <.001 | — | .068 | .932 |
| Endobutton | .971 | .476 | .068 | — | .309 |
| RetroButton | .673 | .008 | .932 | .309 | — |
aP values in parentheses correspond to Tukey post hoc analysis after nonparametric Kruskal-Wallis test.
Position Control Loading
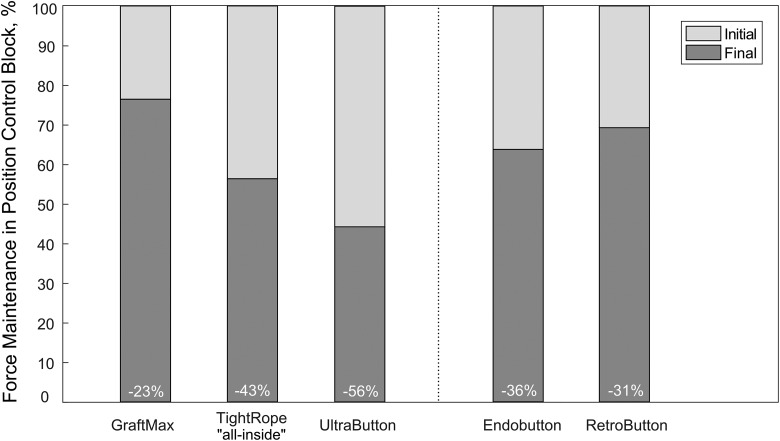
A Kruskal-Wallis test was run to analyze statistical differences in the initial force, as the data did not follow a normal distribution. There was a significant difference between the TR and GM (P < .001), the TR and both FLDs (EB, P = .008; RB, P = .007), and the UB and GM (P = .008) (Figure 4).
Figure 4.
Initial and final force for each device during position-controlled loading block.
The force loss, which was calculated as the difference between the initial and final force data, did not follow a normal distribution; thus, a Kruskal-Wallis test was performed. The change in force for the GM was significantly less than for the UB (P < .001) or the TR (P = .002). The UB had a significantly greater decrease in force than both FLDs. The TR had a significantly greater decrease in force compared with the RB (P = .049) but not the EB.
Force Control Loading
The TR was the only device for which a negative initial elongation was observed. There was a significant difference in initial elongation between the TR and all other devices. No other statistical significance was found among other devices.
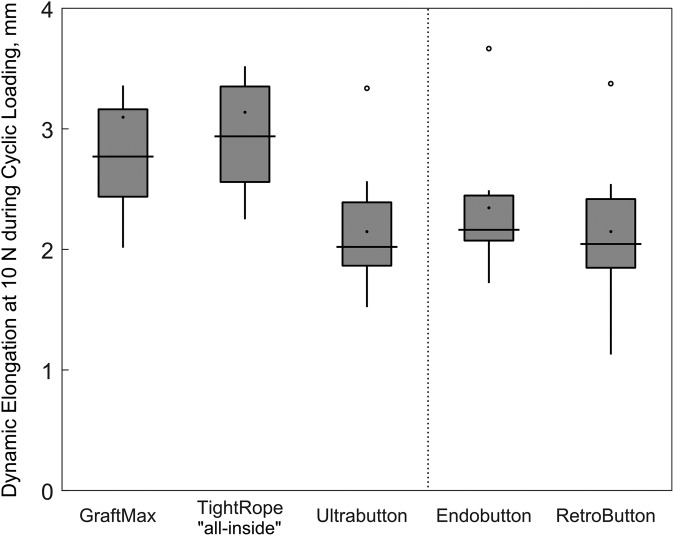
The 1-way ANOVA for dynamic elongation failed the normality test, so a Kruskal-Wallis test was run. No statistically significant differences were found among any of the devices. The power observed for dynamic elongation in the ANOVA was 0.521, which was below the desired power level of 0.8. Dynamic elongation for each device is illustrated in Figure 5.
Figure 5.
Box-and-whisker plot depicting dynamic elongation for each device. The dynamic elongation represents a relative valley elongation during force-controlled cyclic loading. Values are presented as median (line), interquartile range (box), and 95% CI (vertical lines). Black points and circles indicate mean values and outliers, respectively.
For total elongation, the data sets also did not all follow a normal distribution, and a Kruskal-Wallis test was run. There were no significant differences among the groups (P = .094). The GM had the largest total elongation, with 6 of 8 specimens exceeding the 3-mm threshold of clinical failure, followed by the FLDs, TR, and UB (Figure 6). The post hoc power analysis reported that the power for total elongation was 0.546, which was lower than the desired level of 0.8.
