Abstract
In the event of an improvised nuclear device or “dirty bomb” in a highly populated area, potentially hundreds of thousands of people will require screening to ensure that exposed individuals receive appropriate treatment. For this reason, there is a need to develop tools for high-throughput radiation biodosimetry. Gene expression represents an emerging approach to biodosimetry and could potentially provide an estimate of both absorbed dose and individual radiation-induced injury. Since approximately 2–4% of humans are thought to be radiosensitive, and would suffer greater radiological injury at a given dose than members of the general population, it is of interest to explore the potential impact of such sensitivity on the biodosimetric gene expression signatures being developed. In this study, we used wild-type mice and genetically engineered mouse models deficient in two DNA repair pathways that can contribute to radiation sensitivity to estimate the maximum effect of differences in radiosensitivity. We compared gene expression in response to a roughly equitoxic (LD50/30) dose of gamma rays in wild-type C57BL/6 (8 Gy) and DNA double-strand break repair-deficient Atm−/− (4 Gy) and Prkdcscid (3 Gy) mutants of C57BL/6. Overall, 780 genes were significantly differentially expressed in wild-type mice one day postirradiation, 232 in Atm−/− and 269 in Prkdcscid. Upstream regulators including TP53 and NFκB were predicted to be activated by radiation exposure in the wild-type mice, but not in either of the DNA repair-deficient mutant strains. There was also a significant muting of the apparent inflammatory response triggered by radiation in both mutant strains. These differences impacted the ability of gene expression signatures developed in wild-type mice to detect potentially fatal radiation exposure in the DNA repair-deficient mice, with the greatest impact on Atm−/− mice. However, the inclusion of mutant mice in gene selection vastly improved performance of the classifiers.
INTRODUCTION
There is a perceived need to develop tools for high-throughput biodosimetry (1) to prepare for the possibility of accidental or intentional radiation events. This was clearly demonstrated in the 1987 radiation incident in Goiânia, Brazil, in which a medical radiation source was scavenged from an abandoned hospital and breached. Approximately 130,000 individuals were screened within the first days after discovery of the event, but only 20 of these people required hospitalization (2). In the case of an improvised nuclear device (IND) or “dirty bomb” in a major city, potentially hundreds of thousands of people would require screening to ensure appropriate treatment for exposed individuals, and to help reassure the “worried well” (3).
Gene expression represents an emerging approach to biodosimetry, and could potentially provide estimates of both absorbed dose and the severity of radiation-induced injury. Several different model systems have been used for development of this approach. Human-based models have used peripheral blood or lymphocytes irradiated ex vivo (4–8), or blood from patients undergoing total-body irradiation prior to hematopoietic stem cell transplantation (8–12). Mice have also been used as a model for elucidation of radiation-induced responses and construction of biodosimetric gene expression profiles (8, 10, 12–14).
Through clinical experience and epidemiological studies of populations exposed to radiation, it has become apparent that some individuals have heightened radiation sensitivity, which may be at least partly due to genetic factors (15–19). In fact, approximately 2–4% of the human population is estimated to be radiosensitive (20). Alterations in important radiation response signaling pathways, including DNA repair pathways, are known to directly compromise the ability of an organism to tolerate radiation exposure, and can result in dire consequences for the irradiated individual. Due to altered DNA damage response signaling, radiosensitivity may impact both the general gene expression radiation response and the performance of the biodosimetric signatures currently being developed based on gene expression.
In this study, we used wild-type mice (WT) and genetically engineered mutant mouse models, deficient in pathways that impact radiation survival, radiation signaling and DNA double-strand break (DSB) repair, to investigate the effect of DNA repair deficiency on the in vivo gene expression response to radiation. Non-homologous end joining (NHEJ) is one of the two major pathways for DSB repair. Mutation in the gene coding for DNA-PKcs (DNA-dependent protein kinase catalytic subunit, gene Prkdc) precludes NHEJ, severely impacting both immune cell development and survival after irradiation. In both humans and mice, such mutations result in severe combined immunodeficiency (SCID), a condition that is characterized by immunodeficiency and radiation sensitivity. The SCID mice have an LD50/30 of 3 Gy, compared to an LD50/30 of 8 Gy in the parental WT C57BL/6 mice (21). The ataxia telangiectasia mutated [(ATM) gene Atm] is a protein kinase crucial for DNA damage signaling and for DSB repair. Mutations in the ATM gene produce ataxia telangiectasia, which is characterized by neurodegeneration, a weakened immune system, increased cancer risk and radiation sensitivity. In this study, we used Atm−/− mice (also on a C57BL/6 background), which have an LD50/30 of 4 Gy (22). As an initial point of comparison, all genotypes were gamma irradiated with their respective LD50/30 dose, representing equitoxic damage in a range relevant to triage and medical treatment. At 24 h later, which represents a time relevant for large-scale screening, whole genome gene expression was compared with that in nonirradiated controls.
The animal models used in this study represent a “worst-case” scenario, and should be viewed as an extreme example of radiation sensitivity. Both SCID and ataxia telangiectasia are rare and very serious medical conditions in humans, and represent severe radiosensitivity. Most radiosensitive members of the general population will not have full-blown DNA repair deficiency syndromes; however, study of these extreme models represents a first step in defining the maximum likely impact of DNA repair deficiency on gene expression after radiation exposure.
