Abstract
In this study, anti-hyperglycemic and anti-inflammatory activities of polyphenolic-rich extract of Syzygium cumini leaves in alloxan-induced diabetic rats were determined. Diabetes was induced by a single intraperitoneal injection of alloxan (150 mg/kg body weight) in female Wistar rats. The rats were orally administered with 400 mg/kg free phenol, 400 mg/kg bound phenol, and 5 mg/kg metformin, respectively. On the 14th day of oral administration, the animals were sacrificed, anti-hyperglycemic and anti-inflammatory were assessed. Fasting blood glucose and glycated hemoglobin levels; homeostasis model assessment–insulin resistance scores, lipid peroxidation concentration, glucose-6-phosphatase activity, and all concentrations of anti-inflammatory studied in alloxan-induced diabetic rats were significantly (P < .05) reduced with the administration of polyphenolic-rich extract of Syzygium cumini leaves. Also there was significant (P < .05) increase in glycogen and insulin concentrations, pancreatic β-cell scores, antioxidant enzymes and hexokinase activities, as well as glucose transporter levels in diabetic animals administered with polyphenolic-rich extract of S cumini leaves. The results indicate that S cumini leaves possess anti-hyperglycemic and anti-inflammatory activities.
Keywords: anti-hyperglycemic, anti-inflammatory, Syzygium cumini leaves
Introduction
Diabetes mellitus is a disease that affects many people in this century and is acknowledged as the fifth leading cause of death worldwide.1 It is a metabolic disease characterized with hyperglycemia as a result of defects in insulin secretion, insulin action, or both as the case may be.2 The symptoms of diabetes mellitus due to persistent hyperglycemia are polyuria, polyphagia, excessive thirst, weight loss, blurred vision among others.3 According to Chatterjea and Shinde,4 diabetes mellitus is a disease-associated damage, dysfunction, and failure of different organs (such as the eyes, kidneys, nerves, heart, and blood vessels). Leading to long-term complications such as retinopathy (loss of vision); nephropathy (renal failure); gastrointestinal discomfort, cardiovascular problem amongst others.5 The initiator of these complications is free radical generation, cause insulin resistance probably due to persistent hyperglycemia in such patients.6 In addition, Jung et al7 documented that both oxidative stress and inflammation are involved in the pathogenesis of diabetes, suggesting the importance of antioxidants and anti-inflammatory in defending the body against such harmful processes.
The vast majority cases of diabetes mellitus is type II, caused by resistance to insulin action or inadequate compensatory insulin secretory response or insufficient insulin secretion.5 Diabetes mellitus affects about 415 million people worldwide and more than 14 million people in Africa, with more than 1.56 million cases in Nigeria as at 2015.8 It has been projected that by 2040, this figure will be more than double worldwide, if necessary action are not taken.9
Ssenyange et al10 reported that traditional health care is a significant part of medical care throughout the world, especially in Africa and this represent the first line of therapy for majority of the population. Many herbs are used in the management of diabetes mellitus, especially in the rural areas, probably due to side effects of available convectional drugs. Example of such plant is Syzygium cumini (java plum) whose seeds, leaves, bark, and fruits have been observed as antidiabetic,11 with scanty information on anti-hyperglycemic and anti-inflammatory activities of polyphenolic-rich extract of S cumini Linn leaves in alloxan-induced diabetic rats, which is the focus of this study.
Materials and Methods
Chemicals
All chemicals and reagents used were bought from Sigma-Aldrich Inc (St Louis, MO, USA).
Plant Material and Authentication
Syzygium cumini leaves were obtained from the premises of Zamani College, Kaduna, Kaduna State, Nigeria. It was identified and authenticated at the herbarium of the Department of Botany, Ahmadu Bello University, Zaria, Kaduna, Nigeria. The leaves were blended with the aid of electric blender to a powder form, which was used for various analyses.