Figure 6.
Box-and-whisker plot depicting initial and total elongation for each device. The initial elongation was measured as the valley elongation from the start of testing until the first cycle of the force control block was completed. The total elongation is the sum of initial and dynamic elongation. Values are presented as median (line), interquartile range (box), and 95% CI (vertical lines). Black points and circles indicate mean values and outliers, respectively.
Ultimate Failure Load
The largest pull-to-failure force was observed for the TR, which was statistically significantly different than all other devices (Figure 7). No other statistically significant differences were observed. Regarding ultimate stiffness, the TR had a significantly decreased stiffness when compared with the UB (P < .001) and the RB (P = .008). The methods of failure differed among the devices. The main failure mode was suture slippage for the GM, suture rupture for the TR, and button breakage combined with femoral bone breakage for the UB. For the FLDs, the main methods of failure were either femoral bone breakage or graft slippage.
Figure 7.
Box-and-whisker plot depicting ultimate failure load for each device. Values are presented as median (line), interquartile range (box), and 95% CI (vertical lines). Black points indicate mean values.
Discussion
This full–ACL construct study included a test methodology with intraoperative graft preconditioning containing precycling and retensioning according to a surgical technique workflow under in vitro loading parameters that replicate the in vivo ACL environment. All clinically relevant treatment options for FLDs and ALDs were utilized to objectively compare the devices and allow for graft optimization. Retensioning is a major benefit for ALDs, which is not possible for FLDs after primary fixation. During precycling, which simulates intraoperative knee flexion, primary elongations are due to the settling effects of the ACLR. Apart from possible adverse effects of excessive graft tensioning, such as abnormal articulation and cartilage or graft degeneration,3,34 the benefit of retensioning ALDs was shown to eliminate these elongations and further optimize graft tension to mitigate ultimate knee laxity.29
Retensioning allows for ALDs to establish a higher initial force level as compared with an FLD within the position control block. During simulated knee flexion in the position control block, a slack was introduced within the graft, similar to ACL behavior during midflexion angles.4,31,37 This repetitive graft loading-unloading situation (0 N) creates an unfavorable loading condition for an ALD to experience loop lengthening after ACLR, which could be a reason for a greater force loss at reaching the end of position control. An FLD is not affected by a complete unloading situation, owing to its continuous loop design. Within this study, the all-inside TR constructs demonstrated the highest initial force and, at a force loss of 43%, the highest absolute final force. Because our test protocol included position-controlled cycling simulating early rehabilitation, it can be assumed that the high tension that was built up during intraoperative preconditioning would also be preserved in the patient’s knee over the first days subsequent to ACLR.
In our study, the UB and the GM revealed the highest and lowest force loss during position-controlled loading, with a 54% and 23% decrease as referenced to initial force, respectively. The GM could not be retensioned to achieve the desired 200-N level in full extension and had an absolute lower initial force of 95.5 N (see the online Video Supplement). The GM button with its locking mechanism design limits the retensioning. During loop shortening, the pulling sutures lift up the locking suture loop to allow for adjustable loop shortening. After the pulling sutures are released, the locking suture loop above the button is given slack and moves downward with the pulling sutures into the button-locking pocket when tension is applied on the graft side. Although retensioning was performed several times, no further increase of the initial force was possible, owing to this locking-unlocking mechanism. Only the GM utilizes a button-locking mechanism, while the TR and UB utilize a suture-locking mechanism. The lower absolute initial force level as well as the difference in the locking mechanism might explain the decreased force loss during position control cycling.
However, there are differences among the ALDs, as evidenced by the amount of initial elongation. For TR constructs, a negative initial elongation was observed. This finding suggests that retensioning was more effective for this device, resulting in the highest initial force level. Combined with a decreased force loss, less elongation was needed to reach the valley load of 10 N after the first peak of 250 N in force-controlled cyclic loading. This resulted in a significant difference in initial elongation between an all-inside graft with TR and all other devices utilizing tibial screw fixation (P = .002 for EB; P < .001 for GM, UB, and RB).