METHODS
Animal Studies
Wild-type (WT) C57BL/6 and Atm−/− mice (22) (B6.129S6-Atmtm1Awb/J; stock number: 008536) were obtained from Jackson Laboratory (Bar Harbor, ME) and bred at Georgetown University (Washington, DC). Prkdcscid (SCID) mice (23) (B6.CB17-Prkdcscid/SzJ; stock number: 001913) were ordered from the Jackson Laboratory and acclimated at Georgetown University for at least one week prior to irradiations. All mice were housed under specific pathogen-free conditions in standard 12:12 h light-dark schedules and provided with water and food ad libitum. The study was conducted according to NIH guidelines and with approval from the Georgetown University Institutional Animal Care and Use Committee. Male mice between 8 and 10 weeks of age were whole-body gamma irradiated from a 137Cs source at a dose rate of ~1.45 Gy/min. Equitoxic radiation doses were used, and the absorbed doses were 8 Gy, 4 Gy and 3 Gy for WT, Atm−/− and SCID genotypes, respectively, based on their published LD50/30 doses (21, 22). An equal number of nonirradiated controls were used for each genotype. Mice were sacrificed 24 h postirradiation and blood was collected in PAXgene™ blood RNA stabilization solution (BD Biosciences, San Jose, CA), frozen at −80°C and shipped to Columbia University (New York, NY) for RNA isolation.
RNA Isolation and Whole Genome Gene Expression Analysis
RNA isolation, whole genome gene expression analysis and bioinformatics were performed as described elsewhere (13). Briefly, RNA was isolated from the collected blood using the PAXgene Blood RNA kit (QIAGEN®, Valencia, CA) and globin transcripts were depleted using the GLOBINclear™-Mouse/Rat Kit (Ambion®, Foster City, CA). RNA quality was assessed using the Agilent Bioanalyzer (Agilent Technologies Inc., Santa Clara, CA) and samples with RIN ≥8.5 (average 9.1) were hybridized to Agilent Whole Mouse Genome Microarrays (4 × 44K v2) using the single-color workflow. The Agilent DNA Microarray Scanner (G2505B) was used to image the slides, and data was extracted from the images using the Agilent Feature Extraction Software version 9.1 with default settings. The full data set represented five animals in each dose-genotype group, with the exception of the WT controls and the Atm−/− irradiated group, which each had an additional sample. Study size (n = 5) was selected to provide 90% power to detect twofold changes, based on an analysis of our previous mouse microarray data using the methods of Lee and Whitmore (24).
The class comparison tool within BRB-ArrayTools was used to determine differential expression between irradiated and control animals for each genotype. Genes with P < 0.001 were considered statistically significant, and henceforth are referred to as “regulated”. Multiple comparisons were corrected for using the Benjamini-Hochberg false discovery rate (FDR) method to limit false discoveries to below 10% (25). Full microarray expression data are available in the Gene Expression Omnibus (accession no. GSE99176). Independent validation of individual gene expression was not performed due to limited RNA. However, our prior experience has shown that commercial microarray technology is now very robust, and the general patterns of response in previous experiments agree well between microarray and qRT-PCR measurements. For instance, in our broadest validation study, 93% of 71 genes assayed confirmed the microarray response by qRT-PCR, with an overall correlation between the two sets of measurements of 0.96 (26).
Class Prediction
We used the class prediction tool within BRB-ArrayTools to select genes and build predictors of radiation exposure status, and to test the potential impact of DNA repair deficiency on their performance. Training sets were defined, and the greedy pairs method (27) was used to select the 12 pairs of genes that best discriminated between control and irradiated samples in each training set. After feature selection, seven classification methods (compound covariate predictor, linear discriminant analysis, 1- and 3-nearest neighbors, nearest centroid, support vector machines, and Bayesian compound covariate predictor) were used with the selected feature sets to predict the irradiation status of the remaining samples. The percentage correct classification was calculated for each approach.
Gene Ontology Analysis
The DAVID bioinformatics resource tool (http://david.abcc.ncifcrf.gov/) was used for functional annotation of the transcriptional response (28, 29). Up- and downregulated genes were imported and analyzed separately in DAVID version 6.7 using default settings. Statistical significance in DAVID is determined by comparing the proportion of regulated genes associated with a specific term with the fraction of all genes associated with the same term. Fisher’s exact test was performed, and a Benjamini-corrected P value <0.1 was used as the cut-off to determine statistically significant gene ontology or pathway terms.
Expression values of statistically significantly regulated genes were imported into the Ingenuity Pathway Analysis software (IPA; Ingenuity Systems, Redwood City, CA), which was used to predict potential upstream regulators of the transcriptional response and for pathway analysis. Additionally, IPA was used to construct networks for specific genes or targets. IPA uses previously reported experimental data to predict activation or inhibition of upstream regulators and for pathway analysis. In the IPA analysis, a P value cut-off of 0.05 was used, and when predicting activation or inhibition, a z-score ≥2 or ≤−2 was considered statistically significant.