Extraction of Free Phenol
The powdered sample was soaked in acetone for 72 hours, sieved, and concentrated to a small volume according to the method described by Chu et al.12
Extraction of Bound Phenol
The residues obtained from the above process were dried and hydrolyzed using NaOH. The pH of mixture was lowered by concentrated HCl, and thereafter ethylacetate was used to extract the bound phenol as described by Chu et al.12
Experimental Animals
Forty (40) female Wistar rats weighing between 120 and 170 g were used to ascertain the potential of polyphenolic-rich extract of S cumini on anti-hyperglycemia and anti-inflammatory in alloxan-induced diabetic rats. The animals were obtained from Afe Babalola University Animal House and were maintained under standard conditions of temperature. All animals were allowed free access to standard laboratory food and water 7 days before starting the experiment and during the period of the experiment. All animals were fed with pelletized diet and water ad libitum.
Induction of Diabetes
Diabetes was induced in the rats by a single intraperitoneal injection of freshly prepared alloxan of 150 mg/kg body weight in normal saline. Two days after alloxan administration, blood samples were obtained from the tips of the rat’s tail and the fasting blood glucose levels were determined using OneTouch Ultra glucometer (LifeScan, Milpitas, CA, USA) to confirm diabetes.
Animal Grouping
Rats were divided randomly into 5 groups as follows:
Group 1: Nondiabetic control rats received distilled water
Group 2: Untreated diabetic rats received distilled water
Group 3: Diabetic rats received 400 mg/kg body weight of free phenol of plant extract
Group 4: Diabetic rats received 400 mg/kg body weight of bound phenol of plant extract
Group 5: Diabetic control rats received metformin (5 mg/kg body weight)
Single dose of the extracts were used based on oral glucose tolerance test carried out by the authors.
Determination of Fasting Blood Glucose
Fasting blood glucose was determined using the Accu-chek Advantage II Clinical Glucose meter.13 Briefly, blood was collected from the tips of the rats’ tails and a drop was placed on the test area of the glucose meter to obtain the fasting blood levels in each rat.
Determination of Glycated Hemoglobin
This was carried out by the method of Sudhakar and Pattabiraman.14
Determination of Serum Insulin, Glucose Transporter 2, and Some Anti-Inflammatory Agents
The serum insulin, glucose transporter 2 (GLUT 2), interleukin-1α (IL-1α), tumor necrosis factor-α (TNF-α), and nuclear factor κB (NF-κB) concentrations were measured by an enzyme-linked immunosorbent assay (ELISA) method using an ultrasensitive rat insulin ELISA kit in a multiple reader as described by Voller et al.15
Also, homeostatic model assessment (HOMA-IR and HOMA-β) scores were calculated at the end of the intervention according to the following formula:
Conversion factor: insulin (1 U/L = 7.174 pmol/L).
Determination of Lipid Peroxidation
This was done using the method of Varshney and Kale.16 Briefly, an aliquot of 0.4 mL of the sample was mixed with 1.6 mL of Tris-KCl buffer to which 0.5 mL of 30% trichloroacetic acid was earlier added. Thereafter, 0.5 mL of 0.75% thiobarbituric acid was added and placed in a water bath for 45 minutes at 80°C. This was then cooled and centrifuged at 3000 g for 5 minutes. The clear supernatant was collected and the absorbance was measured against blank of distilled water at 532 nm. The malondialdehyde level was calculated according to the formula of Ádám-Vizi and Seregi.17
Determination of Catalase Activity
The method described by Sinha18 was used in this determination. Briefly, 4 mL of H2O2 solution was added to 5 mL of phosphate buffer at pH 7.0 in a 10-mL flat bottom flask. Then, 1 mL of the properly diluted sample was mixed with the reaction mixture by a gentle swirling motion at 25°C (room temperature). Thereafter, 1-mL portion of the reaction mixture was withdrawn and blown into 2 mL of dichromate/acetic acid reagent at 60-second intervals. The hydrogen peroxide contents of the withdrawn samples were determined by reading the absorbance at 570 nm.
