Abstract
Fibroblast growth factor receptor (FGFR) 2 and its downstream signaling cascades, PI3K/AKT/mTOR is playing an important role in cell survival and proliferations. In this study, we firstly found that picrasidine Q (PQ), an alkaloid component extracted from Angelica keiskei species, has the capacity of anti-cell transformation and anti-cancer. After ligand shape similarity approach of PQ, we found that PQ targeted FGFR 2 and verified by FGFR2 kinase assay as well as computational docking model. FGFR2 highly expressed in esophageal cancer tissues and PQ inhibited fibroblast growth factor (FGF)-induced cell transformation. Furthermore, PQ inhibited cell proliferation and induced cell cycle arrest and apoptosis in KYSE30, KYSE410 and KYSE450 esophageal squamous cell carcinoma (ESCC) cells. It was confirmed by detecting of biological markers such as cyclinD1, cyclinD3 and cyclinB1 for cell cycle or cleaved caspase-7, caspase-3 and PARP for apoptosis. PQ targeting of FGFR2 kinase activities suppressed downstream target proteins including phosphorylation of AKT and mTOR but not MEK/ERK signaling pathways. Taken together, our results are the first to identify that PQ might be a chemopreventive and chemotherapeutic agent by direct targeting FGFR2 and inhibiting cell proliferation of ESCC cells.
Keywords: Picrasidine Q (PQ), FGFR2, Esophageal squamous cell carcinoma, AKT/mTOR signaling pathways
Introduction
ESCC is the eighth most frequently diagnosed cancer worldwide (1). Rates vary broadly among countries (2), with approximately half of all cases occurring in China. It is about three times more common in male than in female (1). Five-year survival rates of ESCC are round 13% to 18% (3).
To date, the FGFs belong to a family consists of FGFR1-4 with many isoforms (4) which have been cloned and characterized. FGF receptors are transmembrane tyrosine kinase receptors involved in signaling when stimulate by FGF family. FGFR2 displays biological activity towards cell proliferation, tumorigenesis, metastasis, and angiogenesis (4-6). Recently, it has been reported that many diseases are related to the mutation and the expression of different subtypes of FGFR2 (5,7-9). FGFR2 mutation or amplification was found in varied cancers like colon, gastric, endometrial, esophageal and cholangiocarcinoma (10-15), it also has a close relationship with tumorigenesis (16). The activated FGFR2 can trigger the down-stream signaling pathways such as RAS-MAPK and PI3K-AKT (4-6). Therefore, the development of natural compounds which could inhibit FGFR2 kinase was not only able to inhibit its kinase activity and tumorigenesis, but also avoid the resistance of synthesized chemotherapeutic agents.
Oriental medicinal herbs contain varied beneficial natural compounds and have been widely utilized for identifying novel compounds that may have therapeutic value in human diseases. Angelica keiskei is known under the Japanese name of Ashitaba. Traditionally it is considered as a major contributor to the supposedly healthier, extended lives of the local residents. Possibly because of it contains many kinds of various flavonoids, coumarins, phenolics, acetylenes, sesquiterpene, diterpene, and triterpenes (17,18). So far, it was identified around 43 kinds of components, but still not cleared (18). Angelica keiskei is reported to exhibit cytotoxic, antidiabetic, antioxidative, anti-inflammatory, antihypertensive, and antimicrobial (17-23) properties via in vitro studies though the efficacy of these qualities have yet to be confirmed in vivo (18). Among current researches, it shows potentially usefulness in cancer, as well as its potential as a nerve growth factor etc (24,25). PQ is the one of compounds extracted from the bark of Picrasma quassioides, which first being reported as a natural product in 1993 (26). However, there is no reported PQ extracted from the Angelica keiskei and rare investigation was carried out research on its biological effects, especially anticancer effects.
The aim of this study was to clarify the anticancer effects of PQ from Angelica keiskei, in the ability to suppress cell proliferation, induce cell apoptosis and G1 phase arrest in ESCC, and directly inhibit FGFR2 kinase activity.
Materials and methods
Reagents and materials
PQ (molecular weight 266.26, purity > 95%) was purified by modification of the processes in previous reports (27). It was analyzed and authenticated by nuclear magnetic resonance (NMR) spectroscopy. The other hands, PQ (purity > 98%) purchased from Wuhan Chem Faces Biochemical Co., Ltd. PQ was prepared with a stock solution (100 mM) by dissolving in dimethyl sulfoxide (DMSO, ≥99.7%, Sigma-Aldrich Co. LLC), after which it was aliquoted and stored in a light-shield container at -20°C. PQ was freshly diluted in DMSO before treatment of cells by medium exchange with PQ premixed cell culture medium. Human recombinant FGF was purchased from Sigma-Aldrich. Antibodies, phospho-FGFR2, total FGFR, phospho-Akt (pAKT), total AKT, phospho-GS3β (pGS3β), total GS3β, phospho-mTOR (pmTOR), total mTOR, cyclinB1, cyclinD1, cyclinD3, cleaved caspase 3, cleaved caspase 7 and cleaved PARP were obtained from Cell Signaling Technology (Beverly, MA).
Cell culture
The human esophageal cancer cell lines (KYSE30, KYSE410 and KYSE450) were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in RPMI-1640 containing penicillin (100 units/mL), streptomycin (100 μg/mL), and 10% FBS (BI, Israel). Human immortalized normal esophageal epithelial cell line, SHEE was donated by Dr. Enmin Li in laboratory of tumor pathology, Shantou University Medical College (28). Cells were maintained at 5% CO2, 37°C in a humidified atmosphere. All cells were cytogenetically tested and authenticated before the cells were frozen. Each vial of frozen cells was thawed and maintained in culture for a maximum of 8 weeks.