RESULTS
Differentially Expressed Genes for Wild-Type, Atm−/− and Prkdcscid Phenotypes
Wild-type, Atm−/− and Prkdcscid (SCID) mice were gamma irradiated with equitoxic absorbed doses (LD50/30 doses of 8, 4 and 3 Gy, respectively). As shown in Table 1 and Supplementary Table S1 (http://dx.doi.org/10.1667/RR14862.1.S1), WT mice responded with both a larger number of statistically significant (P < 0.001; FDR < 0.1) genes (780 genes) compared to both Atm−/− (232 genes) and SCID (269 genes). Downregulation was more common than upregulation for all three phenotypes, but most pronounced in SCID (97%), followed by Atm−/− (87%) and the least pronounced in WT (67%) mice. The gene expression response in WT mice involved typical p53-regulated genes such as Bbc3 (up 3.4-fold), Cdkn1a (up 18-fold), Mdm2 (up 2.8-fold) and Phlda3 (up 6.5-fold). The response in Atm−/− and SCID mice lacked much of the typical p53-response pattern (although Cdkn1a and Phlda3 were upregulated 7.8-fold and 3.4-fold, respectively, in SCID) and involved fewer genes overall.
TABLE 1.
Differentially Expressed Genes in Blood from WT, SCID and Atm−/− mice 24 h Postirradiation with an LD50/30 Dose (8, 3 and 4 Gy, respectively)
| Phenotype | Regulated genesa | Upregulated | Downregulated |
|---|---|---|---|
| Wild-type | 780 | 254 | 526 |
| Atm−/− | 232 | 31 | 201 |
| SCID | 269 | 9 | 260 |
Statistical cut-off: P value < 0.001; FDR < 0.1
The numbers of radiation-responsive genes shared between different genotypes are shown in Fig. 1. Wild-type mice demonstrated the highest proportion of genes not responding in either of the other phenotypes, with 469 genes (68%) significant only in the WT response. Ninety-six genes, representing 45% of the total response, were significant only in Atm−/− mice and 85 genes (35% of the response) were significant only in SCID mice. Two genes (Myo1d, Gapvd1) were radioresponsive in both Atm−/− and SCID, but not in WT mice. Both these genes showed stronger downregulation in SCID compared to Atm−/−, especially Myo1d, with fold changes of −10 and −3, respectively.
FIG. 1.
Venn diagram showing the distribution of statistically significant differentially expressed genes. The numbers in each section represent numbers of statistically significant regulated genes, which are also represented by area. The diagrams were made using eulerAPE (46).
A total of 57 genes, all of which were downregulated, were differentially expressed after irradiation in all three genotypes (Fig. 1). Generally, the response of these genes was strongest in WT mice, which also received the highest absorbed dose (8 Gy). SCID mice (3 Gy) generally demonstrated slightly stronger downregulation than Atm−/− (4 Gy), suggesting that individual sensitivity may also contribute to the response of some genes, in addition to absorbed dose.
Gene Ontology Analysis
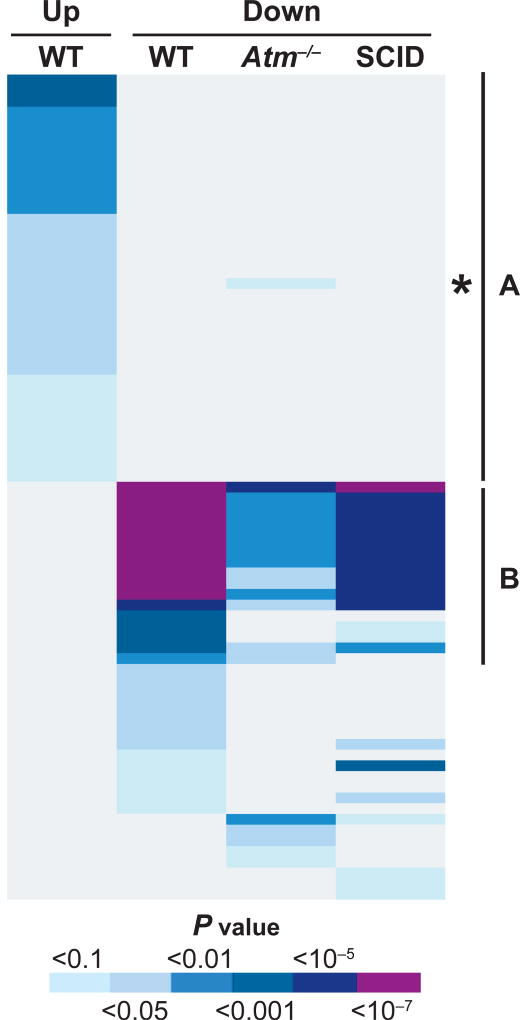
The DAVID bioinformatics resource tool (28, 29) was used to find overrepresented GO terms and KEGG pathways among the responding genes. In all, 52, 16 and 17 terms were statistically significantly overrepresented (Benjamini-corrected P < 0.1) for the WT, Atm−/− and SCID phenotypes, respectively (Fig. 2, Supplementary Table S2; http://dx.doi.org/10.1667/RR14862.1.S2). Nine annotation terms (all related to ubiquitination and catabolism) were enriched among downregulated radiation-responsive genes in all three genotypes. Terms exclusively enriched among upregulated genes in WT mice (27 terms) were dominated by the p53 pathway, apoptosis and nucleic acid metabolism, suggesting a strong impact of both Atm−/− and SCID on these central radiation response pathways. Five annotation terms were significant only in the Atm−/− response (related to cell cycle and division, immune system development and hematopoiesis), whereas three annotation terms were exclusively enriched in SCID mice (functions related to acetylation, histone H3 and mitochondria.)
