Abstract
Background:
The indications for the use of platelet-rich plasma (PRP) are vaguely defined despite the frequency of its use as a treatment for athletes. While select studies have advocated for its efficacy, the majority of orthopaedic research conducted on the topic has been equivocal.
Purpose:
To define the use of PRP in elite athletes by team physicians from professional sports leagues.
Study Design:
Cross-sectional study.
Methods:
A survey assessing treatment timing, usage patterns, indications, and complications was generated by fellowship-trained sports medicine orthopaedic surgeons. The survey was distributed to team physicians from the National Football League, National Basketball Association, Major League Baseball, National Hockey League, Major League Soccer, and the “Power 5” Division I conferences of the National Collegiate Athletic Association. From a compilation of publicly available email addresses and those available from professional team physician associations, 149 team physicians were sent this PRP assessment tool.
Results:
Of the 149 professional and collegiate team physicians contacted, 59 started the survey and 46 completed it, resulting in a 39.6% participation rate and a 30.9% completion rate. Approximately 93% of physicians stated that they use PRP in their practices, and 72% use ultrasonography for injection guidance. On average, collegiate team physicians and National Football League physicians treated the most players per season with PRP (69.4 and 60.4 players, respectively), while National Hockey League physicians treated the fewest (18.0 players). The majority of respondents reported no complications from PRP injections (70%), with pain being the most common complication reported (26%). There was no consensus on the most important aspect of PRP formulation, with the top 2 responses being platelet concentration (48%) and white blood cell concentration (39%). When grading the importance of indications to use PRP, physicians found athlete desire on average (7.5 ± 2.2 [SD]; out of 10) to be more important than reimbursement (2.2 ± 2.2) (P < .001). Importantly, physicians stated that they moderately (5.4 ± 2.3) believed in the evidence behind PRP. Physicians listed hamstring injuries as the most common injury treated with PRP. Hamstring injuries were treated with a mean 3.14 PRP injections, as opposed to 2.19 injections for nonhamstring injuries.
Conclusion:
Professional and collegiate team physicians frequently use PRP despite a lack of consensus regarding the importance of the formulation of the product, the timing of treatment, and the conditions that would most benefit from PRP treatment.
Keywords: platelet-rich plasma, biologic healing enhancement, PRP injection, hamstring
Derived from whole blood, platelet-rich plasma (PRP) is an autologous form of blood plasma that contains high concentrations of platelets and growth factors that have been implicated in accelerating natural healing processes. PRP utilizes centrifugation to remove erythrocytes and concentrate platelets and growth factors in a plasma base before injection.3 PRP is used in musculoskeletal injuries prior to operative treatment, intraoperatively, or following surgery to potentiate the response of cytokines and growth factors at the wound site, which can improve healing in soft and hard tissue.4 Orthopaedic surgery, oral surgery, dermatology, and plastic surgery are among the various specialties that utilize this technique.11
Despite the modern, widespread use of PRP by orthopaedic surgeons, little is known about the explicit benefits of the therapy on the musculoskeletal system.12 Presently, PRP has been investigated for use in the treatment of several musculoskeletal pathologies, the most commonplace of which is tendinopathy. One meta-analysis of 18 studies examining PRP use concluded that a single injection of PRP under ultrasonography was supported as treatment for tendinopathy.6 Given that up to 50% of all activity-related injuries are tendon disorders, these authors advocated for PRP’s utility as a sports medicine modality.2 Within the subset of tendon disorders, PRP has been specifically explored for its value as a therapy for rotator cuff tears and tendinopathy, ulnar collateral ligament tears and ruptures, hamstring strains, Achilles tendinopathy, lateral epicondylitis, and patellar tendinitis.15
Specifically, PRP usage has been linked to lower rotator cuff retear rates,19 faster return-to-play time in ulnar collateral ligament ruptures,16 faster return to play following hamstring injuries,13 extended relief and improved healing in lateral epicondylitis,8,20 and improved pain control and function in patellar tendinitis.5 These studies are in contrast with numerous tendinous studies demonstrating no benefit in the usage of PRP.10,15
While there are broad indications for PRP usage for tendinous injuries in athletes, the evidence behind usage in specific etiologies is often equivocal. Despite the extensive usage of PRP, little is known regarding how team physicians use this treatment modality in elite athletes. Based on mixed literature and broad indications, the goal of this study was to define the treatment criteria, composition, timing, and complications of PRP usage for elite athletes. We hypothesized that team physicians use different formulations of PRP with varying timing and treatment criteria among elite athletes.
