Abstract
Background:
The influence of graft type on recovery after anterior cruciate ligament reconstruction (ACLR) has not been adequately studied in pediatric patients.
Purpose:
To describe lower extremity functional recovery parameters at the 6-month mark after ACLR across 3 distinct groups of skeletally immature patients: pediatric male patients with transphyseal hamstring grafts (PM-HS), pediatric female patients with transphyseal hamstring grafts (PF-HS), and pediatric male patients with extraphyseal iliotibial band grafts (PM-ITB).
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Thigh circumference, knee range of motion, lower extremity strength, dynamic balance, and hop test performance were assessed in all patients 6 months postoperatively. All participants were ≤15 years of age with open physes. The limb symmetry index was used to compare deficits between the operated and uninvolved limbs for all 3 groups (PM-HS, PF-HS, and PM-ITB). Analysis of variance with post hoc correction was employed.
Results:
A total of 93 pediatric patients who underwent ACLR (PM-HS: n = 21 [mean age, 13.6 ± 1.0 years]; PF-HS: n = 33 [mean age, 13.4 ± 0.7 years]; PM-ITB: n = 39 [mean age, 12.5 ± 1.3 years]) were examined. There was no statistically significant difference in thigh circumference, range of motion, dynamic balance, or hop test performance between the groups. Of the various additional comparisons analyzed, there were statistical differences in hamstring strength deficits among the 3 groups (P = .004). The PM-HS group showed a greater hamstring strength deficit (–32.2% relative to healthy limb) than the PM-ITB group (–5.4% relative to healthy limb) (P = .012). The hamstring strength deficit of the PF-HS group (–18.7% relative to healthy limb) was less than that of the PM-HS group and greater than that of the PM-ITB group but not statistically significant in either case.
Conclusion:
Significant hamstring strength deficits were detected in the PM-HS group compared with the PM-ITB group at 6 months following ACLR. Such findings may influence decisions regarding graft selection, timing of return to sports, and postoperative rehabilitation regimens.
Keywords: ACL reconstruction, pediatric ACL, return to sports, transphyseal, extraphyseal, physeal-sparing
Anterior cruciate ligament (ACL) tears can be devastating knee injuries for physically active children. Such injuries are on the rise based on multiple epidemiological reports in the past decade.10,16 One study noted an approximately 9-fold increase in the number of ACL reconstruction (ACLR) procedures being performed on patients younger than 15 years.9 Although new clinical procedures to treat ACL tears may be emerging, such as ACL repair,18,30,47 ACLR remains the established gold standard approach for knee stabilization.6 Conservative/nonsurgical treatment options still exist after an ACL tear. However, several studies have documented a lower risk of secondary meniscus tears,28 greater knee stability,27 and better patient-reported outcomes2 in patients who undergo ACLR compared with those who undergo nonoperative treatment.
While various ACLR techniques have been described for prepubescent, skeletally immature athletes with significant growth remaining, a method devised by Micheli et al29 consists of a combined intra-articular/extra-articular extraphyseal, or “physeal-sparing,” approach that utilizes a distal tendinous iliotibial band (ITB) autograft. This technique protects the integrity and function of the distal femoral and proximal tibial physes while also providing stability in the knee joint.19,20 Recent clinical investigations have reported positive outcomes of the ITB autograft procedure in pediatric patients, including a 3.8% revision rate, favorable patient-reported functional outcome scores (including mean International Knee Documentation Committee and Lysholm scores of 96.5 ± 2.9 and 95.0 ± 6.1, respectively),46 and no growth disturbances, angular deformities, or limb-length discrepancies at a 3-year postoperative follow-up time point.46
Patients with open physes and less than 2 years of growth remaining, however, may also be suitable candidates for more traditional transphyseal reconstruction techniques with minor modifications designed to protect physeal function, despite the establishment of transphyseal tunnels. Provided that soft tissue grafts are utilized and fixation implants do not span the physes, such techniques have been shown to provide knee stability without adverse clinical sequelae for a subset of adolescents with open physes.21 Other authors have even contended that transphyseal techniques are suitable for children of any age, with several small series showing low complication rates.33,42
Despite additional research emerging to support the trend toward ACLR in skeletally immature patients, this important subpopulation demonstrates relatively higher retear rates compared with their adult counterparts.22 While large-scale clinical trials have shown the prophylactic effectiveness of neuromuscular training in an effort to reduce the occurrence of ACL injuries, especially in the female population,11,15,22,26,43 there remains a lack of investigations related to postoperative rehabilitation, functional recovery, and safe timing of return to sports after surgery. Moreover, no studies have compared the characteristics of postoperative recovery between different ACLR techniques in the skeletally immature population. Therefore, the purpose of this study was to describe a variety of lower extremity recovery parameters, including thigh circumference, knee range of motion (ROM), strength, dynamic balance, and functional hop test performance, among 3 distinct groups of skeletally immature patients: pediatric male patients who underwent ACLR with transphyseal hamstring autografts (PM-HS), pediatric female patients with transphyseal hamstring grafts (PF-HS), and pediatric male patients with extraphyseal ITB grafts (PM-ITB). There were few pediatric female patients who had extraphyseal ITB grafts, so this group was not available.
Methods
Study Design
An institutional review board approved this retrospective case-control study (level of evidence, 3). Thigh circumference, knee ROM, strength (quadriceps, hamstring, hip abductor, and hip extensor), dynamic balance, and functional hop test performance were assessed 6 months postoperatively at an injury prevention center as part of the institution’s standard-of-care follow-up protocol for ACLR. The 6-month time point was used to assess postoperative recovery and to determine return-to-play decisions for those who were willing to return to sports activities. A review of each patient’s electronic medical record was conducted to collect demographic information, such as height, weight, and age, as well as clinical information, such as concomitant meniscus tears.
Participants
Patients who were skeletally immature and ≤15 years of age at the time of surgery from 2014 to 2017, and who had sustained a complete ACL tear, underwent ACLR, and completed a set of 6-month postoperative assessments were included in this study. Skeletal maturity was evaluated through available diagnostic imaging tools such as radiography and magnetic resonance imaging, and the status of the physis of each patient was checked by an orthopaedic physician (B.E.H.). Exclusion criteria included age (>15 years), previous ipsilateral or contralateral ACL injuries or knee surgery, congenital ACL absence, and multiligamentous knee reconstruction. Based on a priori power analysis with α = .05 and β = .80, a minimum of 20 pediatric patients in each group was established as a requirement for the study.