FIG. 2.
Gene ontology analysis. The DAVID bioinformatics resource tool (28, 29) was used to find overrepresented gene ontology (GO) terms. GO terms with a Benjamini-corrected P < 0.1 were considered statistically significant. Only terms at GO level 2, 3 and 4 were included in the analysis. The color of a cell indicates a P value as in the key. Region A, representing significant processes among genes upregulated only in WT mice was dominated by the p53 pathway, apoptosis and nucleic acid metabolism. The exception here is the GO term “immune response”, marked with an asterisk. This category was significantly enriched among upregulated genes in WT and among downregulated genes in Atm−/−. Region B, representing GO categories overrepresented among downregulated genes in all genotypes, was exclusive to functions of ubiquitination and catabolism.
Differences in Radiation-Induced Inflammatory Pathways between WT, Atm−/− and SCID
We next used the upstream regulator analysis tool in IPA, initially focusing on upstream regulators with significant predictions of altered activity in response to radiation, having a z-score ≥2 or ≤−2 in at least one genotype. Lipopolysaccharide, a general inducer of inflammatory response, had the highest z-score in WT mice, and the lowest in Atm−/−. We found that an additional 11 upstream regulators showed this trend of opposite directions of activity between the WT and DNA repair-deficient mice (Table 2A and Supplementary Table S2; http://dx.doi.org/10.1667/RR14862.1.S2). Most were predicted to be activated by radiation in WT and unchanged or suppressed in the mutant mice. These upstream regulators included TP53 and NFκB, but were predominantly molecules directly involved in inflammatory response (CSF2, IFNG, IL5, IL1B, IL6, IL21, IRF7). An additional 11 regulatory molecules related to inflammation were found to have a significant prediction of radiation activation or suppression in the WT and no predicted response to radiation in either of the mutant strains (Table 2B). The data suggest that radiation affects the inflammatory response in the blood of WT mice, and that this response is greatly muted in both the Atm−/− and SCID phenotypes.
TABLE 2.
IPA-Predicted Upstream Regulators in Different Genotypes
| Upstream regulator | Description | WT | Atm−/− | SCID |
|---|---|---|---|---|
| Opposite activity trends | ||||
| Lipopolysaccharide | Lipopolysaccharide | 3.6a | −3.0 | N/A |
| IFNG | Interferon gamma | 3.1 | −1.2 | N/A |
| IKBKB | Inhibitor of nuclear factor kappa B kinase subunit beta | 2.5 | −1.0 | N/A |
| TP53 | Tumor protein p53 | 2.0 | −0.1 | 0.2 |
| IL21 | Interleukin 21 | 2.0 | −0.5 | N/A |
| IRF7 | Interferon regulatory factor 7 | 1.8 | −2.0 | N/A |
| CSF2 | Colony stimulating factor 2 (GM-CSF) | 0.3 | −2.8 | −2.1 |
| NFkB (complex) | Nuclear factor kappa-light-chain-enhancer of activated B cells | 0.2 | −2.7 | N/A |
| IL1B | Interleukin 1 beta | 0.1 | −2.2 | N/A |
| IL6 | Interleukin 6 | 0.1 | −2.0 | N/A |
| IL5 | Interleukin 5 | 0.0 | −2.7 | N/A |
| mir-21 | microRNA 21 | −0.6 | 2.0 | 2.2 |
| Significant only in wild-type | ||||
| IFNA | Interferon alpha | 2.7 | N/A | N/A |
| Ifnar1 | Interferon (alpha and beta) receptor 1 | 2.6 | N/A | N/A |
| TLR4 | Toll like receptor 4 | 2.6 | N/A | N/A |
| IFNA2 | Interferon alpha 2 | 2.5 | N/A | N/A |
| IRF8 | Interferon regulatory factor 8 | 2.5 | N/A | N/A |
| IFNB1 | Interferon beta 1 | 2.3 | N/A | N/A |
| IRF3 | Interferon regulatory factor 3 | 2.2 | N/A | N/A |
| IFN type 1 | Interferon subgroup | 2.1 | N/A | N/A |
| TLR3 | Toll like receptor 3 | 2.0 | N/A | N/A |
| IL1A | Interleukin 1 alpha | 2.0 | N/A | N/A |
| SOCS1 | Suppressor of cytokine signaling 1 | −2.6 | N/A | N/A |
Note. N/A no prediction of activity.
z-scores of activity prediction by IPA. Negative z-scores (in italics) indicate predicted suppression of a regulatory factor after irradiation; positive z-scores indicate predicted activation. |Z| ≥ 2 (values in bold) is considered significant.
Impact of Impaired DNA Repair Pathways on Prediction of Radiation Exposure
We next used class prediction methods in BRB-Array Tools to test whether impairment of DNA repair influenced the ability of a gene panel to predict exposure to radiation. The greedy pairs method for feature selection was used to identify the 12 top-performing pairs of genes for discrimination between irradiated and control samples. Each genotype was used in turn as the training set for the gene selection process, and then the remaining samples were used as a test set. A fourth training set, comprised of two control and two irradiated samples from each genotype (the “mixed” set), was also tested. The use of the WT samples to build the classifier resulted in the poorest performance in classifying the Atm−/− mice, while classifiers constructed using the SCID and mixed training sets resulted in 100% correct classification of the test samples (Table 3). The composition of the classifiers and summary of their performance are available in Supplementary Table S3 (http://dx.doi.org/10.1667/RR14862.1.S3).
