Abstract
The hippocampus plays an important role in memory function relying on information interaction between distributed brain areas. The hippocampus can be divided into the anterior and posterior sections with different structure and function along its long axis. The aim of this study is to investigate the effects of normal aging on the structural covariance of the anterior hippocampus (aHPC) and the posterior hippocampus (pHPC). In this study, 240 healthy subjects aged 18–89 years were selected and subdivided into young (18–23 years), middle-aged (30–58 years), and older (61–89 years) groups. The aHPC and pHPC was divided based on the location of uncal apex in the MNI space. Then, the structural covariance networks were constructed by examining their covariance in gray matter volumes with other brain regions. Finally, the influence of age on the structural covariance of these hippocampal sections was explored. We found that the aHPC and pHPC had different structural covariance patterns, but both of them were associated with the medial temporal lobe and insula. Moreover, both increased and decreased covariances were found with the aHPC but only increased covariance was found with the pHPC with age (p < 0.05, family-wise error corrected). These decreased connections occurred within the default mode network, while the increased connectivity mainly occurred in other memory systems that differ from the hippocampus. This study reveals different age-related influence on the structural networks of the aHPC and pHPC, providing an essential insight into the mechanisms of the hippocampus in normal aging.
Keywords: network, structural covariance, normal aging, anterior hippocampus, posterior hippocampus, MRI
Introduction
With the population aging, understanding normal brain changes are as important as understanding demented diseases. Memory decline is a typical characteristic of normal aging. The hippocampus is considered critical in human memory and spatial navigation (Scoville and Milner, 1957; Buzsáki and Moser, 2013). Evidence suggests that hippocampal volume changes throughout the lifespan, which stays relatively stable until the age of 60 shows a sharp decline (Raz et al., 2010; Schuff et al., 2012; Fjell et al., 2013). Functional imaging studies have revealed and hypometabolism (de Leon et al., 2001; Wu et al., 2008) of the hippocampus in aging. Moreover, a reduced fractal dimension of hippocampal dynamics with age was reported (Goldberger et al., 2002; Wink et al., 2006).
The hippocampus differs in structure and function along its longitudinal axis (Poppenk et al., 2013). The anterior hippocampus (aHPC) and posterior hippocampus (pHPC) vary in pyramidal cell density (Babb et al., 1984; King et al., 2008) and have different developmental trajectories (DeMaster et al., 2014). Compared with young adults, both the aHPC and the pHPC showed volumetric atrophy in old adults (Pruessner et al., 2001; Chen et al., 2010; Rajah et al., 2010), and their rates of atrophy were different (Malykhin et al., 2008; Chen et al., 2010). Besides, an fMRI study reported the functional connectivity of the aHPC and pHPC were differentially affected in aging (Damoiseaux et al., 2016).
For structural connectivity, the structural covariance network (SCN) approach provides an effective way to characterize inter-regional structural covariance pattern of gray matter (GM) morphological properties (Mechelli et al., 2005; Modinos et al., 2009; Seeley et al., 2009; Zielinski et al., 2010; Montembeault et al., 2012; Li et al., 2013; DuPre and Spreng, 2017). The GM morphological covariance may result from direct white matter connection or neuronal co-activation (Alexander-Bloch et al., 2013). Studies have revealed a consistency among SCNs, anatomical connectivity networks, and functional connectivity networks, which provides strong support for using SCN mapping approach to assess network integrity. Age-related alteration of structural covariance in sensorimotor and cognitive networks has been found (Montembeault et al., 2012; Li et al., 2013). However, the effects of aging on the structural covariance of the aHPC and pHPC remain to be studied, which may provide insights into the hippocampal-related mechanism of aging and demented diseases.
In this study, we utilized a seed-based SCN approach to investigate the anterior and posterior hippocampal structural networks in 240 healthy subjects that were subdivided into young, middle-aged, and elderly groups. We first defined aHPC and pHPC based on the location of uncal apex in the MNI space. Then, we identified the SCNs seeding from aHPC and pHPC and compared the structural covariance differences between age groups. We expected the SCNs of the aHPC and pHPC have different patterns and were differently affected by age.
Materials and Methods
Participants
The MRI data were obtained from the publicly available Open Access Series of Imaging Studies (OASIS) database (Marcus et al., 2007). The OASIS database consists of 416 subjects aged 18–96, including 100 mild dementia and 316 healthy subjects. Based on the age distribution of the OASIS database, we selected 240 participants from the healthy subcohort and grouped them into young (18–23 years), middle-aged (30–58 years), and elderly (61–89 years) groups, with 80 participants in each group (see Table 1). All the subjects are right-handed and cognitively normal, with the Mini-Mental State Examination scores (Folstein et al., 1975) above 29 and the Clinical Dementia Rating scores (Folstein et al., 1975) equal zero. The same group of subjects was used in our previous study (Li et al., 2013).
Table 1.