Methods
Exemption from institutional review board evaluation was granted, as no patient-specific information was gathered or analyzed. A survey assessing treatment timing, usage patterns, indications, and complications was generated by fellowship-trained sports medicine orthopaedic surgeons. The survey consisted of demographic questions regarding league, university, and team affiliations in addition to 14 clinical questions. Questions assessed timing, frequency, motivations behind usage, and etiology-specific items. While some items utilized a 1-10 Likert scale, others were multiple choice or free-form. The survey is available in the Appendix.
The survey was distributed to team physicians from the National Football League (NFL), National Basketball Association, Major League Baseball, National Hockey League (NHL), Major League Soccer, and the “Power 5” Division I conferences of the National Collegiate Athletic Association. Publicly available email addresses and those available from professional team physician associations were compiled, and these team physicians were sent the PRP assessment survey. All responses were anonymous to encourage honest completion. As the study aimed to assess orthopaedic team physician usage patterns, all other specialties, including neurosurgery and primary care, were excluded.
Statistical Analysis
Responses were collected via independent survey software (Qualtrics). Unpaired Student t tests were utilized for 2-array comparisons of continuous data. For comparison across ≥3 subsets, 1-way analysis of variance was utilized. Correlations were also tested among categorical and quantitative responses. Statistical significance was set at a P value <.05.
Results
Of the 149 professional and collegiate team physicians contacted, 59 started the survey and 46 completed it, resulting in a 39.6% participation rate and a 30.9% completion rate. Approximately 93% of physicians surveyed stated that they currently use PRP in their practices and that they began using this product in 2010 ± 2 years (median ± interquartile range). Approximately 98% of physicians stated that they use PRP injections in the office setting, as opposed to 41% who endorsed using it in the operating room. Three physicians reported no longer using PRP in practice; however, they had previously injected patients in the office setting. Finally, more than half (56%) of physicians stated that they would treat all levels of athletes with PRP (recreational through professional). These demographics are summarized in Table 1.
TABLE 1.
Demographics of Responding Physicians (N = 46)a
| Respondents | Currently Use PRP | Use US to Guide PRP | Inject PRP in the Office | Inject PRP in the OR | Use PRP in All Athlete Levels |
|---|---|---|---|---|---|
| n (%) | 43 (93) | 33 (72) | 45 (98) | 19 (41) | 26 (56) |
aOR, operating room; PRP, platelet-rich plasma; US, ultrasonography.
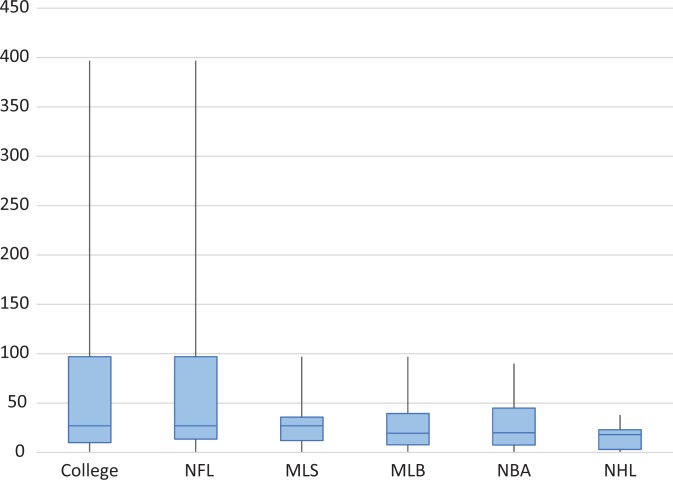
Physician respondents treated a mean ± SD of 44 ± 67 athletes with PRP in their careers. Collegiate team physicians (with 69.4 ± 100 athletes) and NFL physicians (with 60.4 ± 87.8 athletes) treated the most players in their careers with PRP, while NHL physicians (with 18.0 ± 12.7 athletes) treated the fewest. These data are in contrast with the medians and interquartile ranges for such groups: collegiate (30 ± 87 athletes), NFL (30 ± 83.5 athletes), and NHL (20 ± 20 athletes). The median number of players treated by physicians based on sports affiliation is depicted in Figure 1.