TABLE 3.
Performance of Seven Classifier Algorithms Using Greedy Pairs Gene Selection with Separate Training and Test Sets
| Percentage correct classification | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Training set | Test set | CCPa | LDA | 1-NN | 3-NN | NC | SVM | BCCP |
| WT | Atm−/− | 73 | 64 | 64 | 73 | 73 | 64 | 73 |
| SCID | 100 | 100 | 90 | 100 | 90 | 90 | 100 | |
| Atm−/− | WT | 100 | 100 | 91 | 91 | 100 | 91 | 91 |
| SCID | 90 | 90 | 80 | 80 | 90 | 80 | 80 | |
| SCID | WT | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Atm−/− | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Mixeda | WT | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Atm−/− | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| SCID | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
Notes. CCP = compound covariate predictor; LDA = linear discriminant analysis; 1-NN = 1-nearest neighbor. 3-NN = 3-nearest neighbors; NC = nearest centroid; SVM = support vector machines; BCCP = Bayesian compound covariate predictor.
The mixed training set used two irradiated and two control samples from each genotype.
DISCUSSION
In this study, we used male WT C57BL/6 mice and genetically engineered mouse models deficient in pathways known to impact radiation survival, radiation signaling and DSB repair (Atm−/−), and non-homologous end joining (Prkdcscid, acronym: SCID) to explore the potential effect of DNA repair deficiency on the gene expression response to radiation and its implications for radiation biodosimetry. These genotypes were chosen to represent extreme disruption in two of the major DNA damage repair pathways, to delimit the greatest effect that deficiencies in these pathways would be likely to have on gene expression after irradiation. Individual variations in the efficiency of these DNA repair pathways within the general population would be expected to be much more subtle, presumably with a more subtle influence on biodosimetry.
Since the primary focus of this study was the potential impact of DNA repair pathway defects on biodosimetry end points, we examined gene expression at 24 h postirradiation, a time when first responders may be able to reach casualties, and when medical treatment could still be effective. Because it is known that the cascade of gene expression changes resulting from radiation exposure is both complex and temporally dynamic, in future studies it would also be of interest to compare the responses of these strains at later postirradiation times. This would enable assessment of the influence of early DNA damage signaling on later responses and determination of correlations between gene expression and late physiological manifestations of radiation toxicity.
As might be expected, the 24 h gene expression response in WT mice was marked by a prominent response of TP53-regulated genes such as Cdkn1a, Aen, Phlda3, Bbc3 and Mdm2. Furthermore, gene ontology (GO) analysis indicated p53 signaling was the top pathway among those enriched for upregulated genes in the WT mice, while it was not significantly enriched among genes responding in either Atm−/− or SCID. Similarly, IPA upstream regulator analysis predicted activation of the TP53 transcription factor only in WT mice. The response in both Atm−/− and SCID mice lacked this classic p53-related response, although some genes, such as Cdkn1a and Phlda3, were upregulated, although to a lesser degree, in SCID mice
After DSB formation, ATM is known to phosphorylate p53, among many other substrates, and is required both for homologous recombination and for activation of the p53 signaling pathway (30). The observed loss of the p53 response in Atm−/− mice is consistent with these expectations. DNA-PKcs also interacts directly with p53 after radiation exposure. It can bind to the promoters of p53 effector genes, including Cdkn1a, and modify gene expression response in a context-dependent fashion, possibly favoring apoptosis over cell cycle arrest (31). Inhibition of DNA-PKcs in human cancer cells has been shown to delay and ablate the induction of MDM2 protein by gamma rays, which in turn alters the timing and magnitude of CDKN1A mRNA response (32). In the current study, we found ablation of the p53 effector gene response in SCID mice compared to WT mice at the same time after exposure to equitoxic doses of radiation, consistent with a large effect of DNA-PKcs activity on the transcriptional activity of p53.
In addition to the p53 response, the majority of the upstream regulators predicted by IPA to be involved in the gene expression responses in this study were related to immune response or inflammation. Strikingly, all the significant predictions in this area were of activation by radiation exposure in WT mice and/or inhibition in Atm−/− or SCID (Table 2). Inflammation is known to be a major component of the normal radiation response (33, 34), and is mediated by regulation and release of cytokines such as tumor necrosis factor, interleukins and interferons (35, 36). The effect of the different DNA repair genotypes on the inflammatory response to a LD50/30 dose of gamma rays can be clearly visualized in network maps of the genes in the current study that were either upstream or downstream of IFNG (Supplementary Table S2; http://dx.doi.org/10.1667/RR14862.1.S2). In SCID mice, no activation or inhibition of any of the interferons was predicted, and as seen in the IFNG pathway map, few radiation-responsive molecules were related to IFNG signaling. Similarly, in Atm−/−, a trend towards inhibition of IFNG was predicted, and the network map shows response of many downregulated genes, but no upregulated genes at all.