3-[4,5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazoliumbromide (MTT) assay
To detect the cytotoxicity on normal esophageal cells, SHEE (8 × 103) were seeded into 96-well plates in 100 μl of RPMI-1640/10% FBS and incubated for 24 h at 37°C in a 5% CO2 incubator, and then treated with vehicle or PQ at indicated concentration, the absorbance was measured at 570 nm wavelength using MTT in the time point of 24, 48 and 72 h. Briefly, 20 μl of the MTT solution was added to the cells in each well, and were incubated for 1 h at 37°C in a 5% CO2 incubator, then discard the supernatant and add 100 μl of DMSO to dissolve the formazan crystals before the absorbance measurement. The percentage of cell viability was determined as the ratio of the absorbance of the sample versus the control. Six replicate wells were used for each compound concentration. To measure cell proliferation, KYSE30 (1 × 103), KYSE410 (2 × 103) or KYSE450 (1 × 103) ESCC cells were seeded into 96-well plates and treated with vehicle or PQ for 24, 48 and 72 h. After incubation, cells examined as described above subsequently. Each vial of frozen cells was thawed and maintained for about 10 passages.
Anchorage-independent cell transformation/proliferation assay
FGF-induced cell transformation was investigated in JB6 Cl41 cells. Briefly, cells (8 × 103) were exposed to vehicle, FGF (10 ng/ml) or FGF plus different concentrations of PQ in 0.3% upper Basal Medium Eagle agar based by 0.5% bottom agar. KYSE30, KYSE410 or KYSE450 cells (each 8 × 103) were suspended in 0.3% RPMI-1640 agar solution with vehicle, 20, 40 or 60 μM PQ were poured over 0.5% hard-bottomed agar previously in each 6-well plate. The cultures were maintained in a 37°C, 5% CO2 incubator for 2 or 3 weeks and the cell colonies were scored using Image-Pro 6.0 (Media Cybernetics, Silver Spring, MD).
Cell cycle analysis
KYSE30 (1.5 × 105), KYSE410 (2.7 × 105) and KYSE450 (2 × 105) cells were seeded into 60 mm diameter dishes and cultured overnight at 37°C in a 5% CO2 incubator. To examine the cell cycle under normal cell culture conditions, KYSE30 cells were treated with the indicated concentrations of PQ in complete cell culture medium for 48 h. While KYSE410 cells were starved with serum deficient culture medium for 24 h before treated with PQ as KYSE30. All the cells were trypsinized, fixed overnight and then stained with propidium iodide (20 μg/ml) for 15 min at 4°C. The cell cycle distribution was measured by FACScan flow cytometry (BD FACS Calibur flow cytometer).
Apoptosis assay
Apoptosis in the presence or absence of PQ was examined using flow cytometry by staining the cells with annexin V-FITC and propidium iodide (BoiLegend, Nagoya, Japan) labeling. KYSE30 (1.5 × 105), KYSE410 (2.7 × 105) and KYSE410 (2 × 105) cells were seeded into 60 mm diameter dishes and cultured at 37°C in a 5% CO2 incubator. After treatment of PQ for 72 h, cells were harvested and stained with annexin V-FITC and propidium iodide, and then analyzed by FACScan flow cytometry.
In silico target identification and molecular docking modeling
To identify potential binding target of PQ, a shape similarity method, ROCS (29) from the OpenEye tool kits, was used to search for potential biological targets of PQ. Several target libraries together with our in-house database were used in this screening. Based on the result we can identify potential target of PQ (30). For the predicted docking model of PQ and FGFR2, first the three-dimensional (3-D) structure of FGFR2 was derived from the Protein Data Bank (31) (PDB ID:3RI1). The structure was an X-ray crystal structure with a 2.1Å resolution of human FGFR2 kinase domain in complex with ARQ 069 (32). This raw PDB format structure was converted into an all-atom, fully prepared receptor model structure for docking using the Protein Preparation Wizard in Schrödinger Suite 2016 (33). Hydrogen atoms were added consistent with a pH of 7 and all water molecules were removed. The ATP binding pocket based grid file was generated for docking studying.
The compound of PQ was prepared for docking by default parameters using the LigPrep program. Then, the docking of PQ with FGFR2 was accomplished with default parameters under the extra precision (XP) mode using the program Glide. Herein, we could get the best-docked representative structures.
Western blotting
Samples containing equal amounts of protein were resolved by 10, 12 or 15 % SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. The membranes were incubated in blocking buffer containing 5% skim milk and then were probed with phospho-specific antibodies against phospho-Akt, total Akt, phospho-GS3β, total GS3β, phospho-mTOR, total mTOR, cyclinB1, cyclinD1, cyclinD3, cleaved caspase-3, cleaved caspase-7, cleaved PARP and β-actin. After incubation of the blots at 4°C for 18 h, blots were washed three times with 1X PBS-T buffer, followed by the incubation with the appropriate horseradish peroxidase-linked immunoglobulin G (IgG). Western blots were visualized with a chemiluminescence detection reagents using Amersham Imager 600 (GE Healthcare life Science, Pittsburgh, PA).