Determination of Superoxide Dismutase Activity
Misra and Fridovich19 method was employed in this determination. Briefly, an aliquot of the sample was added to 2.5 mL of 0.05 M carbonate buffer at (pH 10.2) to equilibrate in the spectrophotometer. The reactions were inhibited by the addition of 0.3 mL freshly prepared 0.3 mM adrenaline to the mixture which was quickly mixed by inversion. The reference cuvette contained 2.5 mL buffer, 0.3 mL of adrenaline, and 0.2 mL of water. The increase in absorbance was read at 480 nm at every 30 seconds for 150 seconds.
Determination of Hexokinase
The activity of this enzyme was measured using the modified method of Akinyosoye et al.20 Concisely, 2 mL of 0.2 M Tris buffer, 0.2 mL of 0.09 g/mL glucose, 0.1 mL of 10 mM ATP and 0.3 mL of 10 mM MgCl2 were added to the blank and test tubes after which 0.1 mL of the sample was added to the test and 0.1 mL of distilled water was added to the blank. The mixture was mixed and incubated for 15 minutes at 30°C. Thereafter, 0.5 mL of 5% trichloroacetic acid was added to both blank and test to stop the reaction.
Data analysis
The results were expressed as mean ± standard error of mean. The means were analyzed using 1-way analysis of variance and Duncan test was used for the post hoc treatment.21
Results
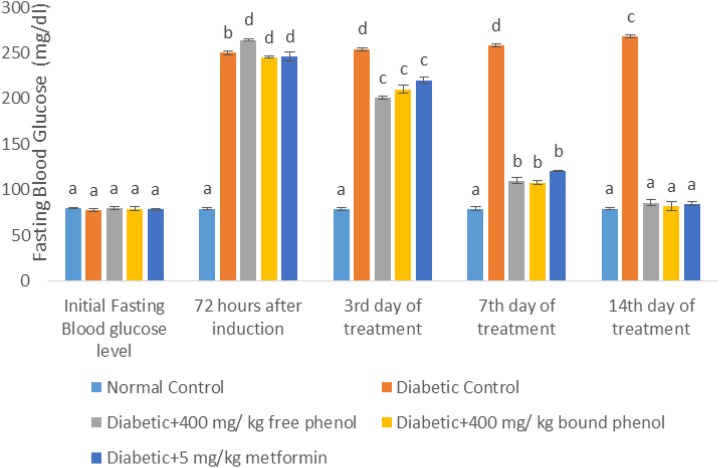
The effect of S cumini leaves on fasting blood glucose is shown in Figure 1. At 72 hours of diabetes induction, fasting blood glucose levels in diabetic control, diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol and diabetes + 5 mg/kg metformin groups significantly (P < .05) increased compared with the normal control. At third and seventh days of extract administration, there was a reduction in fasting blood glucose levels of diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol and diabetes + 5 mg/kg metformin groups. Also, on the 14th day of extract administration, there was significant (P < .05) decrease in fasting blood glucose levels in diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol and diabetes + 5 mg/kg metformin compared to diabetic control rats, with no significant (P > .05) difference in normal control, diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol and diabetes + 5 mg/kg metformin.
Figure 1.
Fasting blood glucose (FBG) level (mg/dL) of alloxan-induced diabetic rats after administration of polyphenol-rich extract of Syzygium cumini. Each value is a mean of 8 determinations ± standard error of mean (SEM). Values with different superscripts are significantly different (P < 0.05).