The observed lack of response of inflammation-related genes in SCID mice may be explained by the direct effect of Prkdc mutation on the immune system. Since DNA-PKcs is also required for V(D)J recombination, a critical process in the development of mature T and B cells, SCID mice lack functional lymphocytes and are severely immunocompromised. DNA-PKcs may also play a direct role in signaling through the NF-κB pathway after radiation exposure (37–39).
Although ataxia telangiectasia patients generally retain a functional immune system, loss of ATM function can also have substantial and variable effects on both the cellular and humoral immune systems (40, 41). In addition, ATM plays a direct role in regulating the expression of inflammatory cytokine genes through phosphorylation of several members of the NF-κB family, including RelA, NEMO, and IκB-α (38, 39, 42). Our finding that the activity of many upstream regulators involved in inflammatory response appear to be suppressed below control levels in irradiated Atm−/− mice, may suggest these responses to DNA damage are even more severely dysregulated in Atm−/− than in SCID mice.
Inflammation-related genes have often been proposed as biomarkers of radiation exposure, or for use in biodosimetric panels (8, 43–45). Although immune and inflammation-related genes often dominate the gene expression responses seen after in vivo exposure, we have previously suggested caution against excessive reliance on such genes for radiation biodosimetry (11). The initial concern was for possible confounding with generalized stress responses due to infection, burn, trauma or chronic inflammatory conditions. The current results suggest that individual differences in DNA repair capacity may also affect the response of these genes to radiation. While the extreme impairments of DNA repair considered here are rare in the human population, the effect of less extreme variations in genetic radiosensitivity on gene expression and inflammatory response deserves further study in the context of biodosimetry.
Despite the broad differences in gene expression responses, we were able to construct radiation classifiers that worked well for all genotypes. Overall, we found that classifiers built using only data from WT mice performed relatively poorly in mice with compromised DSB repair, especially in the Atm−/− mice. Training the classifiers using the Atm−/− samples provided some improvement, producing correct classification rates of 90% or better in the other genotypes, while training using either the SCID samples or a mixed training set representing two controls and two irradiated mice from each genotype, yielded 100% correct classification in the test sets for all genotypes. This finding suggests that making efforts to ensure that individuals representing a range of DNA repair capacities and radiation sensitivities are represented in biodosimetry development may eliminate some of the most confounded genes and is likely to lead to a more robust algorithm. We have shown that it is possible to reliably detect an LD50 radiation exposure, even in mice with severely compromised DNA repair pathways. Further work will be needed to characterize the effect of both extreme and more subtle radiosensitivity on dose estimation, and to determine if physiological sensitivity translates as an apparent elevation in dose.
In future studies, it may be interesting to survey a panel of mouse strains with a broad range of mutations in these and other DNA repair pathways to identify genes with radiation responses that correlate directly with DNA repair capacity or residual chromosomal damage. A study of metabolomic responses to radiation in the same mouse strains is also underway, and it will be interesting to compare those results with the gene expression responses reported here.
CONCLUSION
In this study, we used WT mice and genetically engineered mouse models deficient in two different DNA repair pathways, Atm−/− (DSB repair) and Prkdcscid (non-homologous end joining), in an initial investigation into the effect of DNA repair deficiency on the gene expression response to radiation in the context of biodosimetry. We found that ablation of DNA repair pathways has a substantial effect on gene expression and inferred downstream biological functions 24 h after exposure to equitoxic LD50/30 absorbed dose levels. DNA repair deficiency showed a strong influence on inflammatory responses after irradiation. This may impair the performance of dosimetric signatures developed using only individuals of average sensitivity, suggesting that sensitive subpopulations need to be considered and included at the gene selection level to produce biodosimetric classifiers that are robust to variations in DNA repair capacity and radiation sensitivity.
Supplementary Material
Table S1. BRB-ArrayTools output of differentially expressed genes. Results for each genotype are in a separate tab.
Table S2. Gene ontology and network analyses. Tab 1: Annotation of Fig. 2 with gene ontology categories and terms, as well as actual Benjamini-adjusted P values for categories significantly over-represented among up- or downregulated genes in the different genotypes. Tab 2: An IPA-generated network for IFNG showing differential gene expression in the three genotypes.
Table S3. Tab 1: Composition of the classifiers trained using data from WT, Atm−/−, SCID or a mixed-genotype training set. Tab 2: Performance of the classifiers on the new samples in the test sets.
Acknowledgments
We thank Dr. Sunirmal Paul for beginning the RNA work, and Pelagie Ake, Steven Strawn, Bo-Hyun Moon and Yi-Wen Wang for breeding and genotyping of the mice and for sample collection. Analyses were performed using BRB-ArrayTools developed by Dr. Richard Simon and the BRB-ArrayTools development team. This work was supported by the Center for High-Throughput Minimally-Invasive Radiation Biodosimetry, National Institute of Allergy and Infectious Diseases (grant no. U19AI067773).
Footnotes
The online version of this article (DOI: 10.1667/RR14862.1) contains supplementary information that is available to all authorized users.