In vitro kinase assay
In vitro FGFR2 kinase assay was carried out using CycLex FGFR2 Kinase Assay/Inhibitor Screening Kit (CycLex, Japan) according to the manufacturer’s instructions together with staturosporine, free base (â99%, LC Laboratories, Woburn, MA) as a positive control.
Results
PQ targeted FGFR2 and inhibited its kinase activity
To investigate the new component of Angelica keiskei, we extracted the components of fractions and finally found the PQ which is an alkaloid type of compound (Fig. 1A). To elucidate potential targets of PQ, we first conducted in silico screening by using a shape similarity approach. Screening results showed that PQ was very similar to, a FGFR2 inhibitor, which implied that FGFR2 was a possible molecular target for PQ (Fig. 1B). For the understanding of PQ interacts with FGFR2, we docked it ATP binding pocket of FGFR2 through several protocols in the Schrödinger Suite 2016. Based on the computational docking model result, we found that PQ formed some hydrogen bonds with FGFR2 at the binding pocket (Fig. 1B). These indicated that PQ might be a potential inhibitor of FGFR2 (images were generated with the UCSF Chimera program (34)). To determine whether PQ can inhibit the FGFR2 kinase activity, we conducted an in vitro kinase assay with recombinant FGFR2 in the presence of vehicle, 12.5, 25 or 50 μM concentrations of PQ. Staurosporin, a well-known non-specific FGFR2 inhibitor was used as a positive control (Fig. 1C). PQ suppressed the kinase activity of FGFR2 in a dose-dependent manner (Fig. 1C). To identify the effect of other kinases, we investigated kinase assay of other kinases such as EGFR (epidermal growth factor), ErbB2 (Erb-B2 Receptor Tyrosine Kinase 2, Her-2), ErbB4, Lyn (LYN Proto-Oncogene, Src Family Tyrosine Kinase). PQ inhibited 66±3.47% of kinase activity FGFR2 but 36% of EGFR, 24% of ErbB2 or 12% of ErbB4, 22% of Lyn, respectively. Therefore, PQ mainly targets to FGFR2 (Supplementary Figure 1).
Figure 1.
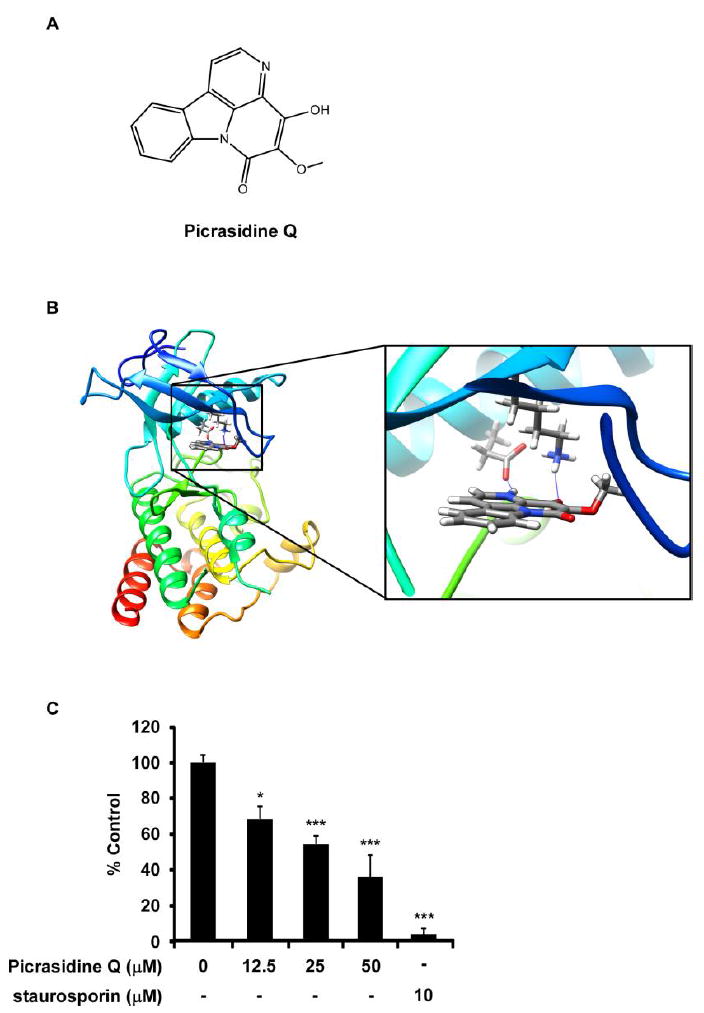
PQ was obtained from barks of Angelica keiskei targets FGFR2. (A) Chemical structure of PQ. (B) Modeling of FGFR2 binding with PQ. Left; PQ binding with FGFR2 at the ATP binding pocket; Right: enlarged view of PQ binding with FGFR2. FGFR2 structure are shown as ribbon representation, PQ is shown as stick. Hydogen bonds are shown as blue line. (C) Effects of PQ on FGFR2 in vitro kinase activity. PQ inhibited FGFR2 kinase activity at 12.5, 25, 50 μM compared to vehicle control (*P < 0.05, ***P < 0.001).