Surgical Technique
ACLR for the PM-HS and PF-HS groups was performed by 1 of 6 different surgeons in a manner previously described.21 Five of the 6 surgeons perform both transphyseal hamstring and extraphyseal ITB techniques; the algorithmic approach of the surgical procedure selection has been previously summarized.20 Briefly, the semitendinosus and gracilis tendons were harvested in a routine fashion from a 2- to 3-cm incision just medial to the tibial tubercle. Transphyseal femoral and tibial tunnels were established with the intra-articular tunnel apertures within the footprints of the femoral and tibial ACL fibers, respectively. Suspensory fixation button-type implants with either a closed or adjustable loop were utilized for femoral graft fixation, while interference screw fixation was utilized for tibial graft fixation but with the screw lengths adjusted to ensure metaphyseal fixation with no crossing of the proximal screw tip across the proximal tibial physis.
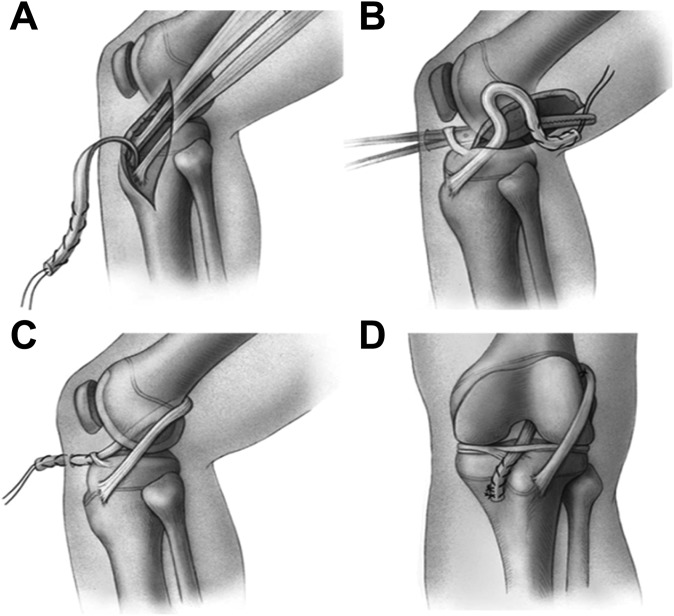
ACLR for the PM-ITB group was performed by 1 of 3 different surgeons in a manner also previously described in a separate study (Figure 1).20 The central portion of the ITB, measuring 10 to 14 mm in width and 200 to 250 mm in length, was harvested through a 4-cm lateral knee incision centered over the lateral epicondyle, leaving the distal portion attached to the Gerdy tubercle. An aperture in the posterolateral knee capsule just posterior to the native femoral ACL footprint was made with a long curved clamp introduced through the anteromedial portal and passed through the intercondylar notch. The free end of the graft was advanced through the aperture and placed provisionally in the anteromedial portal. A 3-cm incision just medial to the tibial tubercle at the level of the pes anserinus insertion was used to create a 3-mm bony trough in the anteromedial tibial metaphysis, below the level of the physis, and a rat-tailed rasp was used to create a second trough in the proximal tibial epiphysis at the native ACL tibial footprint. The free end of the graft was then advanced into the tibial incision site, running through the notch and under the intermeniscal ligament. The graft was secured with 2 to 3 figure-of-8 sutures to the capsular soft tissues at the posterolateral aspect of the lateral femoral condyle, with the knee in 90° of flexion, and on the tibial side to the thick periosteum on either side of the previously made tibial metaphyseal bony trough, with the knee in full extension.
Figure 1.
Extraphyseal iliotibial band techniques. (A) The iliotibial band graft is harvested free proximally and left attached to the Gerdy tubercle distally. (B) The graft is brought through the knee in the over-the-top position posteriorly. (C) The graft is brought through the knee and under the intermeniscal ligament anteriorly. (D) The graft is fixed to the intermuscular septum on the femoral side and to the periosteum of the proximal part of the tibia on the tibial side. Reprinted with permission from Kocher et al.20
Measurements
All data were collected by certified athletic trainers and strength and conditioning specialists at an injury prevention research and training center that was adjacent to a sports medicine and orthopaedic center of a large, academic, tertiary-level pediatric hospital. A tape measure and goniometer were used to measure thigh circumference and knee ROM, respectively, with thigh circumference (measured in cm) being measured at a point 10 cm proximal to the superior pole of the patella. The thigh circumference and knee ROM measurements were performed 1 time.
A handheld dynamometer (Hoggan Scientific) was used to evaluate the isometric strength of each patient’s quadriceps, hamstrings, hip extensors, hip abductors, and hip extensors. For quadriceps strength, participants were asked to sit down on the edge of the treatment table with 90° of knee flexion and arms crossed in front of the chest. Then, the dynamometer was applied to the anterior side of the distal tibia above the dome of the talus, and participants were asked to extend their knees with maximum effort. For hamstring strength, participants were asked to lie down on their stomach with 90° of knee flexion. The dynamometer was applied at the posterior side (Achilles tendon side) of the distal tibia, and participants were asked to further flex their knees toward the hip with maximum effort. For hip abductor strength, participants were asked to lie down on their side, and the targeted leg was slightly pulled toward the posterior and downward directions. The dynamometer was applied to the lateral aspect of the leg just above the lateral malleolus, and participants were asked to move their legs to the ceiling with maximum effort (direction of hip abduction). For hip extensor strength, participants lay on their stomach with 90° of knee flexion, the dynamometer was applied at the middle one-third of the posterior thigh (hamstring side), and participants were asked to move their flexed legs toward the ceiling.