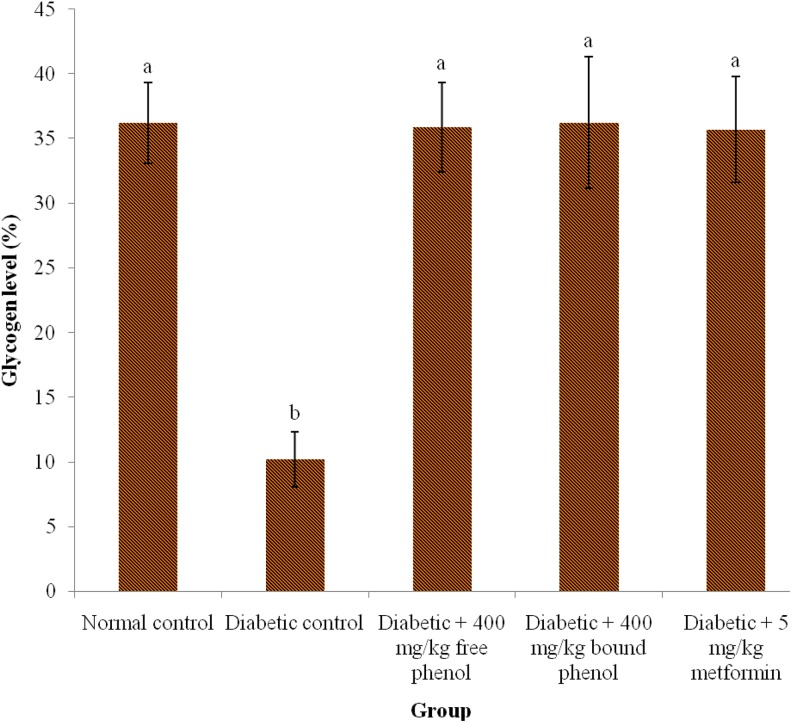
The effect of the polyphenolic-rich extracts of S. cumini leaves on glycogen concentration significantly (P < .05) reduced in diabetic control compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups (Figure 2). There was no significant (P > .05) difference in normal control, diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin.
Figure 2.
Glycogen level in alloxan-induced diabetic rats after administration of polyphenolic-rich extracts of Syzygium cumini leaves for 14 days. Values are represented as means ± standard error of mean (SEM) of 8 determinations. Values with different superscripts are significantly different (P < .05).
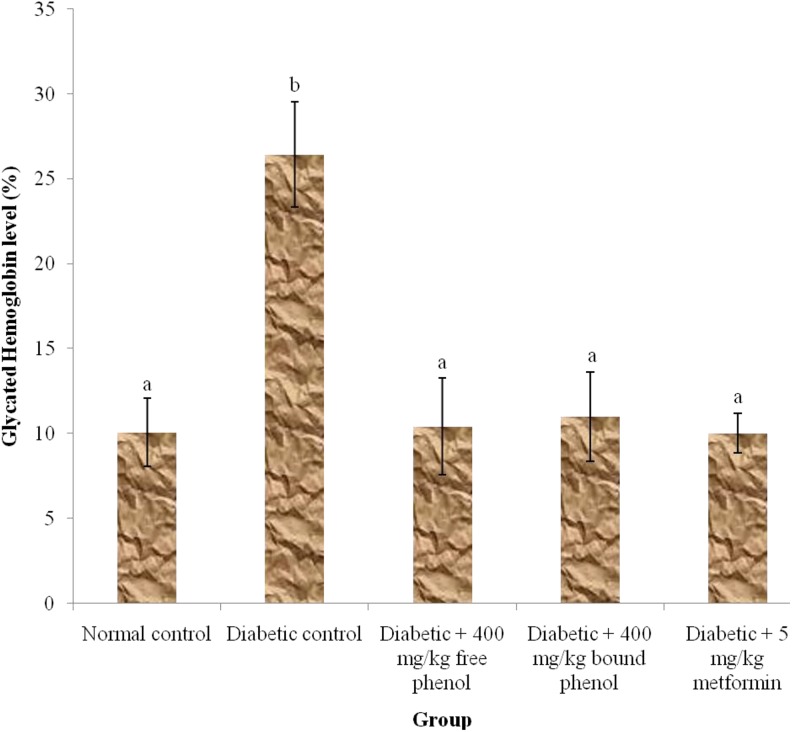
Figure 3 shows the effect of the polyphenolic-rich extracts of S cumini leaves on glycated hemoglobin in alloxan-induced diabetic rats. The diabetic controls showed significant (P < .05) increase in glycated hemoglobin concentration when compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups. There was no significant (P > .05) increase in normal control, diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups.