Participant characteristics by age group.
| Group | Sample size (Females) | Age in years (mean ± SD) |
|---|---|---|
| Young | 80 (50) | 18–23 (20.66 ± 1.47) |
| Middle-aged | 80 (50) | 30–58 (47.43 ± 8.23) |
| Old | 80 (55) | 61–89 (73.75 ± 7.12) |
Data Acquisition
All MRI scans were performed on 1.5 Tesla Siemens scanners. For each individual, three to four T1-weighted images were acquired using a magnetization-prepared rapid gradient echo (MPRAGE) sequence with the following parameters: repetition time = 9.7 ms; echo time = 4 ms; inversion time = 20 ms; delay time = 200 ms; flip angle = 10°; matrix = 256 × 256; field of view = 256 mm; slices = 128; slice thickness = 1.25 mm. After motion corrected, the images of each subject were averaged to improve the contrast-to-noise ratio.
Image Preprocessing
We used the VBM8 toolbox1 runs within SPM8 to implement voxel-based morphometry analysis (Ashburner and Friston, 2000) of the structural images. The acquired anatomical images were tissue classified into GM, white matter and cerebrospinal fluid images using tissue priors. Then, the segmented images were bias corrected and registered to a standard space using an affine transformation and a high-dimensional non-linear registration approach (Ashburner and Friston, 2005). Next, modulation of the segmented images was performed to correct for different individual brain size by using the non-linear registration parameters. Finally, the modulated GM segments were smoothed using an isotropic 12 mm full-width at half maximum Gaussian kernel for the structural covariance analysis.
Definition of the Hippocampal Seeds
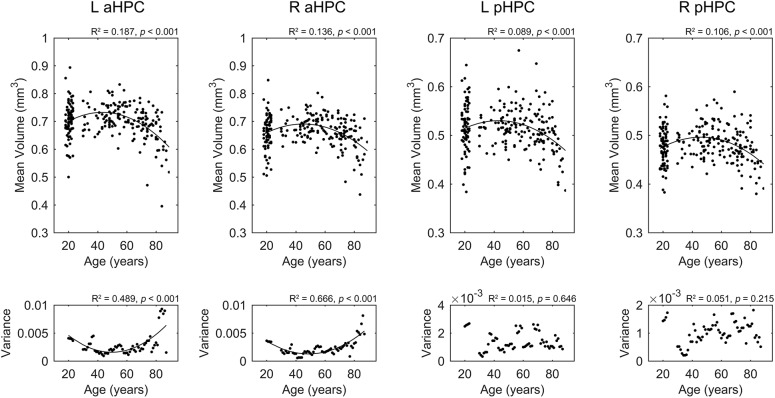
Following previous studies (Poppenk et al., 2013; Persson et al., 2014), we adopted a MNI-coordinate-based segmentation method to partition the hippocampus. The hippocampus was identified using the Harvard-Oxford subcortical structural atlas (Desikan et al., 2006) from the FSL Software Library (Smith et al., 2004). Next, the left and right hippocampi were divided into the anterior and posterior sections separately based on the location of uncal apex in the MNI space (i.e., Y = -21 mm) (Poppenk et al., 2013). To avoid contamination effects between the aHPC and the pHPC, we removed a 2-mm coronal slice from each of the two adjacent ends (see Figure 1). For each subject, we measured the mean volumes of the hippocampal subfields from the modulated GM images using the MarsBar ROI toolbox2. Then a quadratic regression model was used to investigate age effects on the mean volumes of the anterior and posterior hippocampal segments. We also assessed the age-related hippocampal volumetric dispersion. To do so, for age = t, we calculated the variance of hippocampal volumes of subjects with age ∈ [t-2, t+2] and examined its quadratic relationship with age.
FIGURE 1.
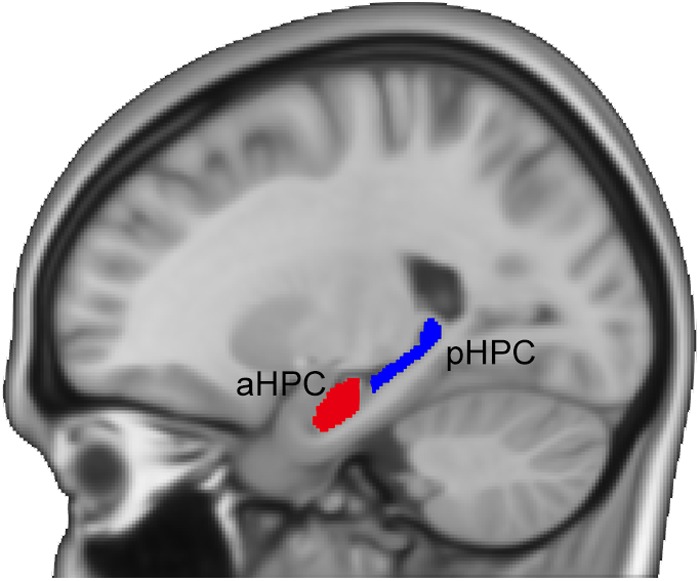
Illustration of the anterior and posterior hippocampal seeds. aHPC, anterior hippocampus; pHPC, posterior hippocampus.
Structural Covariance Analysis
Four separate regression analyses were executed on the modulated GM images data to map SCNs of the bilateral aHPC and pHPC in the young group. The model fitted the target voxel GM volume Y as:
where β0 is the intercept term, β1 model the relationship between the target voxel volume and the seed volume, and the Gender term was included as a nuisance variable. Total intracranial volume was not included because the modulation step already considered the brain size differences. These statistical analyses enable us to determine voxels that expressed a significant positive correlation with each seed. The criterion for significance was set at height and extent thresholds of p < 0.05, family-wise error (FWE) corrected for multiple comparisons. The resulting correlation maps were displayed on a standard brain template using the BrainNet Viewer (Xia et al., 2013) to allow qualitative comparisons the structural covariance patterns of hippocampal seeds.