The strength test was performed 2 times per muscle group bilaterally. Intraclass correlation coefficients were obtained to evaluate interrater and intrarater reliability before the study, which indicated the following: interrater reliability of thigh circumference: 97.8% (95% CI, 83.0%-100.0%), knee ROM: 99.2% (95% CI, 90.0%-99.9%), and muscle strength: 96.6% (95% CI, 88.6%-99.3%) and intrarater reliability of tester A: 90.8% (95% CI, 71.6%-97.0%), tester B: 98.9% (95% CI, 96.8%-99.7%), and tester C: 99.5% (95% CI, 98.5%-99.8%).
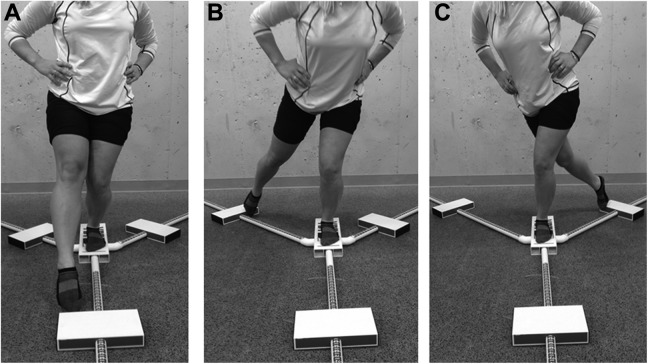
Dynamic balance was quantified using a commercially available Y-balance assessment system (Functional Movement Systems) as previously described.24 Participants were instructed to stand at the center of the equipment and push a plastic piece in the anterior, posteromedial, and posterolateral directions. This was performed 3 times in each direction bilaterally (Figure 2). The mean distance of the 3 trials, in each of the 3 directions, was used for analysis.
Figure 2.
Dynamic balance test. (A) Anterior reach: Participants stand at the center of the equipment and push a plastic piece to the anterior direction. (B) Posteromedial reach: Participants stand at the center of the equipment and push a plastic piece to the posteromedial direction. (C) Posterolateral reach: Participants stand at the center of the equipment and push a plastic piece to the posterolateral direction.
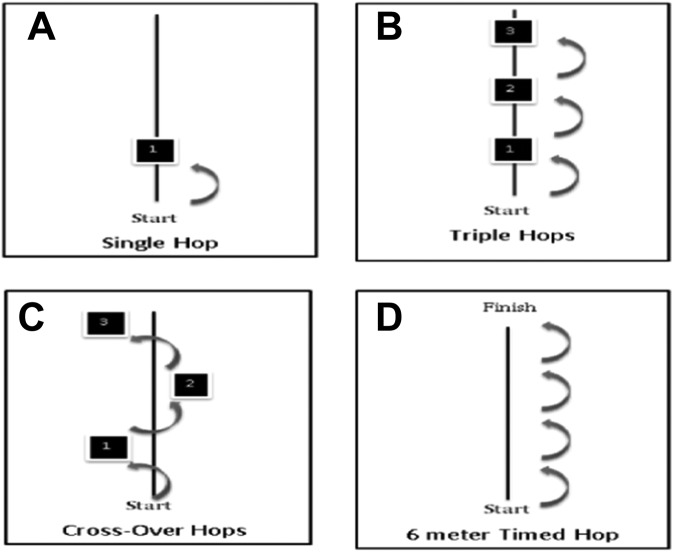
Four types of functional hop tests were performed, including single hop for distance, triple hop for distance, crossover hop for distance, and 6-m timed hop (Figure 3). In the single hop test, participants hopped with a single leg and were instructed to maintain balance upon landing for several seconds. For the triple hop test, participants hopped 3 times consecutively with a single leg and were asked to maintain balance for several seconds upon landing. In the crossover hop test, participants hopped 3 times over a middle tape measure sequentially (medial, lateral, and medial sequences) using a single leg and were again asked to maintain balance for several seconds upon landing. For the 6-m timed hop test, participants were asked to hop 6 m on a single leg as many times as necessary in one direction to cover 6 m. According to previous studies,48 a tape measure and stopwatch were used to perform the test. All hop tests were performed twice on each leg, and the mean value was calculated for data analysis. All participants wore their prescribed, custom-fit, postoperative functional knee brace during the hop tests because it simulated the condition at the time when they return to their athletic activities.
Figure 3.
Functional hop tests. (A) Single hop: Participants hop 1 time with single leg. (B) Triple hops: Participants hop 3 times consecutively with a single leg. (C) Crossover hops: Participants hop 3 times with zigzag figures (medial, lateral, and medial sequences) using a single leg. (D) Six-meter timed hops: Participants hop a 6-meter distance as fast as possible.
Data Analysis
To evaluate the involved limb’s recovery based on the uninvolved limb, limb symmetry index (LSI) scores were used. The LSI score is calculated by dividing the performance of the ACLR limb by the performance of the uninvolved limb and multiplying by 100, with 100% indicating perfect symmetry between the ACLR and uninvolved limbs.7 This technique has been employed frequently when reporting postoperative recovery status using lower extremity functional tests in previous studies with patients who have undergone ACLR.7,12 Deficits were calculated by subtracting 100 from each LSI score. Percentages were used to express both LSI scores and deficits, with negative scores indicating a deficit.
Statistical Analysis
Outcome variables analyzed included thigh circumference, knee ROM, strength (quadriceps, hamstring, hip abductor, and hip extensor), dynamic balance (anterior reach, posteromedial reach, and posterolateral reach), and functional hop test performance (single hop, triple hop, crossover hop, and 6-m timed hop). The independent variables used were the 3 pediatric patient groups: PM-HS, PF-HS, and PM-ITB. One-way analysis of variance was performed to compare differences in outcome variables among the PM-HS, PF-HS, and PM-ITB groups. When statistical significance was indicated, 3 pairwise Bonferroni post hoc comparisons ([1] PM-HS vs PF-HS, [2] PM-HS vs PM-ITB, and [3] PF-HS vs PM-ITB]) were performed. To correct inflated P values and to avoid type I errors, statistical significance within the pair comparison was reduced to P = .017 (.017 = .05/3). SPSS statistical software (version 21; IBM) was used for all analyses.