Figure 3.
Glycated hemoglobin level in alloxan-induced diabetic rats after administration of polyphenol-rich extract of Syzygium cumini for 14 days. Values are represented as means ± standard error of mean (SEM) of 8 determinations. Values with different superscripts are significantly different (P < .05).
Table 1 shows the effect of polyphenolic-rich extract of S cumini leaves in alloxan-induced diabetic rats on insulin concentration, HOMA-IR and HOMA-β. Insulin concentration and HOMA-β levels were significantly (P < .05) increased in diabetic + 400 mg/kg free phenol, diabetic + 400 mg/kg bound phenol, and diabetic + 5 mg/kg metformin groups compared with diabetic control group. However, HOMA-IR levels was significantly (P < .05) reduced in diabetic + 400 mg/kg free phenol, diabetic + 400 mg/kg bound phenol, and diabetic + 5 mg/kg metformin groups compared with diabetic control group. In addition, there was no significant (P > .05) increase in normal control, diabetic + 400 mg/kg free phenol, diabetic + 400 mg/kg bound phenol, and diabetic + 5 mg/kg metformin groups in insulin and HOMA-IR concentrations. Also, there was no significant (P > .05) elevation in normal control and diabetic + 400 mg/kg free phenol; diabetic + 400 mg/kg bound phenol and diabetic + 5 mg/kg metformin groups in HOMA-β concentrations.
Table 1.
Serum Insulin Concentration, HOMA-IR, and HOMA-β, in Alloxan-induced Diabetic Rats After Administration of Polyphenolic-rich Extract of Syzygium cumini Leaves for 14 Days.*
| Group | Insulin (pmol/L) | HOMA-IR | HOMA-β |
|---|---|---|---|
| Normal control | 86.42 ± 1.02a | 2.36 ± 0.2a | 264.8 ± 2.4a |
| Diabetic control | 30.78 ± 2.46b | 2.84 ± 0.1b | 7.73 ± 2.12c |
| Diabetic + 400 mg/kg free phenol | 85.48 ± 2.14a | 2.53 ± 0.05a | 186.25 ± 5.42b |
| Diabetic + 400 mg/kg bound phenol | 85.96 ± 3.2a | 2.43 ± 0.06a | 226.04 ± 4.22a |
| Diabetic + 5 mg/kg metformin | 86.22 ± 2.1a | 2.51 ± 0.12a | 202.03 ± 6.32b |
Abbreviations: HOMA-IR, homeostatic model assessment–insulin resistance; HOMA-β, homeostatic model assessment–β cell.
*Each value is a mean of 8 determinations ± standard error of mean (SEM). Values with different superscripts along the column are significantly different (P < .05).
Table 2 shows the effect of polyphenolic-rich extract of S cumini leaves on antioxidant activities in alloxan-induced diabetic rats. Oxidative stress was assessed by determining the levels of antioxidant enzymes, which are superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) as well as malonaldehyde (MDA), which is a measure of lipid peroxidation in pancreas. The diabetic control rats showed significantly (P < .05) reduced liver SOD, CAT, and GPx activities compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol and diabetes + 5 mg/kg metformin groups. Diabetes + 400 mg/kg bound phenol group has a significantly (P < .05) higher antioxidant enzyme activity than both diabetes + 400 mg/kg free phenol and diabetes + 5 mg/kg metformin groups. However, the diabetic control showed significant (P < .05) increase in pancreatic MDA levels compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups.
Table 2.