We further assessed the influence of age on the regional structural covariance between the hippocampus and the rest brain regions by using a classic linear interaction model (Lerch et al., 2006). For any two age groups, the target voxel volume Y was modeled as follows:
where β0 is the intercept term, β1 ∼ β4 models the relationship between the target voxel volume and the seed volume, group term, gender term, and interaction term (group by seed), respectively. To obtain between-group differences, specific t contrasts were established to test the statistical significance of the interaction term. Clusters with height and extent thresholds set at p < 0.05 (FWE corrected) were considered significant.
Results
Hippocampal Volume Analyses
Results for the regression analysis of anterior and posterior hippocampal mean GM volumes versus age are presented in Figure 2. Similar nonlinear relationship between the bilateral hippocampal volumes and age were found: the volumes slightly increased before the age of 50 and then decreased sharply (left aHPC: R2 = 0.187, p < 0.001; right aHPC: R2 = 0.136, p < 0.001; left pHPC: R2 = 0.089, p < 0.001; right pHPC: R2 = 0.106, p < 0.001). Moreover, the results suggested that the mean volume of the aHPC was larger than the pHPC, and the left hippocampal volume was slightly greater than the right side. In addition, we found the variance of the bilateral anterior hippocampal volumes has an age-related U-shaped relationship (left aHPC: R2 = 0.489, p < 0.001; right aHPC: R2 = 0.666, p < 0.001). Specifically, the anterior hippocampal volumes of the young and old subjects were more dispersed than the middle age. However, the variance of the posterior hippocampal volumes did not significantly relate to age (left pHPC: R2 = 0.015, p = 0.646; right pHPC: R2 = 0.051, p = 0.215).
FIGURE 2.
Life-span trajectories of the anterior and posterior hippocampal mean gray matter volumes. The lower row shows the relationship between age and the variance of hippocampal volumes in a small age range. aHPC, anterior hippocampus; pHPC, posterior hippocampus; L, left, R, right.
Structural Covariance Networks of the Anterior and Posterior Hippocampus
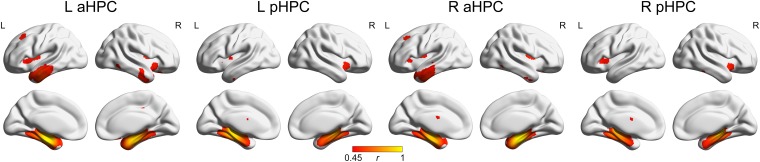
The SCNs seeding from the aHPC and pHPC in the young participants are presented in Figure 3 (p < 0.05, FWE corrected). The aHPC correlated with the bilateral temporal lobe (including the superior, middle and inferior temporal, parahippocampal gryi, entorhinal cortex, fusiform and temporal pole), amygdalae, insula and posterior cingulate gyrus, orbitofrontal cortex, as well as left superior frontal gyrus. For the pHPC, its covariance maps involved the bilateral medial temporal regions (including the parahippocampal gyrus, entorhinal cortex and fusiform), amygdalae and insula. Noted that the regions correlated with both the aHPC and pHPC were mainly located in the medial temporal lobe and insula.
FIGURE 3.
Structural covariance networks of the anterior and posterior hippocampus in the Young group. Regions with PFWE < 0.05 are presented as correlation coefficient values. aHPC, anterior hippocampus; pHPC, posterior hippocampus; L, left; R, right.
Age-Related Differences Within the Anterior Hippocampal Network
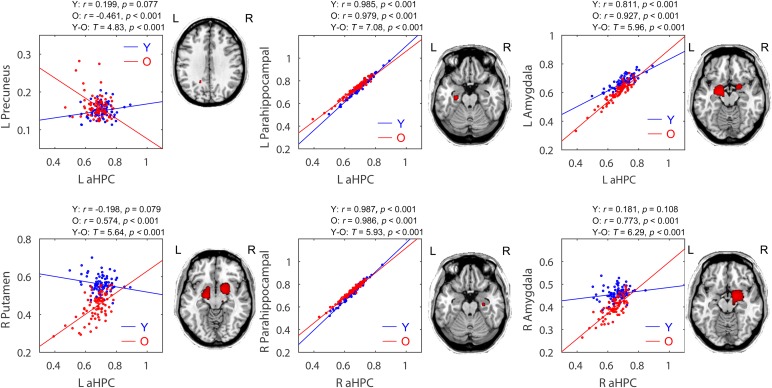
Within the anterior hippocampal network, significant between-group differences were only observed between the young group and the old group (p < 0.05, FWE corrected, Figure 4 and Table 2). Specifically, the left and right aHPC showed decreased positive correlation with the ipsilateral parahippocampus and increased positive correlation with the ipsilateral amygdala in the old group relative to the young group. Moreover, compared to the young group, the left aHPC exhibited lower structural covariance with the left precuneus and greater structural covariance with the right putamen in the old group.
FIGURE 4.