PQ suppressed FGF-induced cell transformation
Because of PQ targeted to FGFR, it was examined the effect of PQ on FGF-induced cell transformation of JB6 Cl41 cells. We treated PQ on JB6 Cl41 cells with indicated concentration under the FGF-stimulated condition and counted its promoted cell transformation. FGF obviously induced cell transformation compared to DMSO treated, and PQ significantly inhibited 71.8, 100% of FGF-induced cell transformation at 40 or 60 μM, respectively compared to DMSO control (Fig. 2A, B). Furthermore, JB6 Cl41 cells were induced S phase of cell cycle by treatment of FGF and then escaped from the G1 phase. Conversely cells treated with PQ were arrested 19.6% in G1 phase of cell cycle at 60 μM (Fig. 2C).
Figure 2.
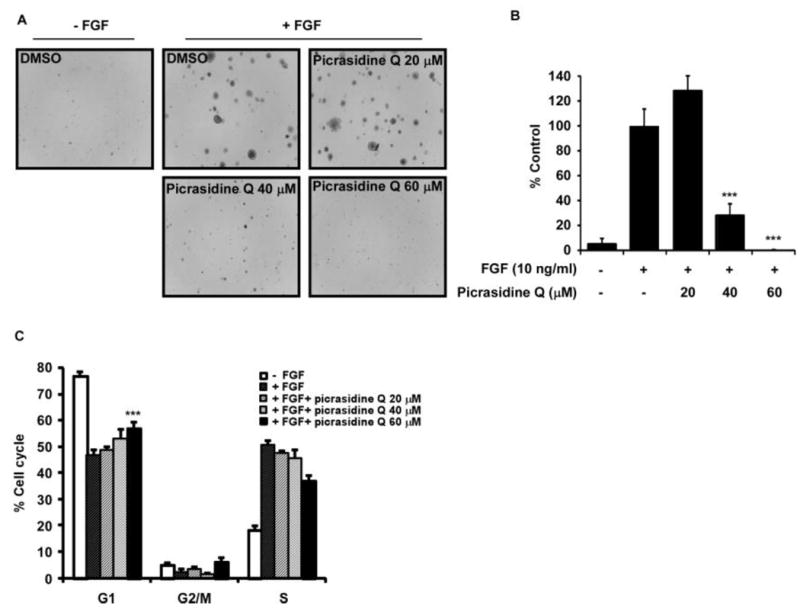
PQ inhibited proliferation by inhibition of G1/S cell cycle transition in JB6 Cl41 cells. Cells (2 × 105) were seeded into 60 mm cell culture dishes and cultured overnight. The cells were starved for 24 h supplemented with 0.1% FBS/MEM and pretreated for 30 min with 0, 20, 40 or 60 μM concentration of PQ and then added with FGF (10 ng/ml) for 12 h. The cell cycle population was measured by FACS. Data are presented as the mean ± SD of values from triplicate experiments, and statistical significance was determined using Student’s t-test (***P < 0.001).
PQ inhibited the proliferation of ESCC cells
To clarify the importance of FGFR2 pathway in ESCC, we first examined the expression of FGFR2 (Fig. 3A). Immunohistochemistry data confirmed that FGFR2 expression in ESCC tissues was significantly higher than observed in normal tissues (Fig. 3A).
Figure 3.
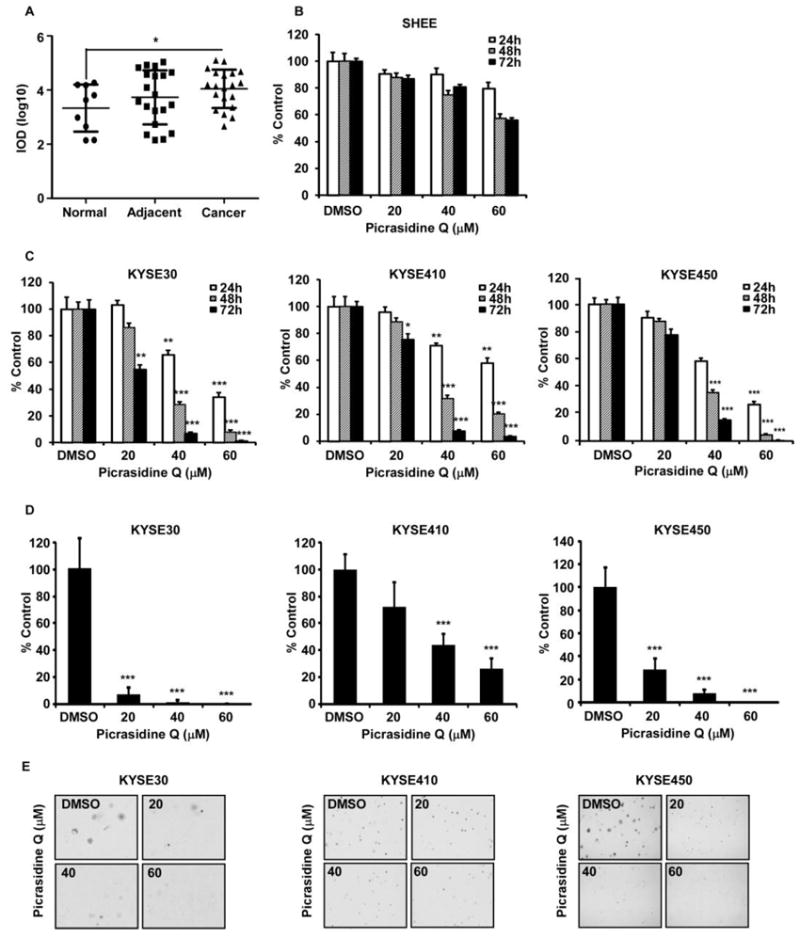
PQ suppressed the growth of ESCC cells. (A) Expression of FGFR2 on ESCC tissues. (B) Toxicity of PQ on normal esophageal cells. (C) Effects of PQ on the proliferation of KYSE30, KYSE410 and KYSE450 ESCC cells were assessed at 24, 48 and 72 h by MTT assay. The asterisk indicates a significant decrease in proliferation compared with vehicle control (**P < 0.01, ***P<0.001). (D) Effects of PQ on anchorage-independent growth of KYSE30, KYSE410 and KYSE450 ESCC cells were evaluated. The asterisks (***P < 0.001) indicate a significant decrease in colony formation with PQ treatment compared with untreated control. (E) Reflected pictures of anchorage-independent cell growth results.