References
- 1.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 2.The radiological accident in Goiania. STI/PUB/815. Vienna: International Atomic Energy Agency; 1988. [Google Scholar]
- 3.Goransson Nyberg A, Stricklin D, Sellstrom A. Mass casualties and health care following the release of toxic chemicals or radioactive material–contribution of modern biotechnology. Int J Environ Res Public Health. 2011;8:4521–49. doi: 10.3390/ijerph8124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amundson SA, Do KT, Shahab S, Bittner M, Meltzer P, Trent J, et al. Identification of potential mRNA biomarkers in peripheral blood lymphocytes for human exposure to ionizing radiation. Radiat Res. 2000;154:342–6. doi: 10.1667/0033-7587(2000)154[0342:iopmbi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Amundson SA. Development of gene expression signatures for practical radiation biodosimetry. Int J Radiat Oncol Biol Phys. 2008;71:1236–44. doi: 10.1016/j.ijrobp.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtoi A, Brown I, Oskamp D, Schneeweiss FH. Early gene expression in human lymphocytes after gamma-irradiation-a genetic pattern with potential for biodosimetry. Int J Radiat Biol. 2008;84:375–87. doi: 10.1080/09553000802029886. [DOI] [PubMed] [Google Scholar]
- 7.Boldt S, Knops K, Kriehuber R, Wolkenhauer O. A frequency-based gene selection method to identify robust biomarkers for radiation dose prediction. Int J Radiat Biol. 2012;88:267–76. doi: 10.3109/09553002.2012.638358. [DOI] [PubMed] [Google Scholar]
- 8.Lucas J, Dressman HK, Suchindran S, Nakamura M, Chao NJ, Himburg H, et al. A translatable predictor of human radiation exposure. PLoS One. 2014;9:e107897. doi: 10.1371/journal.pone.0107897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amundson SA, Grace MB, Mcleland CB, Epperly MW, Yeager A, Zhan Q, et al. Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res. 2004;64:6368–71. doi: 10.1158/0008-5472.CAN-04-1883. [DOI] [PubMed] [Google Scholar]
- 10.Dressman HK, Muramoto GG, Chao NJ, Meadows S, Marshall D, Ginsburg GS, et al. Gene expression signatures that predict radiation exposure in mice and humans. PLoS Med. 2007;4:e106. doi: 10.1371/journal.pmed.0040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Barker CA, Turner HC, Mclane A, Wolden SL, Amundson SA. Prediction of in vivo radiation dose status in radiotherapy patients using ex vivo and in vivo gene expression signatures. Radiat Res. 2011;175:257–65. doi: 10.1667/RR2420.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filiano AN, Fathallah-Shaykh HM, Fiveash J, Gage J, Cantor A, Kharbanda S, et al. Gene expression analysis in radiotherapy patients and C57BL/6 mice as a measure of exposure to ionizing radiation. Radiat Res. 2011;176:49–61. doi: 10.1667/RR2419.1. [DOI] [PubMed] [Google Scholar]
- 13.Paul S, Ghandhi SA, Weber W, Doyle-Eisele M, Melo D, Guilmette R, et al. Gene expression response of mice after a single dose of (137)Cs as an internal emitter. Radiat Res. 2014;182:380–9. doi: 10.1667/RR13466.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudqvist N, Spetz J, Schuler E, Langen B, Parris TZ, Helou K, et al. Gene expression signature in mouse thyroid tissue after (131)I and (211)At exposure. EJNMMI Res. 2015;5:59. doi: 10.1186/s13550-015-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor AM. Chromosome instability syndromes. Best Pract Res Clin Haematol. 2001;14:631–44. doi: 10.1053/beha.2001.0158. [DOI] [PubMed] [Google Scholar]
- 16.Baria K, Warren C, Eden OB, Roberts SA, West CM, Scott D. Chromosomal radiosensitivity in young cancer patients: possible evidence of genetic predisposition. Int J Radiat Biol. 2002;78:341–6. doi: 10.1080/09553000110117359. [DOI] [PubMed] [Google Scholar]
- 17.Flint-Richter P, Sadetzki S. Genetic predisposition for the development of radiation-associated meningioma: an epidemiological study. Lancet Oncol. 2007;8:403–10. doi: 10.1016/S1470-2045(07)70107-9. [DOI] [PubMed] [Google Scholar]
- 18.Miles EF, Tatsukawa Y, Funamoto S, Kamada N, Nakashima E, Kodama Y, et al. Biomarkers of radiosensitivity in A-bomb survivors pregnant at the time of bombings in Hiroshima and Nagasaki. ISRN Obstet Gynecol. 2011;2011:264978. doi: 10.5402/2011/264978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornhardt S, Rossler U, Sauter W, Rosenberger A, Illig T, Bickeboller H, et al. Genetic factors in individual radiation sensitivity. DNA Repair (Amst) 2014;16:54–65. doi: 10.1016/j.dnarep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Washington, DC: National Academies Press; 2006. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. [PubMed] [Google Scholar]
- 21.Budach W, Hartford A, Gioioso D, Freeman J, Taghian A, Suit HD. Tumors arising in SCID mice share enhanced radiation sensitivity of SCID normal tissues. Cancer Res. 1992;52:6292–6. [PubMed] [Google Scholar]
- 22.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, et al. ATM-deficient mice: a paradigm of ataxia telangiectasia. Cell. 1996;86:159–71. doi: 10.1016/s0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 23.Beamer WG, Shultz KL, Tennent BJ, Shultz LD. Granulosa cell tumorigenesis in genetically hypogonadal-immunodeficient mice grafted with ovaries from tumor-susceptible donors. Cancer Res. 1993;53:3741–6. [PubMed] [Google Scholar]