The majority of ACLR elongation (dynamic) occurred during the force-controlled cyclic loading, which could be attributed to graft viscoelastic stretching of the soft tissue material and to fixation device elongation. Because no statistically significant differences were found among any of the devices, it could be assumed that the stretching of the soft tissue material plays the major role for ACLR elongation. However, the statistical analysis regarding the dynamic elongation was underpowered. The highest amount of dynamic elongation was assessed for the TR, which might be a result of a longer elongation distance owing to an increased graft construct length with extracortical fixation points and all-inside graft preparation.
Another difference that was noted but not statistically different among devices was total elongation. As clinical failure of ACLR was reported as a side-to-side difference of >3 mm as measured with the KT-1000 during anterior tibial translation, we used this value as the failure threshold for total elongation.13 The GM was the only device with a total elongation >3 mm (6 of 8 specimens exceeded the threshold), which may be explained by the aforementioned retensioning limitation resulting in higher initial elongation.
The pull-to-failure loads for all ALD constructs were greater than the forces experienced during walking and early rehabilitation of the ACL33; therefore, all the ALDs can be interpreted as a suitable fixation option when benchmarked to FLDs. The TR had the largest pull-to-failure load (P ≤ .030 compared with all other constructs), followed by the GM and UB. A possible explanation of this finding is the graft preparation for an all-inside technique with both-sided cortical button fixation rather than an interference screw for tibial graft fixation. Tibial fixation was previously considered to be the weakest link of a construct, with lower pull-to-failure loads. This was also proved in our model.10,25,32,35
Additionally, Chandrashekar et al11 reported a stiffness of 199 ± 88 N/mm and 308 ± 89 N/mm for females and males, respectively, leading to a combined mean stiffness of 250 ± 102 N/mm. Stiffness results of ACL constructs tested in this study were within the overall range (111-397 N/mm), especially for male patients on the lower side. The lowest stiffness was observed for all-inside constructs with TR, which can be explained by the longer elongation distance of this technique. Especially for those less-stiff graft constructs, a high initial tension is beneficial, as shown by Amis and Jakob.3
For the UB and GM, it was difficult to compare findings with other studies, as these devices have not been formally tested at the time of this study. Furthermore, comparison between the test results of our study and others is challenging owing to differences of the test protocol and test setup.
Limitations of this study include the use of porcine tibias and femurs as well as bovine tendons as substitutes for human tissue. However, these have been shown to best resemble human bones in density1,28 and tendons. Force application was done in line with the tunnel axis, which differs from clinical observations but is in accordance with a worst-case loading scenario for ACLR testing.
Statistical analysis regarding dynamic and total elongation did not show significant difference yet was underpowered. This increases the likelihood of not detecting an existing difference and thereby constitutes a further limitation to this study.
Moreover, this study was designed as a surgical technique–based study allowing ideal circumstances for each device. Therefore, the TR was tested as an all-inside construct with graft tunnel docking, and the GM was retensioned at 30° instead of 0° of flexion. This means that devices were used in an application-oriented manner at the expense of full comparability. We felt that the limitation resulting from the chosen study design was acceptable with regard to the benefits.
This time-zero in vitro biomechanical study does not factor in any postoperative bone healing that might occur and cause mitigated knee laxity. Clinical studies are required to further support the GM and UB devices. This biomechanical model may also facilitate additional research as techniques and devices advance.
Conclusion
The results of the current study suggest that ALDs behave comparably with FLDs with regard to biomechanical fixation strength and ultimate knee laxity while also having unique advantages as compared with FLDs, such as an increased bone-tendon interface and a simplified application. However, some differences between the tested ALDs were observed. The GM button design prevented proper retensioning, so the initial force was less than, and total elongation greater than, the TR or the UB. During complete loading-unloading situations, the UB had the largest force loss. Last, the TR achieved the smallest initial elongation with the greatest ultimate failure load as compared with all other devices.
Supplementary Material
Footnotes
A Video Supplement for this article is available at http://journals.sagepub.com/doi/suppl/10.1177/2325967118768743.
One or more of the authors have declared the following potential conflicts of interest or source of funding: P.A.S. is a consultant for Arthrex, has received hospitality payments from Arthrex and DePuy Orthopaedics, has received honoraria from Arthrex, receives royalties from Arthrex, and has received educational support from Elite Orthopedics, LLC. A.B. is a consultant for Smith & Nephew, Pivot Medical, Stryker, and Arthrex; has received hospitality payments from Smith & Nephew and Arthrex; has received honoraria from Smith & Nephew, Arthrex, and CDC Medical LLC; receives royalties from Smith & Nephew; and has received payments for education purposes from Arthrex and CDC Medical LLC. M.P., S.K.S., S.B., and C.A.W. are employed by Arthrex.