The effect of PQ on the cytotoxicity and proliferation of normal esophageal cells and esophageal carcinoma cancer cells were determined by MTT assay (Fig. 3B-E). The result showed that PQ didn’t possess substantial toxicity to N1217 normal esophageal cells (Fig. 3B). The FGFR2 protein was highly expressed in KYSE30, KYSE410 and KYSE450 ESCC cells, whereas it was barely expressed in normal esophageal cells and other ESCC cells. Compared to normal cells, PQ was sharply inhibited cell proliferation of KYSE30, KYSE 410 and KYSE450 ESCC cells in a time- and dose-dependent manner (Fig. 3C). For the further evaluation, we examined the effect of PQ treatment on anchorage-independent growth of ESCC cells, KYSE30, KYSE410 and KYSE450 (Fig. 3D, E). PQ suppressed colonies growth of KYSE30, KYSE410 and KYSE450 cells (Fig. 3D, E). It is also revealed that KYSE30 and KYSE 450 cells were more sensitive to PQ treatment than that of KYSE410 cells.
PQ induces cell cycle and apoptosis of ESCC cells
Inhibition of FGFR2 can result in many effects on cells such as cell cycle and apoptosis. Therefore, we examined whether PQ affects cell cycle progression and apoptosis (Fig. 4). Results revealed that PQ treatment led to G1 phase arrest of cell cycle in KYSE30, KYSE410 and KYSE450 cells (Fig. 4A, Supplementary Fig. 2A). PQ also induced G2/M phase at 60 μM concentration in KYSE30 and KYSE450 cells (Fig. 4A left, right). We also identified that these effects were reflected on the expression of cell cycle markers such as cyclin D1, cyclin D3 and cyclin B1 (Fig. 4B). PQ impaired the expression of cell cycle markers in dose dependent manner (Fig. 4B). To apoptosis analysis, KYSE30, 410 and 450 cells were treated with PQ at the concentration of 0, 20, 40 or 60 µM for 72 h. The results revealed that PQ significantly induced apoptosis at 40 or 60 μM (Fig. 4C, Supplementary Fig. 2B). Furthermore, PQ increased expression of apoptosis markers such as cleaved caspase-7, caspase-3 and PARP (Fig. 4D). In addition, we verified the effects of FGFR2 inhibition to ESCC by treatment of clinical FGFR2 inhibitor, BGJ-398 (Fig. 5). BGJ-398 significantly inhibited cell proliferation as well as anchorged-independent cell growth in a dose dependent manner (Fig. 5A-C).
Figure 4.
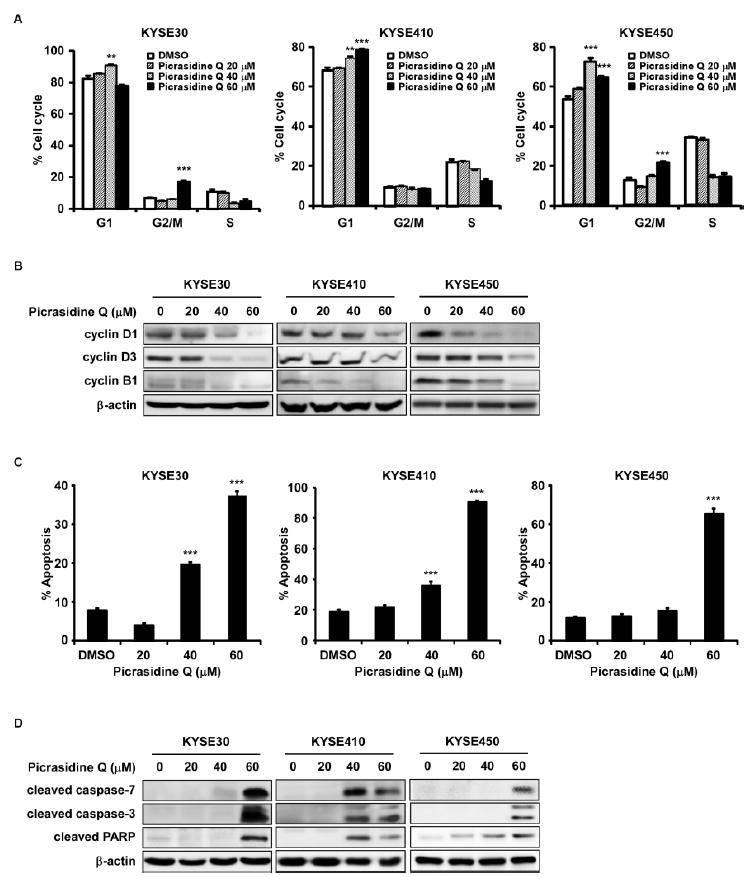
PQ induced cell cycle arrest and apoptosis. Effects of PQ on cell cycle (A, B) and apoptosis (C, D) were in KYSE30, KYSE410 and KYSE450 ESCC cells. Cells were treated with 0, 20, 40 or 60 μM of PQ and then incubated for 48 h (cell cycle analysis, cell cycle marker expression) and 72 h (annexin-V staining assay, apoptosis marker expression). The asterisks (**P < 0.01, ***P < 0.001) indicate a significant difference between untreated control and treated cells.
