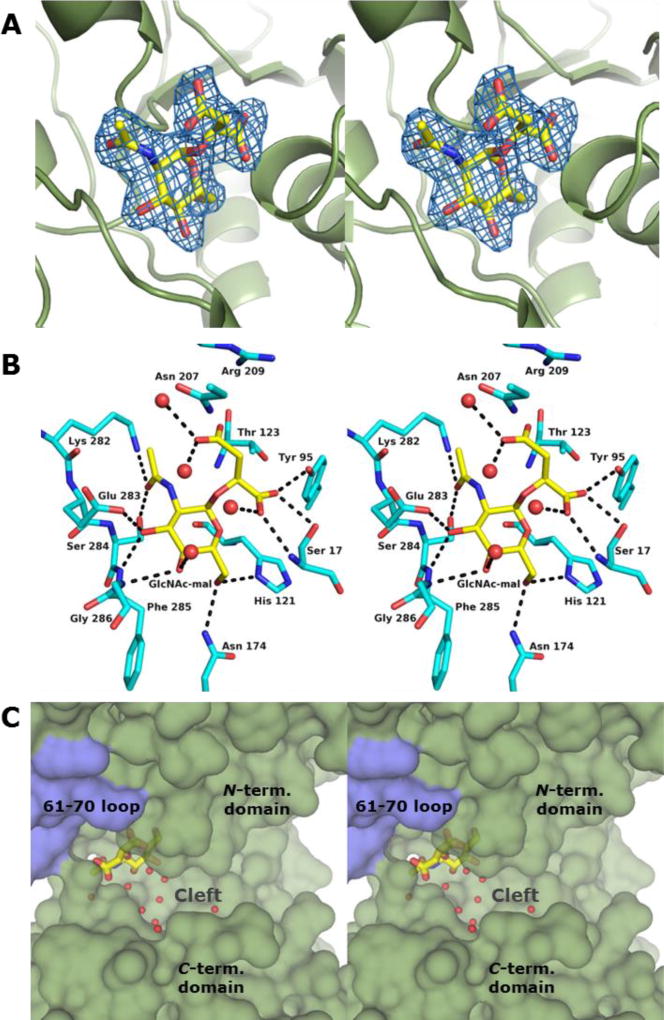
Figure 2. Structure of BshA bound with GlcNAc-mal.
(A) Stereo representation of electron density corresponding to the bound GlcNAc-mal product. The map was calculated with coefficients of the form Fo - Fc with the atoms of the product excluded from the coordinate file and is contoured at 2.5 σ. (B) Close-up stereo view of the active site with the bound product. Amino acid residues lying within ~3.4 Å of the product are shown. Potential hydrogen bonds are highlighted with dashed lines. Water molecules are shown as red spheres. (C) Surface rendering of BshA highlighting the active site cleft and GlcNAc-mal. Subunit A is colored green, whereas Subunit B is colored blue. GlcNAc-mal is shown in sticks with the malyl moiety pointing toward the viewer and the α-face of the hexose pointing into the cleft.