Results
A total of 93 pediatric patients who underwent ACLR were included (PM-HS: n = 21 [mean age, 13.6 ± 1.0 years]; PF-HS: n = 33 [mean age, 13.4 ± 0.7 years]; PM-ITB: n = 39 [mean age, 12.5 ± 1.3 years]). Demographic information (age, height, weight, and body mass index), meniscus injury status (repair vs meniscectomy, trephination, and/or rasping for both the medial and lateral menisci), and time from ACLR to assessments are described in Table 1.
TABLE 1.
Patient Characteristicsa
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | P Value | |
|---|---|---|---|---|
| Age,b y | 13.6 ± 1.0 | 13.4 ± 0.7 | 12.5 ± 1.3 | .000d |
| Height,c cm | 169.8 ± 8.9 | 162.8 ± 6.0 | 157.5 ± 12.2 | .000d |
| Weight, kg | 60.9 ± 9.9 | 62.9 ± 14.6 | 54.7 ± 18.0 | .068 |
| Body mass index, kg/m2 | 21.1 ± 2.8 | 23.6 ± 4.8 | 21.7 ± 5.7 | .120 |
| Meniscal tears,e n (%) | .419 | |||
| No | 14 (66.7) | 16 (48.5) | 21 (53.8) | |
| Yes | 7 (33.3) | 17 (51.5) | 18 (46.2) | |
| Medial meniscus | .492 | |||
| Repair | 2 (100.0) | 7 (77.8) | 1 (50.0) | |
| Meniscectomy, trephination, rasping | 0 (0.0) | 2 (22.2) | 1 (50.0) | |
| Lateral meniscus | .228 | |||
| Repair | 5 (83.3) | 4 (40.0) | 8 (50.0) | |
| Meniscectomy, trephination, rasping | 1 (16.7) | 6 (60.0) | 8 (50.0) | |
| Time from ACLR to measurements, mo | 6.9 ± 4.0 | 6.9 ± 3.9 | 6.9 ± 2.5 | .999 |
aValues are presented as mean ± SD unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts.
bThe PM-ITB group was significantly different from the PM-HS and PF-HS groups (P = .001 for PM-ITB vs PM-HS and P = .001 for PM-ITB vs PF-HS).
cThe PM-HS group was significantly different from the PF-HS and PM-ITB groups (P = .001 for PM-HS vs PM-ITB and P = .022 for PM-HS vs PF-HS).
dP < .05.
eOne participant in the PM-HS group and 2 participants in the PF-HS group had tears on both the medial and lateral menisci.
The mean differences between the uninvolved and ACLR limbs for thigh circumference, ROM, lower extremity strength, dynamic balance, and hop test performance are presented in Tables 2 to 5. There were no statistically significant differences in thigh circumference, ROM, dynamic balance, or functional hop test performance between the groups. However, there were statistical differences in hamstring strength deficits among the 3 groups (P = .004) (Table 6). The PM-HS group showed a greater hamstring strength deficit (–32.2% relative to healthy limb) than the PM-ITB group (–5.4% relative to healthy limb) (P = .012). The hamstring strength deficit of the PF-HS group (–18.7% relative to healthy limb) was less than that of the PM-HS group (–32.2% relative to healthy limb) and greater than that of the PM-ITB group (–5.4% relative to healthy limb) but not statistically significant (P = .038) after Bonferroni correction.
TABLE 2.
Mean Differences in Thigh Circumference and Knee Extension and Flexion ROMa
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | |
|---|---|---|---|
| Thigh circumference, cm | |||
| Uninvolved limb | 43.6 ± 3.9 (41.9-45.4) | 45.2 ± 4.8 (43.5-46.9) | 41.5 ± 5.6 (39.7-43.3) |
| ACLR limb | 42.4 ± 4.2 (40.5-44.3) | 44.2 ± 5.0 (42.4-46.0) | 40.9 ± 5.7 (39.0-42.7) |
| Mean difference | –1.2 | –1.0 | –0.6 |
| Knee extension ROM, deg | |||
| Uninvolved limb | 4.2 ± 2.3 (3.2-5.3) | 4.6 ± 3.0 (3.5-5.6) | 5.0 ± 2.9 (4.0-5.9) |
| ACLR limb | 3.4 ± 2.1 (2.4-4.4) | 4.0 ± 2.7 (3.0-5.0) | 3.9 ± 2.6 (3.0-4.7) |
| Mean difference | –0.8 | –0.6 | –1.1 |
| Knee flexion ROM, deg | |||
| Uninvolved limb | 135.5 ± 6.1 (132.8-138.3) | 138.0 ± 9.7 (134.5-141.4) | 138.8 ± 8.9 (135.8-141.7) |
| ACLR limb | 131.9 ± 7.4 (128.6-135.3) | 134.6 ± 11.2 (130.6-138.6) | 135.7 ± 9.2 (132.7-138.8) |
| Mean difference | –3.6 | –3.4 | –3.1 |
aValues are presented as mean ± SD (95% CI) unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts; ROM, range of motion.
TABLE 3.
Mean Differences in Quadriceps, Hamstring, Hip Abductor, and Hip Extensor Muscle Strengtha
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | |
|---|---|---|---|
| Quadriceps strength, N/kg | |||
| Uninvolved limb | 4.8 ± 1.0 (4.4-5.3) | 4.3 ± 1.3 (3.9-4.8) | 4.6 ± 1.1 (4.3-5.0) |
| ACLR limb | 4.8 ± 0.9 (4.4-5.2) | 4.3 ± 1.2 (3.8-4.7) | 4.4 ± 1.0 (4.1-4.7) |
| Mean difference | 0.0 | 0.0 | –0.2 |
| Hamstring strength,b N/kg | |||
| Uninvolved limb | 2.4 ± 0.6 (2.2-2.7) | 2.1 ± 0.6 (1.9-2.3) | 2.7 ± 0.8 (2.4-3.0) |
| ACLR limb | 1.6 ± 0.6 (1.3-1.9) | 1.6 ± 0.6 (1.4-1.9) | 2.6 ± 0.9 (2.3-2.8) |
| Mean difference | –0.8 | –0.5 | –0.1 |
| Hip abductor strength, N/kg | |||
| Uninvolved limb | 2.0 ± 0.8 (1.6-2.3) | 1.9 ± 0.9 (1.5-2.2) | 1.7 ± 0.5 (1.5-1.9) |
| ACLR limb | 2.1 ± 0.8 (1.7-2.4) | 2.0 ± 1.1 (1.6-2.3) | 1.7 ± 0.7 (1.5-2.0) |
| Mean difference | +0.1 | +0.1 | 0.0 |
| Hip extensor strength, N/kg | |||
| Uninvolved limb | 3.3 ± 1.1 (2.8-3.8) | 2.9 ± 1.0 (2.5-3.3) | 3.4 ± 1.0 (3.1-3.7) |
| ACLR limb | 3.5 ± 1.2 (3.0-4.1) | 2.8 ± 1.1 (2.4-3.2) | 3.4 ± 1.0 (3.1-3.7) |
| Mean difference | +0.2 | –0.1 | 0.0 |
aValues are presented as mean ± SD (95% CI) unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts.