Ethical approval was not sought for the present study.
References
- 1. Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad CS, Gardner TR, Groh M, Arnouk J, Levine WN. Mechanical properties of soft tissue femoral fixation devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):635–640. [DOI] [PubMed] [Google Scholar]
- 3. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998;6(suppl 1):S2–S12. [DOI] [PubMed] [Google Scholar]
- 4. Arnold MP, Verdonschot N, van Kampen A. ACL graft can replicate the normal ligament’s tension curve. Knee Surg Sports Traumatol Arthrosc. 2005;13(8):625–631. [DOI] [PubMed] [Google Scholar]
- 5. Arthrex. Continuous Loop GraftLink ACL Reconstruction Surgical Technique. Vol LT10167B Naples, FL: Arthrex Inc; 2015. [Google Scholar]
- 6. Arthrex. New Approaches to All-Inside ACL RetroConstruction Surgical Technique. Vol LT20180A Naples, FL: Arthrex Inc; 2013. [Google Scholar]
- 7. Arthrex. SpeedWhip Technique With FiberLoop and TigerLoop. Vol LT0135D. Naples, FL: Arthrex Inc; 2011. [Google Scholar]
- 8. Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC. Femoral suspension devices for anterior cruciate ligament reconstruction: do adjustable loops lengthen? Am J Sports Med. 2014;42(2):343–349. [DOI] [PubMed] [Google Scholar]
- 9. Boyle MJ, Vovos TJ, Walker CG, Stabile KJ, Roth JM, Garrett WE., Jr Does adjustable-loop femoral cortical suspension loosen after anterior cruciate ligament reconstruction? A retrospective comparative study. Knee. 2015;22(4):304–308. [DOI] [PubMed] [Google Scholar]
- 10. Brand J, Weiler A, Caborn DNM, Brown CH, Johnson DL. Graft fixation in cruciate ligament reconstruction. Am J Sports Med. 2000;28(5):761–774. [DOI] [PubMed] [Google Scholar]
- 11. Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39(16):2943–2950. [DOI] [PubMed] [Google Scholar]
- 12. Brown CH, Spalding T. Single-Bundle ACL Reconstruction: Medial Portal Technique. Vol 10600926A Andover, MA: Smith & Nephew Inc; 2012. [Google Scholar]
- 13. Daniel DM, Stone ML, Sachs R, Malcom L. Instrumented measurement of anterior knee laxity in patients with acute anterior cruciate ligament disruption. Am J Sports Med. 1985;13(6):401–407. [DOI] [PubMed] [Google Scholar]
- 14. DeBerardino TM, Smith PA, Cook JL. Femoral suspension devices for anterior cruciate ligament reconstruction: letter to the editor. Am J Sports Med. 2014;42(2):NP15–NP16. [DOI] [PubMed] [Google Scholar]
- 15. Eguchi A, Ochi M, Adachi N, Deie M, Nakamae A, Usman MA. Mechanical properties of suspensory fixation devices for anterior cruciate ligament reconstruction: comparison of the fixed-length loop device versus the adjustable-length loop device. Knee. 2014;21(3):743–748. [DOI] [PubMed] [Google Scholar]
- 16. Fuss FK. Anatomy and function of the cruciate ligaments of the domestic pig (Sus scrofa domestica): a comparison with human cruciates. J Anat. 1991;178:11–20. [PMC free article] [PubMed] [Google Scholar]
- 17. Haut Donahue TL, Gregersen C, Hull ML, Howell SM. Erratum: “comparison of viscoelastic, structural, and material properties of double-looped anterior cruciate ligament grafts made from bovine digital extensor and human hamstring tendons” [J Biomech Eng. 2001;123(2):162-169]. J Biomech Eng. 2001;123(5):523. [DOI] [PubMed] [Google Scholar]
- 18. Johnson JS, Smith SD, LaPrade CM, Turnbull TL, LaPrade RF, Wijdicks CA. A biomechanical comparison of femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction under high loads. Am J Sports Med. 2015;43(1):154–160. [DOI] [PubMed] [Google Scholar]
- 19. Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994–1000. [DOI] [PubMed] [Google Scholar]
- 20. Li G, DeFrate LE, Rubash HE, Gill TJ. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthrop Res. 2005;23(2):340–344. [DOI] [PubMed] [Google Scholar]