Figure 5.
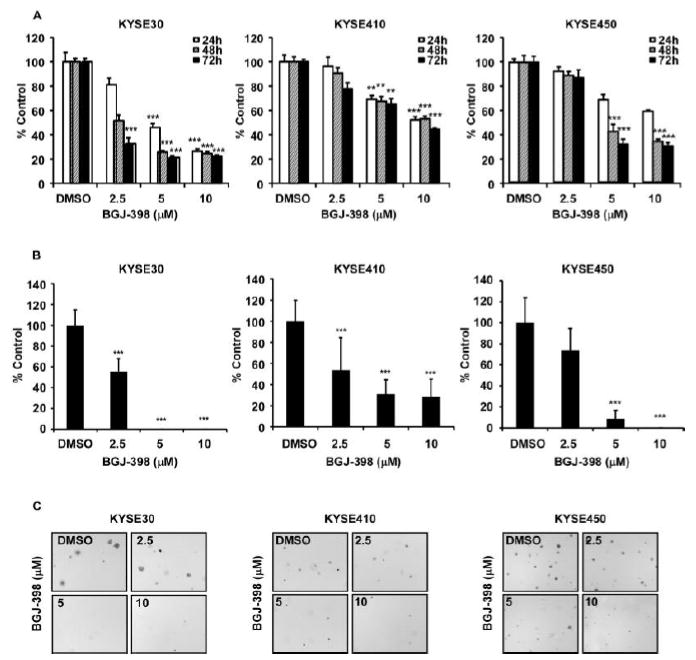
BGJ-398 inhibited the growth of ESCC cells. (A) Effects of BGJ-398 on the proliferation of KYSE30, KYSE410 and KYSE450 ESCC cells were assessed at 24, 48 and 72 h by MTT assay. The asterisk indicates a significant decrease in proliferation compared with vehicle control (**P < 0.01, ***P<0.001). (B) Effects of BGJ-398 on anchorage-independent growth of KYSE30, KYSE410 and KYSE450 ESCC cells were evaluated. The asterisks (***P < 0.001) indicate a significant decrease in colony growth with BGJ-398 treatment compared with untreated control. (C) Reflected pictures of anchorage-independent cell growth results.
PQ inhibited the AKT/mTOR signaling pathways
To investigate the inhibitory signaling pathways of cell proliferation by treatment of PQ, we hypothesized that PQ might suppress the AKT/mTOR or ERKs/RSKs signaling pathway because PQ inhibited cell proliferation and FGF-induced G1/S cell cycle progression (Fig. 2). To examine the hypothesis, we analyzed phosphorylation protein profiles by treatment with or without FGF (Supplementary Fig. 3). FGF induced expression of phosphorylated AKT but not MEK or ERK (Supplementary Fig. 3). It was verified that PQ suppressed the expression of phosphorylated AKT and mTOR in KYSE30, KYSE410 and KTSE450 cells in a concentration dependent manner (Fig. 6).
Figure 6.
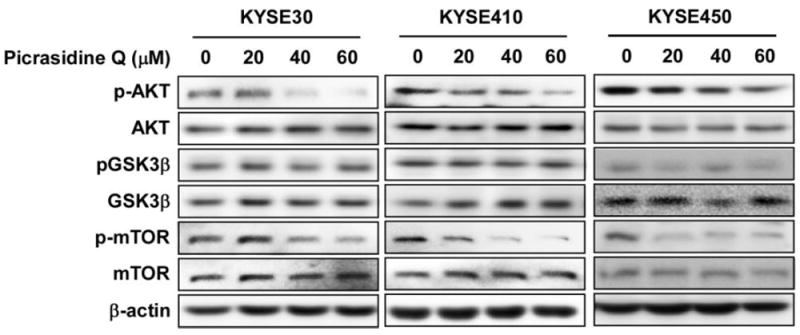
PQ suppressed downstream signaling of FGFR2 in ESCC cells. Cells were treated with the indicated concentration of PQ for 3 h. The proteins were extracted and pAKT, total AKT, pGSK3β, total GSK3β, pmTOR and total mTOR were visualized by western blotting using specific antibodies as indicated. β-Actin was used for the internal control to verify equal protein loading.