bThe PM-HS group was significantly different from the PM-ITB group (P = .003), but there were no differences between the PM-HS and PF-HS groups (P = .304) or between the PF-HS and PM-ITB groups (P = .173).
TABLE 4.
Mean Differences in Anterior Reach, Posteromedial Reach, and Posterolateral Reacha
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | |
|---|---|---|---|
| Anterior reach, cm | |||
| Uninvolved limb | 58.8 ± 6.7 (55.8-61.8) | 57.7 ± 7.2 (55.2-60.3) | 55.4 ± 6.0 (53.5-57.3) |
| ACLR limb | 58.5 ± 5.8 (55.9-61.2) | 56.9 ± 5.8 (54.9-59.0) | 54.7 ± 8.2 (52.1-57.4) |
| Mean difference | –0.3 | –0.8 | –0.7 |
| Posteromedial reach, cm | |||
| Uninvolved limb | 99.4 ± 8.2 (95.6-103.1) | 93.1 ± 9.5 (89.7-96.4) | 95.4 ± 11.3 (91.7-99.1) |
| ACLR limb | 95.4 ± 11.0 (90.4-100.4) | 92.1 ± 11.5 (88.0-96.2) | 92.9 ± 13.9 (88.4-97.4) |
| Mean difference | –4.0 | –1.0 | –2.7 |
| Posterolateral reach, cm | |||
| Uninvolved limb | 93.8 ± 9.8 (89.4-98.3) | 91.7 ± 10.4 (88.0-95.4) | 91.5 ± 13.4 (87.2-95.9) |
| ACLR limb | 91.5 ± 10.0 (87.0-94.9) | 91.3 ± 10.1 (87.7-94.9) | 88.5 ± 16.9 (83.1-93.9) |
| Mean difference | –2.3 | –0.4 | –3.0 |
aValues are presented as mean ± SD (95% CI) unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts.
TABLE 5.
Mean Differences in Functional Hop Test Resultsa
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | |
|---|---|---|---|
| Single hop test, m | |||
| Uninvolved limb | 1.34 ± 0.23 (1.22-1.47) | 1.07 ± 0.23 (0.97-1.16) | 1.06 ± 0.21 (0.98-1.15) |
| ACLR limb | 1.28 ± 0.25 (1.15-1.41) | 0.99 ± 0.23 (0.89-1.08) | 0.95 ± 0.27 (0.84-1.05) |
| Mean difference | –0.06 | –0.08 | –0.11 |
| Triple hop test, m | |||
| Uninvolved limb | 4.50 ± 0.65 (4.16-4.83) | 3.40 ± 0.72 (3.09-3.71) | 3.50 ± 0.49 (3.30-3.70) |
| ACLR limb | 4.02 ± 0.91 (3.53-4.50) | 3.19 ± 0.70 (2.89-3.50) | 3.20 ± 0.64 (2.94-3.46) |
| Mean difference | –0.48 | –0.21 | –0.30 |
| Crossover hop test, m | |||
| Uninvolved limb | 3.73 ± 0.80 (3.27-4.20) | 3.07 ± 0.61 (2.79-3.33) | 2.86 ± 0.60 (2.60-3.12) |
| ACLR limb | 3.47 ± 0.90 (2.96-3.99) | 2.79 ± 0.65 (2.50-3.08) | 2.57 ± 0.94 (2.15-2.98) |
| Mean difference | –0.26 | –0.28 | –0.29 |
| 6-m timed hop test, s | |||
| Uninvolved limb | 2.11 ± 0.25 (1.97-2.25) | 2.60 ± 0.42 (2.42-2.78) | 2.60 ± 0.46 (2.41-2.80) |
| ACLR limb | 2.23 ± 0.34 (2.04-2.41) | 2.65 ± 0.47 (2.45-2.85) | 2.88 ± 0.69 (2.58-3.17) |
| Mean difference | –0.12 | –0.05 | –0.28 |
aValues are presented as mean ± SD (95% CI) unless otherwise specified. ACLR, anterior cruciate ligament reconstruction; PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts.
TABLE 6.
Limb Symmetry Index for Quadriceps, Hamstring, Hip Abductor, and Hip Extensor Muscle Strengtha
| PM-HS (n = 21) | PF-HS (n = 33) | PM-ITB (n = 39) | P Value | |
|---|---|---|---|---|
| Quadriceps, % | 0.8 ± 9.1 (–3.3 to 5.0) | 1.1 ± 14.5 (–4.1 to 6.4) | –3.2 ± 10.8 (–6.6 to 3.4) | .259 |
| Hamstring,b % | –32.2 ± 24.2 (–43.2 to –21.1) | –18.7 ± 40.8 (–33.1 to –4.2) | –5.4 ± 17.6 (–11.1 to 0.4) | .004c |
| Hip abductor, % | 7.4 ± 18.9 (–1.2 to 16.0) | 4.2 ± 16.6 (–1.7 to 10.1) | 2.5 ± 24.8 (–5.5 to 10.5) | .688 |
| Hip extensor,d % | 6.1 ± 13.1 (1.6 to 12.1) | –3.1 ± 14.1 (–8.1 to 1.9) | 1.2 ± 14.5 (–3.5 to 5.9) | .066 |
aValues are presented as mean ± SD (95% CI) unless otherwise specified. Negative values represent strength deficit in the involved limb. PF-HS, pediatric female patients with transphyseal hamstring grafts; PM-HS, pediatric male patients with transphyseal hamstring grafts; PM-ITB, pediatric male patients with extraphyseal iliotibial band grafts.
bThe PM-HS group was significantly different from the PM-ITB group (P = .012).
cP < .05.
dThe PM-HS group was not significantly different from the PF-HS group (P = .038) after Bonferroni correction was made (P = .017).