Age-related group differences in structural covariance of the anterior hippocampus. Correlations between the mean volume of the anterior hippocampus and the regional gray matter volumes extracted from a 4-mm-radius sphere centered on the peak voxel of a significant cluster (PFWE < 0.05, shown on the right) are displayed. Y, young group; O, old group; L, left; R, right.
Table 2.
Significant between-group differences in structural association between hippocampal seeds and other anatomical regions.
| Seed | Contrast | Anatomical region | MNI coordinates | Cluster size | MaxT | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| L aHPC | Y > O | L Parahippocampus | -27 | -16 | -21 | 126 | 7.08 |
| L Precuneus | -18 | -52 | 31 | 18 | 4.83 | ||
| Y < O | L Amygdala | -21 | -7 | -15 | 1314 | 5.96 | |
| R Putamen | 18 | 3 | -11 | 1096 | 5.64 | ||
| R aHPC | Y > O | R Parahippocampus | 27 | -15 | -21 | 36 | 5.93 |
| Y < O | R Amygdala | 16 | -1 | -14 | 1871 | 6.29 | |
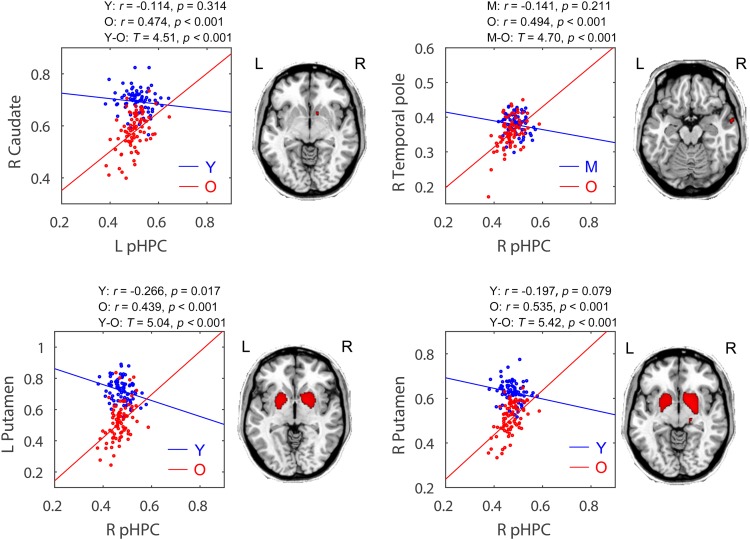
| L pHPC | Y < O | R Caudate | 12 | 10 | -6 | 5 | 4.51 |
| R pHPC | M < O | R Temporal pole | 62 | 0 | -17 | 29 | 4.70 |
| Y < O | R Putamen | 14 | 8 | -6 | 1655 | 5.42 | |
| L Putamen | -22 | 3 | -3 | 1084 | 5.04 | ||
P < 0.05, FWE corrected. Abbreviations: L, left; R, right; aHPC, anterior hippocampus; pHPC, posterior hippocampus; Y, young group; M, middle-aged group; O, old group.
Age-Related Differences Within the Posterior Hippocampal Network
Within the posterior hippocampal network, only increased structural associations were found in the old group relative to younger adults (mainly the young group, p < 0.05, FWE corrected, see Figure 5 and Table 2). For the left pHPC, the old group exhibited significantly increased connectivity with the right caudate related to the young group. For the right pHPC, its connection with bilateral putamen was negative in the young group but was positive in the old group. Similarly, the right pHPC and temporal pole was negatively related in the middle-aged group but positively related in the old group.
FIGURE 5.
Age-related group differences in structural covariance of the posterior hippocampus. Correlations between the mean volume of the posterior hippocampus and the regional gray matter volumes extracted from a 4-mm-radius sphere centered on the peak voxel of a significant cluster (PFWE < 0.05, shown on the right) are displayed. aHPC, anterior hippocampus; pHPC, posterior hippocampus; Y, young group; M, middle-aged group; O, old group; L, left; R, right.
Discussion
Here, we studied the age-related structural covariance alterations of the aHPC and pHPC using a seed-based SCN approach. We found that the SCNs seeding from the aHPC and pHPC in the young adults were different from each other, but both of them related with the medial temporal lobe and insula. In addition, the structural covariance differences within the anterior hippocampal network were mainly between the young group and the old group with both decreased and increased positive structural associations. While compared to the younger adults, only increased structural associations were found in the old group within the posterior hippocampal network.
We observed that the volumes of aHPC/pHPC slightly increased from young to middle age, and then decreased sharply with age. In line with this finding, several morphometric studies reported an inverted U pattern of the hippocampal volume changes with age (Walhovd et al., 2005; Li et al., 2014). As the hippocampus is important in memory processing, this pattern may partially explain the similar age-related memory change trajectory (Nyberg et al., 2012). Interestingly, we found that the anterior hippocampal volumes of the young and old subjects are more dispersed than the middle age, may pointing to stronger heterogeneity memory ability in young and old subjects. Whether this age-related dispersion due to the sample selection or other reasons requires further analysis.
Structural covariance analyses suggested that the aHPC connected with temporal lobe, amygdala, insula, and orbitofrontal cortex (p < 0.05, FWE corrected), which agree with previous studies (Kier et al., 2004; Smith et al., 2009; Catenoix et al., 2011). And the pHPC was covariant with medial temporal amygdala, and insula (p < 0.05, FWE corrected) showing consistent connections with previous studies by using fMRI and tractography (Kahn et al., 2008; Poppenk and Moscovitch, 2011; Poppenk et al., 2013). The common related regions with both aHPC and pHPC were mainly located in the medial temporal lobe where the hippocampus located.