Figure 1.
Number of players treated in career by sport affiliation. Values are presented as median, interquartile range, and 95% CI. MLB, Major League Baseball; MLS, Major League Soccer; NBA, National Basketball Association; NFL, National Football League; NHL, National Hockey League.
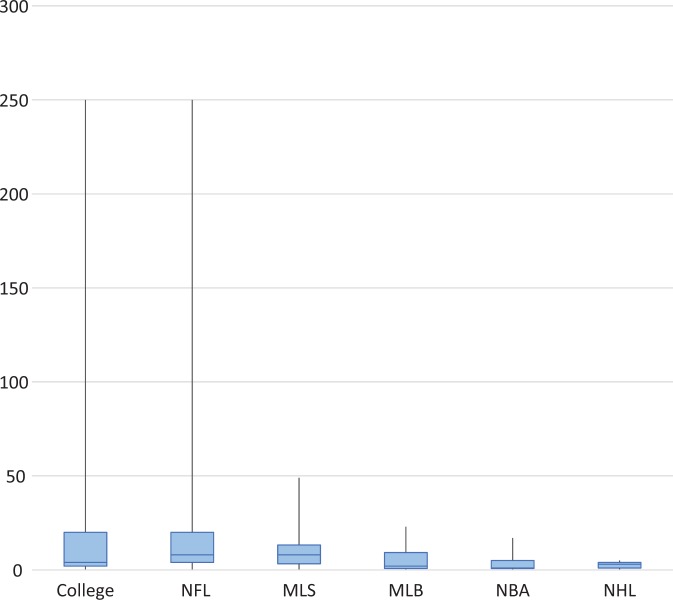
Collegiate physicians also provided the most injections (26.1 ± 60.5) in the previous sport season prior to survey administration, as closely followed by NFL physicians (23.65 ± 55.8) and with NHL physicians (2.6 ± 1.88) again treating the least. These data are again contrasted by the median and interquartile ranges for these groups: collegiate (4 ± 18), NFL (8 ± 16), and NHL (3 ± 3). The median number of injections given by physicians in the previous season based on sports affiliation is depicted in Figure 2.
Figure 2.
Injections given in the past season by sport affiliation. Values are presented as median, interquartile range, and 95% CI. MLB, Major League Baseball; MLS, Major League Soccer; NBA, National Basketball Association; NFL, National Football League; NHL, National Hockey League.
The majority of respondents reported no complications from PRP injections (70%), with pain being the most commonly reported complication (26%). The most serious complications following injection were hamstring rupture and iatrogenic calcification. The hamstring rupture occurred distally following PRP injection in the medial collateral ligament, and the calcification was observed in the hip flexors following core muscle injection. The surgeons who indicated these complications reported utilizing ultrasonography for guidance and providing only 1 injection, on average, for their most common indications for PRP. These results are summarized in Table 2.
TABLE 2.