Discussion
These data demonstrate greater deficits in hamstring strength in the PM-HS group than in the PM-ITB group (Table 6). It is logical to believe that the hamstring strength deficits stem from surgical harvesting of the hamstring tendons. The question is why the significant hamstring deficits were observed in the PM-HS group but not in the PF-HS group. The greater hamstring strength of the uninvolved limb in the PM-HS group relative to the PF-HS group might have facilitated a difficulty in achieving adequate strength recovery after ACLR, although this difference was not statistically significant (see Table 3). Or, perhaps, even though the ages of the PM-HS and PF-HS groups were comparable (see Table 1), skeletal ages might have been different between the PM-HS and PF-HS groups and potentially influenced the current results. Regardless, these data suggest a need for additional rehabilitation or perhaps a greater duration of time before a safe return to play to regain adequate hamstring strength.
Several investigations have shown that ACL reinjury rates in teenage athletes range from 24% to 32%,8,37,44 and an ACL reinjury often occurs within 2 years from ACLR.23,35,36,39 A study conducted by Nomura et al32 showed that a subset of patients who underwent ACLR with hamstring grafts did not demonstrate a regeneration of the semitendinosus after ACLR, and another study indicated muscle deficits 2 years after ACLR.5 The synthesis of this evidence indicates that it may take several more months past this study’s 6-month postoperative time point for these hamstring deficits to diminish. Along with the deficits, a few studies have investigated asymmetry between the involved and uninvolved limbs through biomechanical, neuromuscular, and functional variables.17,40,41,49 Among those studies, an investigation performed by Abourezk et al1 indicated that young adults (mean age, 22.6 ± 4.9 years) who had hamstring autografts with greater hamstring strength asymmetry showed different gait mechanics 3 years after ACLR. The long-term strength asymmetry and mechanical differences may potentially lead to a compensatory mechanism, which may further link to ACL reinjuries in the uninvolved limb. A recent meta-analysis showed that the risk of ACL reinjuries between involved and uninvolved limbs is approximately the same.45
In addition to the effect of graft type and hamstring strength deficits, maturation of the reconstructed graft has also been discussed,31,38 and studies have suggested a longer recovery duration after ACLR to facilitate a safe return to play.31 Another study with a cohort of young adults (mean age, 24.3 ± 7.3 years) reported reductions in ACL reinjury rates when return-to-play timing was extended to 9 months after ACLR.13 In short, to track the recovery process of these strength deficits, a more comprehensive longitudinal investigation is warranted.
Another interesting finding is that there were no statistically significant differences in the strength of other musculature structures (Table 6), dynamic balance, or functional hop test performance. For dynamic balance, all deficits were less than 5%. Similarly, deficits in functional hop test performance were all less than 12%. It seems odd that hamstring strength deficits in this study were not reflective of dynamic balance and functional hop test performance. However, there may be a synergistic development of other muscle groups in the operated lower extremity relative to the affected hamstring. Hip abductor and hip extensor strength of the involved limb in the PM-HS group was actually greater than the uninvolved limb (see Table 3). Although statistical significance was not detected, hip abductor (+7.4%) and hip extensor strength (+6.1%) deficits of the PM-HS group were greater than strength deficits in the PF-HS and PM-ITB groups (Table 6). In addition to the PM-HS group, the PF-HS group (+4.2%) and PM-ITB group (+2.5%) also demonstrated greater hip abductor strength deficits in the involved limb compared with the uninvolved limb (Table 6).
The increased hip musculature may be a compensatory mechanism due to a lack of hamstring strength, or it may simply reflect the training of muscles not recruited or trained as frequently on the nonoperated lower extremity at a baseline level as that which occurred in the postoperative physical therapy program. Other musculature structures might have been recruited to compensate for the lack of strength in weaker hamstring muscles during physical therapy, activities of daily living, and reconditioning training.
Similar findings were reported by Bell et al.3 In their study, young adults (aged 18-25 years) recovering from ACLR who had low quadriceps strength developed significantly greater hip extensor strength approximately 2.5 years postoperatively. This potential compensatory mechanism may also account for the lack of differences in dynamic balance and functional hop test performance among the 3 groups in this study. Further investigation is necessary to understand the long-term effects of decreased hamstring strength and increased hip musculature in the PM-HS group.
While the body of literature investigating recovery after ACLR in strength and function in the skeletally immature population is still in its nascent stages, the hamstring deficits observed in the current study interestingly conflict with those of a previous study. Greenberg et al12 reported that 94% of skeletally immature patients who underwent ACLR (mean age, 12.3 years) with hamstring autografts achieved greater than 90% of strength recovery after 7 months (range, 3.0-12.6 months) postoperatively. The skeletally immature patients in this study had hamstring autografts as well. The difference appears to be a method of strength testing. In that study, isokinetic measurements with a speed of 180 deg/s were used.12 The different muscle strength testing protocols might have influenced the different outcomes. A study performed by Hashemi et al14 stated that there is a minimal effect of hamstring strength deficits on dynamic balance. However, Clagg et al7 identified a linear association between hamstring strength and the posterolateral reach test of dynamic balance. In the current study, there were no statistically significant differences among the 3 groups.
There were no significant differences in functional hop test performance between the 3 study groups (Table 5). Several studies have investigated the impact of quadriceps strength deficits on hop performance,14,34,40,48 but investigations focusing on hamstring strength deficits, hamstring autografts, and ITB autografts are few. In this study, the PM-HS group did not demonstrate significant deficits in the 4 types of functional hop tests, even though there was a notable hamstring strength deficit. The increased hip musculature of the involved limb, which was previously discussed, might have aided hop performance in the PM-HS group.