Age-related decrements in structural covariance were observed in the aHPC-related SCNs (p < 0.05, FWE corrected). In particular, the parahippocampal gyrus and precuneus showed reduced association with the aHPC seed in old adults relative to young adults. The parahippocampal gyrus is considered as a mediator between the cortical DMN subsystem and the hippocampus (Ward et al., 2014), and the integrity of the cortico-parahippocampus-hippocampus circuit is important for learning and episodic memory (Witter et al., 2000; Van Strien et al., 2009). Therefore, the weakened parahippocampus-hippocampus connection may lead to memory deficits in normal elderly, and result in decreased structural covariance between the hippocampus and cortical regions, such as the precuneus found in this study. Besides, the decreased connectivity between the precuneus and hippocampus might result from very early beta-amyloid deposition of the precuneus in elderly subjects (Sheline et al., 2010). The abnormal synaptic activity caused by amyloid deposition might disrupt cortico-hippocampal connectivity, which then results in hippocampal atrophy (Mormino et al., 2009).
Note that the parahippocampal gyrus, precuneus, and hippocampus are all components of DMN (Andrews-Hanna et al., 2014). Thus, our findings may indicate that aging is associated with decreased structural covariance within the DMN, which is in keeping with observations from previous SCN studies (Montembeault et al., 2012; Li et al., 2013; Spreng and Turner, 2013). A previous study reported decreased fractal complexity in DMN with age using multifractal analysis of fMRI series (Ni et al., 2014). Moreover, aging-related decrements in functional connectivity (Damoiseaux et al., 2008; Tomasi and Volkow, 2012) and white matter integrity (Damoiseaux et al., 2009; Brown et al., 2015) of DMN were also reported. Since DMN is known to play a role in episodic memory processing (Greicius et al., 2004, 2009), its decreased integrity could underlie memory impairment in senior populations (Salami et al., 2014).
Additionally, our data suggest that the influence of age on the structural connectivity between the hippocampus and cortical DMN nodes may be limited to the anterior portion of the hippocampus. Similarly, Salami et al. (2014) revealed reduced functional connectivity between the cortical DMN subsystems and more anteriorly located hippocampus with advancing age. Several fMRI studies have demonstrated the aHPC as part of DMN was engaged in episodic memory (autobiographical memory) processing (Zeidman and Maguire, 2016). However, some studies found no age-related differences for the connectivity between the aHPC and DMN regions (Koch et al., 2010; Damoiseaux et al., 2016), while others reported lower connectivity between the pHPC and DMN regions in older adults (Andrews-Hanna et al., 2007; Damoiseaux et al., 2016). These discrepancies may be due to methodological differences, notably in the type of measurements and sample characteristics, which should be further investigated.
Moreover, age-related increments in structural covariance were observed in both the aHPC- and pHPC-related SCNs (p < 0.05, FWE corrected). Particularly, compared to young adults, the putamen and amygdala showed increased associations within the aHPC-related SCNs in old adults. Within the pHPC-related SCNs, the putamen, caudate, and temporal pole showed increased associations in old adults relative to younger adults. The putamen and caudate form the dorsal striatum. In fact, the hippocampus, dorsal striatum, and amygdala belong to different memory systems and play different roles in information acquisition (McDonald and White, 1993). The dorsal striatum and hippocampus cooperate to support episodic memory function (Sadeh et al., 2011), while the amygdala plays a role in regulating these two memory systems (Packard and Teather, 1998). We speculated that the age-related increment in hippocampal structural covariance may reflect the compensatory mechanism or dedifferentiation effects of the brain memory systems during aging (Dennis and Cabeza, 2011; Oedekoven et al., 2015).
The greater structural covariance between the hippocampus and dorsal striatum (caudate-putamen) in older adults may also be related to non-optimal dopamine processing. The CA1 area of the hippocampus receives dopaminergic modulation from the ventral tegmental area, which plays a vital role in synaptic plasticity of the hippocampus (Lisman and Grace, 2005). But the ventral tegmental area suffers from dopamine neurons loss (Siddiqi et al., 1999) and reduced dopamine transporter function (Salvatore et al., 2003) with age. However, the dorsal striatum, another area in the dopamine system, increases its dopamine synthesis capacity in aging (Braskie et al., 2008). Thus, the increased connections between the hippocampus and dorsal striatum during aging suggest compensation for deficits in the ventral tegmental area, which may represent non-optimal dopamine system functioning.
The SCN method used in this study provides an effective way to construct brain networks from medical images, which complements the signal analysis methods (Liu et al., 2015). However, since aging is not only characterized by brain deficits but also decline in multiple organ functions, it is interesting to utilize the integrative approaches within the new filed of network physiology to study the effects of aging on brain–brain or brain–organ networks in future (Bashan et al., 2012; Bartsch et al., 2015; Ivanov et al., 2016). In addition, it is worth noting that brain networks have a fractal property of hierarchical modularity, which confers robustness of network function (Bullmore and Sporns, 2012). Future studies using fractal analysis approaches (Meunier et al., 2010; Xue and Bogdan, 2017) to study the complexity and heterogeneity of hippocampal networks could advance our understanding of the brain in normal aging.