Complications Following Injection of Platelet-Rich Plasma
| Respondents | None | Pain | Tendon Rupture | Calcification |
|---|---|---|---|---|
| n (%) | 32 (70) | 12 (26) | 1 (2) | 1 (2) |
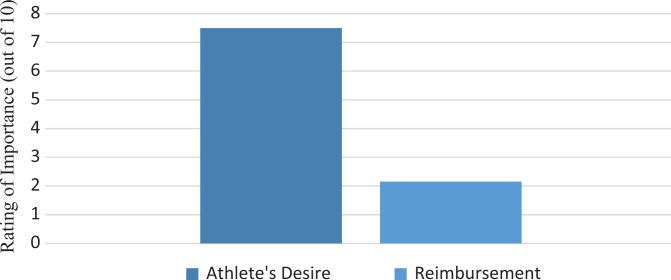
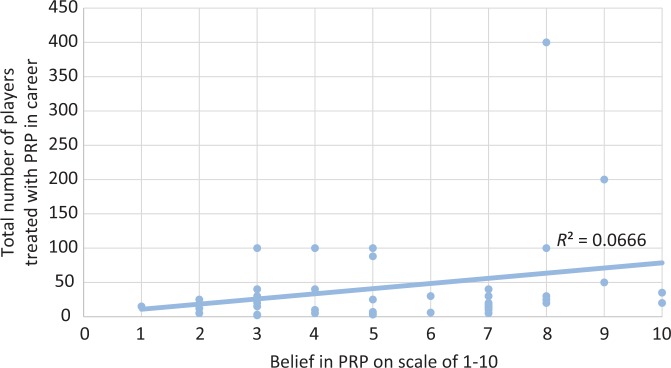
When asked to rank the importance of indications for using PRP (from 0 to 10, with 10 being the most important), physicians stated that the athlete’s desire (7.5 ± 2.2) was more important than reimbursement (2.2 ± 2.2) (P < .001); this finding is displayed in Figure 3. With regard to what aspect or biologic agent in the formulation of PRP was the most important, was there no consensus opinion, with the top 2 responses being platelet concentration (48%) and white blood cell concentration (39%). Physicians stated that they moderately (5.4 ± 2.3) believed in the evidence behind PRP. No correlation existed between the number of players treated in a physician's career and their belief in PRP (Figure 4), nor among the number of players treated and the physician's belief in PRP or the reasons motivating usage.
Figure 3.
Motivations to use platelet-rich plasma. Values are presented as means.
Figure 4.
Correlation between belief in platelet-rich plasma (PRP) and total players injected.
Regarding timing, an equal number of respondents (32.6%) stated they use PRP as first-line treatment versus a last-line treatment for a given injury. Following these answers, 19% said that they use PRP after physical therapy and rehabilitation; 13% after rest, ice, and nonsteroidal anti-inflammatory drugs; and 2% during or after surgery. When asked if they use ultrasonography to guide injections, almost three-quarters (72%) of respondents stated that they do. Physicians who listed league affiliations as both “NFL” and “collegiate” were significantly less likely to use ultrasonography to guide PRP injections as compared with the sample as a whole (P = .036).
Physicians were asked to report the 3 conditions that they most frequently use PRP to treat as well as to quantify how many injections they perform per condition. The most commonly reported condition (50%) was a hamstring strain or tear, for which an average of 3.14 injections were given. This is in contrast to 2.19, the average number of injections for all nonhamstring injuries. The next-most frequently treated conditions (26% each) were lateral epicondylitis and patellar tendinitis, for which an average of 2.73 and 2.08 injections were given, respectively. Achilles tendinopathy was the fourth-most commonly treated condition (20%), for which an average of 2.5 injections were used. These conditions and their associated number of injections are summarized in Table 3.
TABLE 3.
Conditions Most Commonly Treated With Platelet-Rich Plasmaa
| Hamstring Strain/Tear | Patellar Tendinitis | Lateral Epicondylitis | Achilles Tendinopathy | Nonspecific Tendinosis | |
|---|---|---|---|---|---|
| Respondents treating, % | 50 | 26 | 26 | 20 | 13 |
| Injections given, median ± IQR | 2 ± 1 | 1 ± 1.25 | 1 ± 1 | 2 ± 2 | 2 ± 0 |
aIQR, interquartile range.