- 24.Lee ML, Whitmore GA. Power and sample size for DNA microarray studies. Stat Med. 2002;21:3543–70. doi: 10.1002/sim.1335. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 26.Ghandhi SA, Ming L, Ivanov VN, Hei TK, Amundson SA. Regulation of early signaling and gene expression in the alpha-particle and bystander response of IMR-90 human fibroblasts. BMC Med Genomics. 2010;3:31. doi: 10.1186/1755-8794-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bø T, Jonassen I. New feature subset selection procedures for classification of expression profiles. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-4-research0017. RESEARCH0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 30.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–86. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 31.Hill R, Madureira PA, Waisman DM, Lee PW. DNA-PKcs binding to p53 on the p21WAF1/CIP1 promoter blocks transcription resulting in cell death. Oncotarget. 2011;2:1094–108. doi: 10.18632/oncotarget.378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Finzel A, Grybowski A, Strasen J, Cristiano E, Loewer A. Hyperactivation of ATM upon DNA-PKcs inhibition modulates p53 dynamics and cell fate in response to DNA damage. Mol Biol Cell. 2016;27:2360–7. doi: 10.1091/mbc.E16-01-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Candéias SM, Testard I. The many interactions between the innate immune system and the response to radiation. Cancer Lett. 2015;368:173–8. doi: 10.1016/j.canlet.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Hellweg CE. The nuclear factor kappaB pathway: a link to the immune system in the radiation response. Cancer Lett. 2015;368:275–89. doi: 10.1016/j.canlet.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6:702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 36.Di Maggio FM, Minafra L, Forte GI, Cammarata FP, Lio D, Messa C, et al. Portrait of inflammatory response to ionizing radiation treatment. J Inflamm (Lond) 2015;12:14. doi: 10.1186/s12950-015-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basu S, Rosenzweig KR, Youmell M, Price BD. The DNA-dependent protein kinase participates in the activation of NF kappa B following DNA damage. Biochem Biophys Res Commun. 1998;247:79–83. doi: 10.1006/bbrc.1998.8741. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Banin S, Ouyang H, Li GC, Courtois G, Shiloh Y, et al. ATM is required for IkappaB kinase (IKK) activation in response to DNA double strand breaks. J Biol Chem. 2001;276:8898–903. doi: 10.1074/jbc.M009809200. [DOI] [PubMed] [Google Scholar]
- 39.Sabatel H, Pirlot C, Piette J, Habraken Y. Importance of PIKKs in NF-kappaB activation by genotoxic stress. Biochem Pharmacol. 2011;82:1371–83. doi: 10.1016/j.bcp.2011.07.105. [DOI] [PubMed] [Google Scholar]
- 40.Nowak-Wegrzyn A, Crawford TO, Winkelstein JA, Carson KA, Lederman HM. Immunodeficiency and infections in ataxia-telangiectasia. J Pediatr. 2004;144:505–11. doi: 10.1016/j.jpeds.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 41.Staples ER, Mcdermott EM, Reiman A, Byrd PJ, Ritchie S, Taylor AM, et al. Immunodeficiency in ataxia telangiectasia is correlated strongly with the presence of two null mutations in the ataxia telangiectasia mutated gene. Clin Exp Immunol. 2008;153:214–20. doi: 10.1111/j.1365-2249.2008.03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabatel H, Di Valentin E, Gloire G, Dequiedt F, Piette J, Habraken Y. Phosphorylation of p65(RelA) on Ser(547) by ATM represses NF-kappaB-dependent transcription of specific genes after genotoxic stress. PLoS One. 2012;7:e38246. doi: 10.1371/journal.pone.0038246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okunieff P, Chen Y, Maguire DJ, Huser AK. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008;27:363–74. doi: 10.1007/s10555-008-9138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ossetrova NI, Blakely WF. Multiple blood-proteins approach for early-response exposure assessment using an in vivo murine radiation model. Int J Radiat Biol. 2009;85:837–50. [PubMed] [Google Scholar]
- 45.Tucker JD, Joiner MC, Thomas RA, Grever WE, Bakhmutsky MV, Chinkhota CN, et al. Accurate gene expression-based biodosimetry using a minimal set of human gene transcripts. Int J Radiat Oncol Biol Phys. 2014;88:933–9. doi: 10.1016/j.ijrobp.2013.11.248. [DOI] [PubMed] [Google Scholar]
- 46.Micallef L, Rodgers P. EulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9:e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. BRB-ArrayTools output of differentially expressed genes. Results for each genotype are in a separate tab.
Table S2. Gene ontology and network analyses. Tab 1: Annotation of Fig. 2 with gene ontology categories and terms, as well as actual Benjamini-adjusted P values for categories significantly over-represented among up- or downregulated genes in the different genotypes. Tab 2: An IPA-generated network for IFNG showing differential gene expression in the three genotypes.
Table S3. Tab 1: Composition of the classifiers trained using data from WT, Atm−/−, SCID or a mixed-genotype training set. Tab 2: Performance of the classifiers on the new samples in the test sets.