Author Contributions
XL designed and performed the experiments, analyzed the data, and drafted the manuscript. QL and XW helped to analyze the data and to draft the manuscript. DL and SL contributed to the study design, coordination, and final approval of the manuscript. All authors read and approved this version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the OASIS project for making the MRI data freely available.
Funding. This work was supported by the National Natural Science Foundation of China (Grant Nos. 81622025 and 81471731), the Fundamental Research Funds for the Central Universities (Grant No. YWF-17-BJ-J-11), the Innovation Foundation of BUAA for Ph.D. Students, and Academic Excellence Foundation of BUAA for Ph.D. Students. The OASIS project was supported by the NIH Grant Nos. P50 AG05681, P01 AG03991, R01 AG021910, P50 MH071616, U24 RR021382, and R01 MH56584.
References
- Alexander-Bloch A., Giedd J. N., Bullmore E. (2013). Imaging structural co-variance between human brain regions. 14 322–336. 10.1038/nrn3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Snyder A. Z., Vincent J. L., Lustig C., Head D., Raichle M. E., et al. (2007). Disruption of large-scale brain systems in advanced aging. 56 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Smallwood J., Spreng R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. 1316 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J., Friston K. J. (2000). Voxel-based morphometry—the methods. 11 805–821. 10.1006/nimg.2000.0582 [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K. J. (2005). Unified segmentation. 26 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Babb T. L., Lieb J. P., Brown W. J., Pretorius J., Crandall P. H. (1984). Distribution of pyramidal cell density and hyperexcitability in the epileptic human hippocampal formation. 25 721–728. 10.1111/j.1528-1157.1984.tb03483.x [DOI] [PubMed] [Google Scholar]
- Bartsch R. P., Liu K. K., Bashan A., Ivanov P. C. (2015). Network physiology: how organ systems dynamically interact. 10:e0142143. 10.1371/journal.pone.0142143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan A., Bartsch R. P., Kantelhardt J. W., Havlin S., Ivanov P. C. (2012). Network physiology reveals relations between network topology and physiological function. 3:702. 10.1038/ncomms1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie M. N., Wilcox C. E., Landau S. M., O’neil J. P., Baker S. L., Madison C. M., et al. (2008). Relationship of striatal dopamine synthesis capacity to age and cognition. 28 14320–14328. 10.1523/JNEUROSCI.3729-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. A., Hakun J. G., Zhu Z. D., Johnson N. F., Gold B. T. (2015). White matter microstructure contributes to age-related declines in task-induced deactivation of the default mode network. 7:194. 10.3389/fnagi.2015.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. (2012). The economy of brain network organization. 13 336–349. 10.1038/nrn3214 [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Moser E. I. (2013). Memory, navigation and theta rhythm in the hippocampal-entorhinal system. 16 130–138. 10.1038/nn.3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catenoix H., Magnin M., Mauguiere F., Ryvlin P. (2011). Evoked potential study of hippocampal efferent projections in the human brain. 122 2488–2497. 10.1016/j.clinph.2011.05.007 [DOI] [PubMed] [Google Scholar]
- Chen K. H., Chuah L. Y., Sim S. K., Chee M. W. (2010). Hippocampal region-specific contributions to memory performance in normal elderly. 72 400–407. 10.1016/j.bandc.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Beckmann C. F., Arigita E. J., Barkhof F., Scheltens P., Stam C. J., et al. (2008). Reduced resting-state brain activity in the “default network” in normal aging. 18 1856–1864. 10.1093/cercor/bhm207 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Smith S. M., Witter M. P., Sanz-Arigita E. J., Barkhof F., Scheltens P., et al. (2009). White matter tract integrity in aging and Alzheimer’s disease. 30 1051–1059. 10.1002/hbm.20563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J. S., Viviano R. P., Yuan P., Raz N. (2016). Differential effect of age on posterior and anterior hippocampal functional connectivity. 133 468–476. 10.1016/j.neuroimage.2016.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon M. J., Convit A., Wolf O. T., Tarshish C. T., DeSanti S., Rusinek H., et al. (2001). Prediction of cognitive decline in normal elderly subjects with 2-[18F] fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET). 98 10966–10971. 10.1073/pnas.191044198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaster D., Pathman T., Lee J. K., Ghetti S. (2014). Structural Development of the Hippocampus and Episodic Memory: Developmental Differences Along the Anterior/Posterior Axis. 24 3036–3045. 10.1093/cercor/bht160 [DOI] [PubMed] [Google Scholar]