Discussion
The results of this study demonstrate that PRP usage rates, indications, motivations, and beliefs vary considerably among team physicians. Despite only beginning to use PRP as a treatment modality in 2010 on average, the majority of team physicians now use PRP regularly. Regardless of sport, the greatest impetus behind usage appears to be athletes’ desire for PRP, which was found to be significantly more important than reimbursement (P < .001). No consensus existed in the timing of utilization, as an equal number of team physicians stated that they inject PRP as a first-line and as a last-line treatment. Team physicians almost exclusively utilized PRP for tendinous injuries, with hamstring injuries being the most common across sports.
As research behind innovative biologic treatments continues to surge, an increasing number of products are being approved for clinical use. These biologic treatments range from intraoperative rotator cuff and osteochondral defect augments to PRP and other injectable treatment options.7,9 While numerous clinical studies have evaluated the usage of PRP over the past decade, true indications are ill-defined. Moreover, sports media distributed to the lay public portrays PRP injections as a commonplace therapy for athletes and celebrities, without providing details on implementation, efficacy, or limitations.17 This study represents an important step in delineating the current usage of PRP in high-level athletes.
The results of this study demonstrate that despite broad indications and frequent usage reported by team physicians, no concordance exists across surgeons regarding the appropriate timing or frequency of use with PRP. Team physicians inject athletes with PRP as the initial treatment, an intermediate treatment, and a last-resort treatment, with no consensus regarding the number of injections that each condition warrants. Additionally, despite differences in PRP composition per specific preparation, the importance of each component to the physician is unclear. Different physicians selected every factor as the most important in PRP—from growth factor concentration to white blood cell concentration. Although the evidence is not uniform, dedicated studies have demonstrated a potential benefit for PRP usage in hamstring injuries,13 lateral epicondylitis,8,20 and patellar tendinitis,5 which were the 3 most common conditions treated with PRP by team physicians. Additionally, the majority (72%) of physicians stated that they use ultrasonography to guide injections. This practice is congruent with the literature, as double-blinded randomized controlled trials have supported the use of ultrasound-guided PRP injections as a complication-free means of providing significant improvement in athletes with proximal hamstring tendinopathy.1
Sports-specific variation was largely limited to frequency of use. While professional and collegiate football team physicians utilized PRP the most, these physicians care for teams with the largest number of players. Despite similarly sized rosters to basketball, hockey team physicians used the lowest number of injections per season and in their careers. This variation likely reflects a combination of decreased desire from hockey players, lower enthusiasm for PRP from team physicians, and an injury profile with fewer tendinopathies. No significant variation was found behind the motivations behind PRP usage, with all team physicians finding athlete desire to be the most important variable (P < .001).
Regarding complications, the results of this study demonstrated that serious complications from PRP injections were extremely rare. This is congruent with existing studies in the literature that reported no complications with PRP for treatment in chronic epicondylitis and Achilles tendon repair.14,18 Despite broad indications and often-equivocal results, this study demonstrates that complications beyond injection site pain occur with <5% frequency. Regardless, the report of iatrogenic tendon rupture reinforces the importance of safe indications, as serious sequelae are possible although rare.
Several limitations are present in this study. First, this study suffered from a low completion rate, with 46 of 149 team physicians completing the survey in its entirety. Given the broad base to which this survey was distributed, a low response rate was not unexpected but does introduce potential response bias. Next, despite anonymous completion, respondents may not have answered probing questions genuinely, owing to the nature of the study. Finally, the broad goals of the study—to outline PRP usage in elite athletes across different professional sports—likely also contributed to the variation seen in responses. This aspect was intentional, however, as we aimed to gather a more complete profile of usage rather than focus on a specific sport. Future studies may better delineate detailed treatment algorithms by focusing on the approach to specific injuries. Once physicians can agree on the timing, frequency, and composition of PRP treatment, further clinical trials may be able to better quantify the benefit of this broadly utilized biologic.