Discussion
At the beginning, we tried to find new components from Angelica keiskei and idenfy its underlying molecular mechanism in cancer. Finally, we found the new compound, PQ from the many fractions of Angelica keiskei (data not shown) and confirmed by MNR spectroscopy analysis (Supplementary Fig. 4). H.-Y. Li et al., reported the extraction of picrasidine W, X, Y and Q from Picrasma quassioides (26). Other types of picrasidine have exhibited activities of anti-inflammation, anti-metabolic disease, cerebral protection and anti-diabetes (35-41). Although the extracts of Angelica keiskei have used in several diseases, it is not clearly elucidated the effects of PQ on the disease models, even in cancers. Because of ESCC is one of the leading cancer in the worldwide, we examined that PQ inhibited cell proliferation of ESCC cells (Fig. 3C, D). For the investigation of direct target to PQ, we performed ligand shape similarity approach and found FGFR2 as a potential target. After confirmed by kinase assay, FGFR2 was identified as a direct target of PQ (Fig. 1B, C). Also FGF which is a ligand of FGFR2 induced cell transformation and cell cycle promotion, and PQ abrogated its colony numbers as well as arrested G1 phase of cell cycle (Fig. 2). Furthermore, we showed the inhibitory effects of BGJ-398, clinical FGFR2 inhibitor in cell proliferation and similar tendency with PQ treatment in ESCC cells. The specific binding of FGF and FGFR induces its dimerization and phosphorylation at the kinase domain of cytosolic face, resulting in the activation of the intracellular signaling pathway. Activation of FGFR2 promotes the cell survival and proliferation by AKT or ERK signaling pathways (5,6). When treated with FGF in KYSE410 cells which cultured with medium not contained serum, phosphorylation of AKT was increased. Therefore, we detected expressions of AKT/GSK3β/mTOR. PQ suppressed the expressions of AKT and mTOR but not GSK3β (Fig. 6). PQ majorly affects to AKT/mTOR signaling pathway.
In conclusion, we first demonstrate that PQ inhibits the cell growth and induces apoptosis in KYSE30 and KYSE410 ESCC cells by suppressing AKT/mTOR signaling through direct targeting of FGFR2.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health, USA, CA187027, CA196639, CA166011 and Key program of Henan Province, China, Grant NO.161100510300 (Z.G.Dong), and National Natural Science Foundation of China NSFC81672767 (M.H.Lee) and Foreign Young Scientist NSFC 81650110530 (M. Fredimoses), Zhengzhou Intellectual Property Office and Henan Provincial Government, China. Funders had no role in producing this manuscript. We wish to thank Ran Yang in China-US (Henan) Hormel Cancer Institute for supporting experiments
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Montgomery EA, B F, Brennan P, Malekzadeh R. Oesophageal Cancer. International Agency for Research on Cancer; 2014. [Google Scholar]
- 2.Chen S, Zhou K, Yang L, Ding G, Li H. Racial Differences in Esophageal Squamous Cell Carcinoma: Incidence and Molecular Features. BioMed research international. 2017;2017 doi: 10.1155/2017/1204082. 1204082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.F FF. Esophageal Tumors. Maryland Heights, MO: Mosby (Elsevier); 2012. [Google Scholar]
- 4.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature reviews Cancer. 2010;10(2):116–29. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 5.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nature reviews Cancer. 2017;17(5):318–32. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 6.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley interdisciplinary reviews Developmental biology. 2015;4(3):215–66. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee KM, Santos-Ruiz L, Ferretti P. A single-point mutation in FGFR2 affects cell cycle and Tgfbeta signalling in osteoblasts. Biochimica et biophysica acta. 2010;1802(3):347–55. doi: 10.1016/j.bbadis.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Sagong B, Jung DJ, Baek JI, Kim MA, Lee J, Lee SH, et al. Identification of causative mutation in a Korean family with Crouzon syndrome using whole exome sequencing. Annals of clinical and laboratory science. 2014;44(4):476–83. [PubMed] [Google Scholar]
- 9.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, et al. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nature genetics. 2007;39(7):870–4. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smyth EC, Babina IS, Turner NC. Gatekeeper Mutations and Intratumoral Heterogeneity in FGFR2-Translocated Cholangiocarcinoma. Cancer discovery. 2017;7(3):248–49. doi: 10.1158/2159-8290.CD-17-0057. [DOI] [PubMed] [Google Scholar]
- 11.Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, et al. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer research. 2008;68(7):2340–8. doi: 10.1158/0008-5472.CAN-07-5229. [DOI] [PubMed] [Google Scholar]
- 12.Kwak Y, Cho H, Hur W, Sim T. Antitumor Effects and Mechanisms of AZD4547 on FGFR2-Deregulated Endometrial Cancer Cells. Molecular cancer therapeutics. 2015;14(10):2292–302. doi: 10.1158/1535-7163.MCT-15-0032. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, et al. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(12):4017–27. doi: 10.1158/1078-0432.CCR-08-2824. [DOI] [PubMed] [Google Scholar]