Limitations
There are several inherent limitations in this study. First, we did not have access to each patient’s physical therapy record. We attempted to review physical therapy recommendations from medical charts; however, there was considerable variability with regard to exercise prescription documentation. Thus, we could not organize qualitative data (such as the type of rehabilitative exercises) for physical therapy; however, we did verify that all patients had at least a total of 20 physical therapy sessions. Importantly, this is likely highly reflective of most orthopaedic practices, for which there may be a common protocol between different surgeons, but the speed and nature with which such protocols are instituted and phases progressed are quite variable. In reality, most patients who have undergone ACLR and have been investigated in the literature have pursued physical therapy in various local settings for convenience, without standardized physical therapy being rigorously followed across all patients.
A second limitation is that the data from the current study’s assessments were collected by multiple raters, which may have reduced the interreliability and intrareliability values. To conduct reliable data measurement procedures, both interreliability and intrareliability values of thigh circumference, ROM, and muscle strength, but not functional tests, were checked before the measurement, which indicated acceptable levels of reliability.
Third, all strength data were measured in an isometric manner rather than with an isokinetic method. Isokinetic strength measurements generate peak torque, total work, and mean power in defined ROM.4,25 It would have been ideal if lower extremity strength had been measured by an isokinetic machine. However, at the beginning of this study, the machine was not available at the testing site.
Fourth, although skeletal maturity was rigorously checked, significant age differences were still noted among the 3 groups, which may be because of potential selection bias among surgeons. A randomized controlled trial would have been an ideal study design to eliminate those concerns. Moreover, this study did not include joint laxity and imaging modalities. Incorporating a KT-1000/2000 arthrometer for joint laxity and magnetic resonance imaging for anatomic measurements would have strengthened the outcomes of this study. Finally, the study only yielded short-term outcomes. Long-term outcomes including reinjury rates, sports participation, and osteoarthritis development likely offer clinically pertinent data for the skeletally immature population.
Conclusion
The current study demonstrated superior hamstring strength 6 months postoperatively in the PM-ITB group as compared with the PM-HS group among a skeletally immature ACLR population aged ≤15 years. There were no significant differences in other lower extremity strength measurements, including the quadriceps, hip abductor, and hip extensor, dynamic balance, or functional hop test performance. Complementary future studies are needed to investigate the long-term effects of hamstring and ITB autografts on the functional performance, ACL reinjury rate, and sports participation of young, physically active patients after ACLR.
Acknowledgment
The authors are appreciative of each staff member of the Micheli Center for Sports Injury Prevention for their support. The authors are also grateful for Madison N. Maniatis for allowing the use of her dynamic balance image.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.E.H. has stock options in Imagen Technologies Inc and receives royalties from Springer Publishing. M.S.K. is a consultant for Smith & Nephew Endoscopy, OrthoPediatrics, and Ossur and receives royalties from OrthoPediatrics and WB Saunders.
Ethical approval was obtained from Boston Children’s Hospital (protocol No. 00013151).
References
- 1. Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring strength asymmetry at 3 years after anterior cruciate ligament reconstruction alters knee mechanics during gait and jogging. Am J Sports Med. 2017;45(1):97–105. [DOI] [PubMed] [Google Scholar]
- 2. Ardern CL, Taylor NF, Feller JA, Whitehead TS, Webster KE. Sports participation 2 years after anterior cruciate ligament reconstruction in athletes who had not returned to sport at 1 year: a prospective follow-up of physical function and psychological factors in 122 athletes. Am J Sports Med. 2015;43(4):848–856. [DOI] [PubMed] [Google Scholar]
- 3. Bell DR, Trigsted SM, Post EG, Walden CE. Hip strength in patients with quadriceps strength deficits after ACL reconstruction. Med Sci Sports Exerc. 2016;48(10):1886–1892. [DOI] [PubMed] [Google Scholar]
- 4. Changstrom BG, Brou L, Khodaee M, Braund C, Comstock RD. Epidemiology of stress fracture injuries among US high school athletes, 2005-2006 through 2012-2013. Am J Sports Med. 2015;43(1):26–33. [DOI] [PubMed] [Google Scholar]
- 5. Chung KS, Ha JK, Yeom CH, et al. Are muscle strength and function of the uninjured lower limb weakened after anterior cruciate ligament injury? Two-year follow-up after reconstruction. Am J Sports Med. 2015;43(12):3013–3021. [DOI] [PubMed] [Google Scholar]
- 6. Cimino F, Volk BS, Setter D. Anterior cruciate ligament injury: diagnosis, management, and prevention. Am Fam Physician. 2010;82(8):917–922. [PubMed] [Google Scholar]
- 7. Clagg S, Paterno MV, Hewett TE, Schmitt LC. Performance on the modified star excursion balance test at the time of return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2015;45(6):444–452. [DOI] [PubMed] [Google Scholar]
- 8. Dekker TJ, Godin JA, Dale KM, Garrett WE, Taylor DC, Riboh JC. Return to sport after pediatric anterior cruciate ligament reconstruction and its effect on subsequent anterior cruciate ligament injury. J Bone Joint Surg Am. 2017;99(11):897–904. [DOI] [PubMed] [Google Scholar]
- 9. Dekker TJ, Rush JK, Schmitz MR. What’s new in pediatric and adolescent anterior cruciate ligament injuries? J Pediatr Orthop. 2018;38(3):185–192. [DOI] [PubMed] [Google Scholar]
- 10. Dodwell ER, Lamont LE, Green DW, Pan TJ, Marx RG, Lyman S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014;42(3):675–680. [DOI] [PubMed] [Google Scholar]
- 11. Gilchrist J, Mandelbaum BR, Melancon H, et al. A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. Am J Sports Med. 2008;36(8):1476–1483. [DOI] [PubMed] [Google Scholar]