Conclusion
Orthopaedic professional and collegiate team physicians frequently utilize PRP injections, with expanding indications since its introduction approximately a decade ago. While the literature suggests that benefit is often marginal or that there is no benefit at all beyond specific etiologies, PRP has evolved into a standard treatment in the armamentarium of team physicians owing to its biologic potential. According to the study findings, team physicians disagree on the timing of usage, the importance of the PRP composition, and the specific conditions that they treat with PRP. Additionally, these physicians appear to cede to the athlete’s desire for treatment as the most important indication for treatment. Despite the apparent in vivo benefits of treating musculoskeletal injuries with autologous injections with high concentrations of platelets and growth factors, more research is needed to hone the timing, composition, and etiologies treated with PRP to optimize outcomes.
Appendix
Survey: PRP Usage by Team Physicians
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: C.S.A. receives royalties from Arthrex and is a consultant for Arthrex. T.S.L. is a consultant for Smith & Nephew.
Ethical approval was not sought for the present study.
References
- 1. Davenport KL, Campos JS, Nguyen J, Saboeiro G, Adler RS, Moley PJ. Ultrasound-guided intratendinous injections with platelet-rich plasma or autologous whole blood for treatment of proximal hamstring tendinopathy. J Ultrasound Med. 2015;34(8):1455–1463. [DOI] [PubMed] [Google Scholar]
- 2. De Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. [DOI] [PubMed] [Google Scholar]
- 3. Dhurat R, Sukesh MS. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7(4):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114(6):1502–1508. [DOI] [PubMed] [Google Scholar]
- 5. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34(6):909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzpatrick J, Bulsara M, Zheng MH. The effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2017;45(1):226–233. [DOI] [PubMed] [Google Scholar]
- 7. Fortier LA, Chapman HS, Pownder SL, et al. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44(9):2366–2374. [DOI] [PubMed] [Google Scholar]
- 8. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg. 2015;23(1):1–5 [DOI] [PubMed] [Google Scholar]
- 9. Gillespie RJ, Knapik DM, Akkus O. Biologic and synthetic grafts in the reconstruction of large to massive rotator cuff tears. J Am Acad Orthop Surg. 2016;24(12):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton B, Tol JL, Almusa E, et al. Platelet-rich plasma does not enhance return to play in hamstring injuries: a randomised controlled trial. Br J Sports Med. 2015;49(14):943–950. [DOI] [PubMed] [Google Scholar]
- 11. Lacci KM, Dardik A. Platelet-rich plasma: support for its use in wound healing. Yale J Biol Med. 2010;83(1):1. [PMC free article] [PubMed] [Google Scholar]
- 12. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. [DOI] [PubMed] [Google Scholar]
- 13. Mejia HA, Bradley JP. The effects of platelet-rich plasma on muscle: basic science and clinical application. Oper Tech Sports Med. 2011;19(3):149–153. [Google Scholar]
- 14. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774–1778. [DOI] [PubMed] [Google Scholar]
- 15. Mlynarek RA, Kuhn AW, Bedi A. Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop (Belle Mead NJ). 2016;45(5):290–326. [PubMed] [Google Scholar]
- 16. Podesta L, Crow SA, Volkmer D, Bert T, Yocum LA. Treatment of partial ulnar collateral ligament tears in the elbow with platelet-rich plasma. Am J Sports Med. 2013;41(7):1689–1694. [DOI] [PubMed] [Google Scholar]
- 17. Rachul C, Rasko JE, Caulfield T. Implicit hype? Representations of platelet rich plasma in the news media. PloS One. 2017;12(8):e0182496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245–251. [DOI] [PubMed] [Google Scholar]
- 19. Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. [DOI] [PubMed] [Google Scholar]
- 20. Yadav R, Kothari SY, Borah D. Comparison of local injection of platelet rich plasma and corticosteroids in the treatment of lateral epicondylitis of humerus. J Clin Diagn Res. 2015;9(7):RC05–RC07. [DOI] [PMC free article] [PubMed] [Google Scholar]