- 14.Mathur A, Ware C, Davis L, Gazdar A, Pan BS, Lutterbach B. FGFR2 is amplified in the NCI-H716 colorectal cancer cell line and is required for growth and survival. PloS one. 2014;9(6):e98515. doi: 10.1371/journal.pone.0098515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato H, Arao T, Matsumoto K, Fujita Y, Kimura H, Hayashi H, et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. International journal of oncology. 2013;42(4):1151–8. doi: 10.3892/ijo.2013.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CJ, Lee MH, Cho YY. Fibroblast and Epidermal Growth Factors Utilize Different Signaling Pathways to Induce Anchorage-independent Cell Transformation in JB6 Cl41 Mouse Skin Epidermal Cells. Journal of cancer prevention. 2014;19(3):199–208. doi: 10.15430/JCP.2014.19.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kil YS, Pham ST, Seo EK, Jafari M. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Archives of pharmacal research. 2017 doi: 10.1007/s12272-017-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caesar LK, Cech NB. A Review of the Medicinal Uses and Pharmacology of Ashitaba. Planta medica. 2016;82(14):1236–45. doi: 10.1055/s-0042-110496. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Liu JJ, Sun J, Yang XH, Zhao TT, Lu X, et al. Design, synthesis and biological evaluation of novel chalcone derivatives as antitubulin agents. Bioorganic & medicinal chemistry. 2012;20(10):3212–8. doi: 10.1016/j.bmc.2012.03.055. [DOI] [PubMed] [Google Scholar]
- 20.Mahapatra DK, Asati V, Bharti SK. Chalcones and their therapeutic targets for the management of diabetes: structural and pharmacological perspectives. European journal of medicinal chemistry. 2015;92:839–65. doi: 10.1016/j.ejmech.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Yadav VR, Prasad S, Sung B, Aggarwal BB. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. International immunopharmacology. 2011;11(3):295–309. doi: 10.1016/j.intimp.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, Yamashita Y, Yasuda M, Yamamoto N, Ashida H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food & function. 2015;6(1):135–45. doi: 10.1039/c4fo00525b. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa H, Nakashima S, Baba K. Effects of dietary Angelica keiskei on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clinical and experimental pharmacology & physiology. 2003;30(4):284–8. doi: 10.1046/j.1440-1681.2003.03830.x. [DOI] [PubMed] [Google Scholar]
- 24.Oh SR, Kim SJ, Kim DH, Ryu JH, Ahn EM, Jung JW. Angelica keiskei ameliorates scopolamine-induced memory impairments in mice. Biological & pharmaceutical bulletin. 2013;36(1):82–8. doi: 10.1248/bpb.b12-00681. [DOI] [PubMed] [Google Scholar]
- 25.Sumiyoshi M, Taniguchi M, Baba K, Kimura Y. Antitumor and antimetastatic actions of xanthoangelol and 4-hydroxyderricin isolated from Angelica keiskei roots through the inhibited activation and differentiation of M2 macrophages. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2015;22(7-8):759–67. doi: 10.1016/j.phymed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Li HY, Ka K, T O. New Alkaloids Picrasidines W, X and Y from Picrasma quassioides and X-Ray Crystallographic Analysis of Picrasidine Q. Chem Pharm Bull. 1993;41(10):1807–11. [Google Scholar]
- 27.Kimiye Baba KN, Masahiko Taniguchi, Tadashi Kido, Mitsugi Kozawa. Chalcones from Angelica Keiskei. Phytochemistry. 1990;29(12):3907–39010. [Google Scholar]
- 28.Shen ZY, Xu LY, Li EM, Shen J, Zheng RM, Cai WJ, et al. Immortal phenotype of the esophageal epithelial cells in the process of immortalization. International journal of molecular medicine. 2002;10(5):641–6. [PubMed] [Google Scholar]
- 29.ROCS 3.2.1.4, OpenEye Scientific Software, Inc. Santa Fe, NM: ( www.eyesopen.com) [Google Scholar]
- 30.Chen H, Yao K, Nadas J, Bode AM, Malakhova M, Oi N, et al. Prediction of molecular targets of cancer preventing flavonoid compounds using computational methods. PloS one. 2012;7(5):e38261. doi: 10.1371/journal.pone.0038261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic acids research. 2000;28(1):235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eathiraj S, Palma R, Hirschi M, Volckova E, Nakuci E, Castro J, et al. A novel mode of protein kinase inhibition exploiting hydrophobic motifs of autoinhibited kinases: discovery of ATP-independent inhibitors of fibroblast growth factor receptor. The Journal of biological chemistry. 2011;286(23):20677–87. doi: 10.1074/jbc.M110.213736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrödinger. Schrödinger Suite 2016. New York, NY: LLC; 2016. [Google Scholar]
- 34.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25(13):1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 35.Chen KC, Lee WY, Chen HY, Chen CY. In silico investigation of potential mTOR inhibitors from traditional Chinese medicine for treatment of Leigh syndrome. BioMed research international. 2014;2014:139492. doi: 10.1155/2014/139492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen KC, Sun MF, Chen CY. In Silico Investigation of Potential PARP-1 Inhibitors from Traditional Chinese Medicine. Evidence-based complementary and alternative medicine : eCAM. 2014;2014:917605. doi: 10.1155/2014/917605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao WH, Gao H, Li CY, Zhao F, Jiang RW, Wang Y, et al. Quassidines A-D, bis-beta-carboline alkaloids from the stems of Picrasma quassioides. Journal of natural products. 2010;73(2):167–71. doi: 10.1021/np900538r. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Li W, Higai K, Koike K. Canthinone alkaloids are novel protein tyrosine phosphatase 1B inhibitors. Bioorganic & medicinal chemistry letters. 2015;25(9):1979–81. doi: 10.1016/j.bmcl.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki T, Li W, Ohmoto T, Koike K. Evaluation of canthinone alkaloids as cerebral protective agents. Bioorganic & medicinal chemistry letters. 2016;26(20):4992–95. doi: 10.1016/j.bmcl.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhao S, Kanno Y, Li W, Sasaki T, Zhang X, Wang J, et al. Identification of Picrasidine C as a Subtype-Selective PPARalpha Agonist. Journal of natural products. 2016;79(12):3127–33. doi: 10.1021/acs.jnatprod.6b00883. [DOI] [PubMed] [Google Scholar]
- 41.Zhao S, Kanno Y, Li W, Wakatabi H, Sasaki T, Koike K, et al. Picrasidine N Is a Subtype-Selective PPARbeta/delta Agonist. Journal of natural products. 2016;79(4):879–85. doi: 10.1021/acs.jnatprod.5b00909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