- 12. Greenberg EM, Greenberg ET, Ganley TJ, Lawrence JT. Strength and functional performance recovery after anterior cruciate ligament reconstruction in preadolescent athletes. Sports Health. 2014;6(4):309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grindem H, Snyder-Mackler L, Moksnes H, Engebretsen L, Risberg MA. Simple decision rules can reduce reinjury risk by 84% after ACL reconstruction: the Delaware-Oslo ACL cohort study. Br J Sports Med. 2016;50(13):804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hashemi J, Breighner R, Jang TH, Chandrashekar N, Ekwaro-Osire S, Slauterbeck JR. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010;17(3):235–241. [DOI] [PubMed] [Google Scholar]
- 15. Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27(6):699–706. [DOI] [PubMed] [Google Scholar]
- 16. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 17. Ithurburn MP, Paterno MV, Ford KR, Hewett TE, Schmitt LC. Young athletes with quadriceps femoris strength asymmetry at return to sport after anterior cruciate ligament reconstruction demonstrate asymmetric single-leg drop-landing mechanics. Am J Sports Med. 2015;43(11):2727–2737. [DOI] [PubMed] [Google Scholar]
- 18. Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3(2):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg Am. 2005;87(11):2371–2379. [DOI] [PubMed] [Google Scholar]
- 20. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents: surgical technique. J Bone Joint Surg Am. 2006;88 (suppl 1, Pt 2):283–293. [DOI] [PubMed] [Google Scholar]
- 21. Kocher MS, Smith JT, Zoric BJ, Lee B, Micheli LJ. Transphyseal anterior cruciate ligament reconstruction in skeletally immature pubescent adolescents. J Bone Joint Surg Am. 2007;89(12):2632–2639. [DOI] [PubMed] [Google Scholar]
- 22. LaBella CR, Hennrikus W, Hewett TE. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. 2014;133(5):e1437–e1450. [DOI] [PubMed] [Google Scholar]
- 23. Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40(7):1551–1557. [DOI] [PubMed] [Google Scholar]
- 24. Linek P, Sikora D, Wolny T, Saulicz E. Reliability and number of trials of Y Balance Test in adolescent athletes. Musculoskelet Sci Pract. 2017;31:72–75. [DOI] [PubMed] [Google Scholar]
- 25. MacWilliams BA, Wilson DR, DesJardins JD, Romero J, Chao EY. Hamstrings cocontraction reduces internal rotation, anterior translation, and anterior cruciate ligament load in weight-bearing flexion. J Orthop Res. 1999;17(6):817–822. [DOI] [PubMed] [Google Scholar]
- 26. Mandelbaum BR, Silvers HJ, Watanabe DS, et al. Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes: 2-year follow-up. Am J Sports Med. 2005;33(7):1003–1010. [DOI] [PubMed] [Google Scholar]
- 27. Mendiguchia J, Arcos AL, Garrues MA, Myer GD, Yanci J, Idoate F. The use of MRI to evaluate posterior thigh muscle activity and damage during Nordic hamstring exercise. J Strength Cond Res. 2013;27(12):3426–3435. [DOI] [PubMed] [Google Scholar]
- 28. Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10(10):e0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Micheli LJ, Rask B, Gerberg L. Anterior cruciate ligament reconstruction in patients who are prepubescent. Clin Orthop Relat Res. 1999;(364):40–47. [DOI] [PubMed] [Google Scholar]
- 30. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nagelli CV, Hewett TE. Should return to sport be delayed until 2 years after anterior cruciate ligament reconstruction? Biological and functional considerations. Sports Med. 2017;47(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nomura Y, Kuramochi R, Fukubayashi T. Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2015;25(3):301–307. [DOI] [PubMed] [Google Scholar]
- 33. Paletta GA., Jr Complete transphyseal reconstruction of the anterior cruciate ligament in the skeletally immature. Clin Sports Med. 2011;30(4):779–788. [DOI] [PubMed] [Google Scholar]
- 34. Palmieri-Smith RM, Lepley LK. Quadriceps strength asymmetry after anterior cruciate ligament reconstruction alters knee joint biomechanics and functional performance at time of return to activity. Am J Sports Med. 2015;43(7):1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Clin J Sport Med. 2012;22(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of second ACL injuries 2 years after primary ACL reconstruction and return to sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Petersen W, Fink C, Kopf S. Return to sports after ACL reconstruction: a paradigm shift from time to function. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1353–1355. [DOI] [PubMed] [Google Scholar]
- 39. Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. [DOI] [PubMed] [Google Scholar]
- 40. Schmitt LC, Paterno MV, Ford KR, Myer GD, Hewett TE. Strength asymmetry and landing mechanics at return to sport after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2015;47(7):1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schmitt LC, Paterno MV, Hewett TE. The impact of quadriceps femoris strength asymmetry on functional performance at return to sport following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012;42(9):750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shelbourne KD, Gray T, Wiley BV. Results of transphyseal anterior cruciate ligament reconstruction using patellar tendon autograft in Tanner stage 3 or 4 adolescents with clearly open growth plates. Am J Sports Med. 2004;32(5):1218–1222. [DOI] [PubMed] [Google Scholar]
- 43. Walden M, Atroshi I, Magnusson H, Wagner P, Hagglund M. Prevention of acute knee injuries in adolescent female football players: cluster randomised controlled trial. BMJ. 2012;344:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 45. Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Willimon SC, Jones CR, Herzog MM, May KH, Leake MJ, Busch MT. Micheli anterior cruciate ligament reconstruction in skeletally immature youths: a retrospective case series with a mean 3-year follow-up. Am J Sports Med. 2015;43(12):2974–2981. [DOI] [PubMed] [Google Scholar]
- 47. Wilson WT, Hopper GP, Byrne PA, MacKay GM. Anterior cruciate ligament repair with internal brace ligament augmentation. Surg Technol Int. 2016;XXIX:273–278. [PubMed] [Google Scholar]
- 48. Xergia SA, Pappas E, Zampeli F, Georgiou S, Georgoulis AD. Asymmetries in functional hop tests, lower extremity kinematics, and isokinetic strength persist 6 to 9 months following anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2013;43(3):154–162. [DOI] [PubMed] [Google Scholar]
- 49. Zwolski C, Schmitt LC, Quatman-Yates C, Thomas S, Hewett TE, Paterno MV. The influence of quadriceps strength asymmetry on patient-reported function at time of return to sport after anterior cruciate ligament reconstruction. Am J Sports Med. 2015;43(9):2242–2249. [DOI] [PubMed] [Google Scholar]