- Dennis N. A., Cabeza R. (2011). Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. 32 2318.e17–30. 10.1016/j.neurobiolaging.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R. S., Ségonne F., Fischl B., Quinn B. T., Dickerson B. C., Blacker D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. 31 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- DuPre E., Spreng R. N. (2017). Structural covariance networks across the lifespan, from 6-94 years of age. 1 302–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A. M., Westlye L. T., Grydeland H., Amlien I., Espeseth T., Reinvang I., et al. (2013). Critical ages in the life course of the adult brain: nonlinear subcortical aging. 34 2239–2247. 10.1016/j.neurobiolaging.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., Mchugh P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. 12 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Goldberger A. L., Amaral L. A., Hausdorff J. M., Ivanov P. C., Peng C.-K., Stanley H. E. (2002). Fractal dynamics in physiology: alterations with disease and aging. 99 2466–2472. 10.1073/pnas.012579499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Srivastava G., Reiss A. L., Menon V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. 101 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Supekar K., Menon V., Dougherty R. F. (2009). Resting-state functional connectivity reflects structural connectivity in the default mode network. 19 72–78. 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov P. C., Liu K. K., Bartsch R. P. (2016). Focus on the emerging new fields of network physiology and network medicine. 18:100201. 10.1111/ede.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I., Andrews-Hanna J. R., Vincent J. L., Snyder A. Z., Buckner R. L. (2008). Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. 100 129–139. 10.1152/jn.00077.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kier E. L., Staib L. H., Davis L. M., Bronen R. A. (2004). MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. 25 677–691. [PMC free article] [PubMed] [Google Scholar]
- King K. G., Glodzik L., Liu S., Babb J. S., De Leon M. J., Gonen O. (2008). Anteroposterior hippocampal metabolic heterogeneity: three-dimensional multivoxel proton 1H MR spectroscopic imaging—initial findings. 249 242–250. 10.1148/radiol.2491071500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Teipel S., Mueller S., Buerger K., Bokde A. L., Hampel H., et al. (2010). Effects of aging on default mode network activity in resting state fMRI: does the method of analysis matter? 51 280–287. 10.1016/j.neuroimage.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Lerch J. P., Worsley K., Shaw W. P., Greenstein D. K., Lenroot R. K., Giedd J., et al. (2006). Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. 31 993–1003. 10.1016/j.neuroimage.2006.01.042 [DOI] [PubMed] [Google Scholar]
- Li W., Wu B., Batrachenko A., Bancroft-Wu V., Morey R. A., Shashi V., et al. (2014). Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. 35 2698–2713. 10.1002/hbm.22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Pu F., Fan Y., Niu H., Li S., Li D. (2013). Age-related changes in brain structural covariance networks. 7:98. 10.3389/fnhum.2013.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Grace A. A. (2005). The hippocampal-VTA loop: controlling the entry of information into long-term memory. 46 703–713. 10.1016/j.neuron.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Liu K. K., Bartsch R. P., Lin A., Mantegna R. N., Ivanov P. C. (2015). Plasticity of brain wave network interactions and evolution across physiologic states. 9:62. 10.3389/fncir.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin N. V., Bouchard T. P., Camicioli R., Coupland N. J. (2008). Aging hippocampus and amygdala. 19 543–547. 10.1097/WNR.0b013e3282f8b18c [DOI] [PubMed] [Google Scholar]
- Marcus D. S., Wang T. H., Parker J., Csernansky J. G., Morris J. C., Buckner R. L. (2007). Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. 19 1498–1507. 10.1162/jocn.2007.19.9.1498 [DOI] [PubMed] [Google Scholar]
- McDonald R. J., White N. M. (1993). A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. 107 3–22. 10.1037/0735-7044.107.1.3 [DOI] [PubMed] [Google Scholar]
- Mechelli A., Friston K. J., Frackowiak R. S., Price C. J. (2005). Structural covariance in the human cortex. 25 8303–8310. 10.1523/JNEUROSCI.0357-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D., Lambiotte R., Bullmore E. T. (2010). Modular and hierarchically modular organization of brain networks. 4:200 10.3389/fnins.2010.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Vercammen A., Mechelli A., Knegtering H., Mcguire P. K., Aleman A. (2009). Structural covariance in the hallucinating brain: a voxel-based morphometry study. 34 465–469. [PMC free article] [PubMed] [Google Scholar]
- Montembeault M., Joubert S., Doyon J., Carrier J., Gagnon J. F., Monchi O., et al. (2012). The impact of aging on gray matter structural covariance networks. 63 754–759. 10.1016/j.neuroimage.2012.06.052 [DOI] [PubMed] [Google Scholar]
- Mormino E., Kluth J., Madison C., Rabinovici G., Baker S., Miller B., et al. (2009). Episodic memory loss is related to hippocampal-mediated β-amyloid deposition in elderly subjects. 132 1310–1323. 10.1093/brain/awn320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H., Huang X., Ning X., Huo C., Liu T., Ben D. (2014). Multifractal analysis of resting state fMRI series in default mode network: age and gender effects. 59 3107–3113. 10.1007/s11434-014-0355-x [DOI] [Google Scholar]
- Nyberg L., Lövdén M., Riklund K., Lindenberger U., Bäckman L. (2012). Memory aging and brain maintenance. 16 292–305. 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Oedekoven C. S., Jansen A., Keidel J. L., Kircher T., Leube D. (2015). The influence of age and mild cognitive impairment on associative memory performance and underlying brain networks. 9 776–789. 10.1007/s11682-014-9335-7 [DOI] [PubMed] [Google Scholar]
- Packard M. G., Teather L. A. (1998). Amygdala modulation of multiple memory systems: hippocampus and caudate-putamen. 69 163–203. 10.1006/nlme.1997.3815 [DOI] [PubMed] [Google Scholar]
- Persson J., Spreng R. N., Turner G., Herlitz A., Morell A., Stening E., et al. (2014). Sex differences in volume and structural covariance of the anterior and posterior hippocampus. 99 215–225. 10.1016/j.neuroimage.2014.05.038 [DOI] [PubMed] [Google Scholar]