- 21. Lubowitz JH. All-inside anterior cruciate ligament graft link: graft preparation technique. Arthroscopy Techniques. 2012;1(2):e165–e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lubowitz JH, Ahmad CS, Anderson K. All-inside anterior cruciate ligament graft-link technique: second-generation, no-incision anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(5):717–727. [DOI] [PubMed] [Google Scholar]
- 23. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–2370. [DOI] [PubMed] [Google Scholar]
- 24. Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) Cohort Study. Arthroscopy. 2013;29(12):1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayr R, Heinrichs CH, Eichinger M, Coppola C, Schmoelz W, Attal R. Biomechanical comparison of 2 anterior cruciate ligament graft preparation techniques for tibial fixation: adjustable-length loop cortical button or interference screw. Am J Sports Med. 2015;43(6):1380–1385. [DOI] [PubMed] [Google Scholar]
- 26. Monaco E, Bachmaier S, Fabbri M, Lanzetti RM, Wijdicks CA, Ferretti A. Intraoperative workflow for all-inside anterior cruciate ligament reconstruction: an in vitro biomechanical evaluation of preconditioning and knot tying. Arthroscopy. 2018;34(2):538–545. [DOI] [PubMed] [Google Scholar]
- 27. Monaco E, Fabbri M, Lanzetti RM, Del Duca A, Labianca L, Ferretti A. Biomechanical comparison of four coupled fixation systems for ACL reconstruction with bone socket or full-tunnel on the tibial side. Knee. 2017;24(4):705–710. [DOI] [PubMed] [Google Scholar]
- 28. Nagarkatti DG, McKeon BP, Donahue BS, Fulkerson JP. Mechanical evaluation of a soft tissue interference screw in free tendon anterior cruciate ligament graft fixation. Am J Sports Med. 2001;29(1):67–71. [DOI] [PubMed] [Google Scholar]
- 29. Noonan BC, Dines JS, Allen AA, Altchek DW, Bedi A. Biomechanical evaluation of an adjustable loop suspensory anterior cruciate ligament reconstruction fixation device: the value of retensioning and knot tying. Arthroscopy. 2016;32(10):2050–2059. [DOI] [PubMed] [Google Scholar]
- 30. Petre BM, Smith SD, Jansson KS, et al. Femoral cortical suspension devices for soft tissue anterior cruciate ligament reconstruction: a comparative biomechanical study. Am J Sports Med. 2013;41(2):416–422. [DOI] [PubMed] [Google Scholar]
- 31. Rachmat HH, Janssen D, Verkerke GJ, Diercks RL, Verdonschot N. In-situ mechanical behavior and slackness of the anterior cruciate ligament at multiple knee flexion angles. Med Eng Phys. 2016;38(3):209–215. [DOI] [PubMed] [Google Scholar]
- 32. Scheffler SU, Südkamp NP, Göckenjan A, Hoffmann RFG, Weiler A. Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques. Arthroscopy. 2002;18(3):304–315. [DOI] [PubMed] [Google Scholar]
- 33. Shelburne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37(6):797–805. [DOI] [PubMed] [Google Scholar]
- 34. Shinichi Yoshiya JTA, Manley MT, Bauer TW. Graft tension in anterior cruciate ligament reconstruction: an in vivo study in dogs. Am J Sports Med. 1987;15(5):464–470. [DOI] [PubMed] [Google Scholar]
- 35. Smith PA, DeBerardino TM. Tibial fixation properties of a continuous-loop ACL hamstring graft construct with suspensory fixation in porcine bone. J Knee Surg. 2015;28(6):506–512. [DOI] [PubMed] [Google Scholar]
- 36. Smith & Nephew. ULTRABUTTON Adjustable Fixation Device. PN 71577A Andover, MA: Smith & Nephew Inc; 2016. [Google Scholar]
- 37. Wascher DC, Markolf KL, Shapiro MS, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part I: the effect of multiplane loading in the intact knee. J Bone Joint Surg Am. 1993;75(3):377–386. [DOI] [PubMed] [Google Scholar]
- 38. Wolf BR. Curved Anatomic Soft Tissue ACL Reconstruction Using GraftMax Curved Reaming, GraftMax Button, and GENESYS Matryx. Vol M2014713 Utica, NY: ConMed Corporation; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.