- Poppenk J., Evensmoen H. R., Moscovitch M., Nadel L. (2013). Long-axis specialization of the human hippocampus. 17 230–240. 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Poppenk J., Moscovitch M. (2011). A hippocampal marker of recollection memory ability among healthy young adults: contributions of posterior and anterior segments. 72 931–937. 10.1016/j.neuron.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Pruessner J., Collins D., Pruessner M., Evans A. (2001). Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. 21 194–200. 10.1523/JNEUROSCI.21-01-00194.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah M. N., Kromas M., Han J. E., Pruessner J. C. (2010). Group differences in anterior hippocampal volume and in the retrieval of spatial and temporal context memory in healthy young versus older adults. 48 4020–4030. 10.1016/j.neuropsychologia.2010.10.010 [DOI] [PubMed] [Google Scholar]
- Raz N., Ghisletta P., Rodrigue K. M., Kennedy K. M., Lindenberger U. (2010). Trajectories of brain aging in middle-aged and older adults: regional and individual differences. 51 501–511. 10.1016/j.neuroimage.2010.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh T., Shohamy D., Levy D. R., Reggev N., Maril A. (2011). Cooperation between the hippocampus and the striatum during episodic encoding. 23 1597–1608. 10.1162/jocn.2010.21549 [DOI] [PubMed] [Google Scholar]
- Salami A., Pudas S., Nyberg L. (2014). Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. 111 17654–17659. 10.1073/pnas.1410233111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore M. F., Apparsundaram S., Gerhardt G. A. (2003). Decreased plasma membrane expression of striatal dopamine transporter in aging. 24 1147–1154. 10.1016/S0197-4580(03)00129-5 [DOI] [PubMed] [Google Scholar]
- Schuff N., Tosun D., Insel P. S., Chiang G. C., Truran D., Aisen P. S., et al. (2012). Nonlinear time course of brain volume loss in cognitively normal and impaired elders. 33 845–855. 10.1016/j.neurobiolaging.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville W. B., Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. 20 11–21. 10.1136/jnnp.20.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W. W., Crawford R. K., Zhou J., Miller B. L., Greicius M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. 62 42–52. 10.1016/j.neuron.2009.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y. I., Raichle M. E., Snyder A. Z., Morris J. C., Head D., Wang S., et al. (2010). Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. 67 584–587. 10.1016/j.biopsych.2009.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi Z., Kemper T. L., Killiany R. (1999). Age-related neuronal loss from the substantia nigra-pars compacta and ventral tegmental area of the rhesus monkey. 58 959–971. 10.1097/00005072-199909000-00006 [DOI] [PubMed] [Google Scholar]
- Smith C. D., Lori N. F., Akbudak E., Sorar E., Gultepe E., Shimony J. S., et al. (2009). MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. 29 1248–1261. 10.1002/jmri.21692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Jenkinson M., Woolrich M. W., Beckmann C. F., Behrens T. E., Johansen-Berg H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. 23 S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Spreng R. N., Turner G. R. (2013). Structural covariance of the default network in healthy and pathological aging. 33 15226–15234. 10.1523/JNEUROSCI.2261-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N. D. (2012). Aging and functional brain networks. 17 471–558. 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Strien N., Cappaert N., Witter M. (2009). The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. 10 272–282. 10.1038/nrn2614 [DOI] [PubMed] [Google Scholar]
- Walhovd K. B., Fjell A. M., Reinvang I., Lundervold A., Dale A. M., Eilertsen D. E., et al. (2005). Effects of age on volumes of cortex, white matter and subcortical structures. 26 1261–1270. 10.1016/j.neurobiolaging.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Ward A. M., Schultz A. P., Huijbers W., Van Dijk K. R., Hedden T., Sperling R. A. (2014). The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. 35 1061–1073. 10.1002/hbm.22234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink A. M., Bernard F., Salvador R., Bullmore E., Suckling J. (2006). Age and cholinergic effects on hemodynamics and functional coherence of human hippocampus. 27 1395–1404. 10.1016/j.neurobiolaging.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Witter M. P., Naber P. A., Van Haeften T., Machielsen W. C., Rombouts S. A., Barkhof F., et al. (2000). Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. 10 398–410. [DOI] [PubMed] [Google Scholar]
- Wu W., Brickman A. M., Luchsinger J., Ferrazzano P., Pichiule P., Yoshita M., et al. (2008). The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. 64 698–706. 10.1002/ana.21557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet viewer: a network visualization tool for human brain connectomics. 8:e68910. 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y., Bogdan P. (2017). Reliable multi-fractal characterization of weighted complex networks: algorithms and implications. 7:7487. 10.1038/s41598-017-07209-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidman P., Maguire E. A. (2016). Anterior hippocampus: the anatomy of perception, imagination and episodic memory. 17 173–182. 10.1038/nrn.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski B. A., Gennatas E. D., Zhou J., Seeley W. W. (2010). Network-level structural covariance in the developing brain. 107 18191–18196. 10.1073/pnas.1003109107 [DOI] [PMC free article] [PubMed] [Google Scholar]