Hepatic Antioxidant Enzyme Activities and Lipid Peroxidation Level After Administration of Polyphenol-Rich Extract of Syzygium cumini Leaves for 14 Days.*
| Group | SOD (U/mg protein) | CAT (U/mg protein) | GPx (nm/min/mg) | MDA (×10−5 mmol/mL) |
|---|---|---|---|---|
| Normal control | 46.17 ± 4.10a | 82.41 ±3.10a | 65.13 ± 4.10a | 0.86 ± 0.40a |
| Diabetic control | 10.01 ± 2.10c | 18.42 ± 1.01d | 12.10 ± 2.39c | 4.16 ± 1.48c |
| Diabetic + 400 mg/kg free phenol | 44.86 ± 3.10b | 78.21 ± 2.40b | 64.68 ± 3.10a | 0.94 ± 0.46b |
| Diabetic + 400 mg/kg bound phenol | 46.42 ± 4.18a | 80.14 ± 1.40a | 65.48 ± 2.44c | 0.88 ± 0.41a |
| Diabetic + 5 mg/kg metformin | 43.46 ± 3.10b | 72.18 ± 1.49c | 57.94 ± 3.10b | 0.92 ± 0.46b |
Abbreviations: SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; MDA, malondialdehyde.
*Each value is a mean of 8 determinations ± standard error of mean (SEM). Values with different superscripts along the column are significantly different (P < .05).
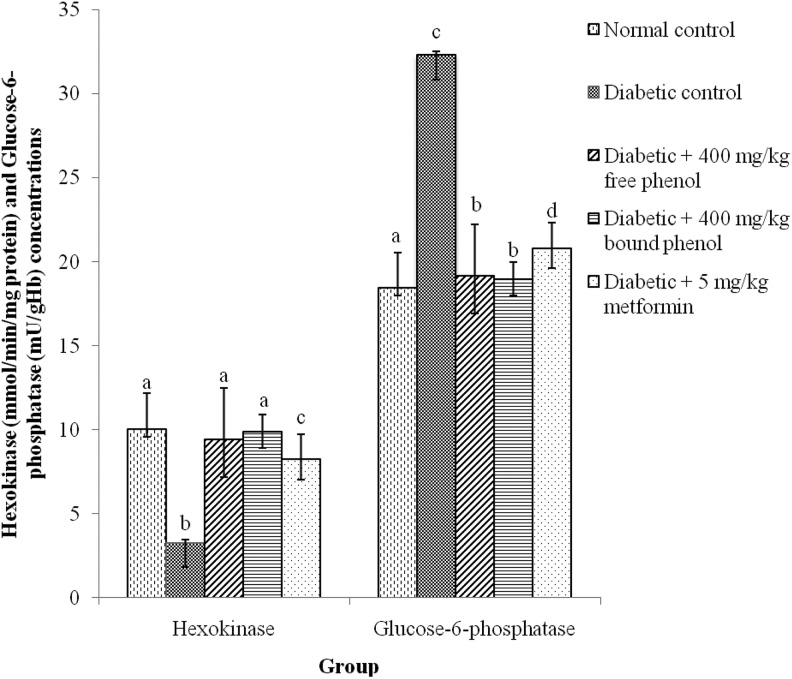
Figure 4 shows the effect of administration of polyphenols of S cumini leaves on the activities of carbohydrate metabolic enzymes. The effect of polyphenolic extracts of S cumini on the activity of hexokinase significantly (P < .05) reduced in the diabetic control rats compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups, whereas, the effect of polyphenolic extracts of S cumini on the activity of glucose-6-phosphatase significantly (P < .05) increased in the diabetic control rats compared with diabetes + 400 mg/kg free phenol, diabetes + 400 mg/kg bound phenol, and diabetes + 5 mg/kg metformin groups (Figure 4). Diabetes + 400 mg/kg bound phenol group exhibited a significant (P < .05) increase hexokinase activity and a decrease in glucose-6-phosphatase activity compared with diabetes + 400 mg/kg free phenol and diabetes + 5 mg/kg metformin groups.
Figure 4.
Some carbohydrate metabolic enzymes activities after administration of polyphenolic-rich extract of Syzygium cumini leaves in alloxan-induced diabetic rats for 14 days. Values are represented as means ± standard error of mean (SEM) of 8 determinations. Values with different superscripts are significantly different (P < .05).
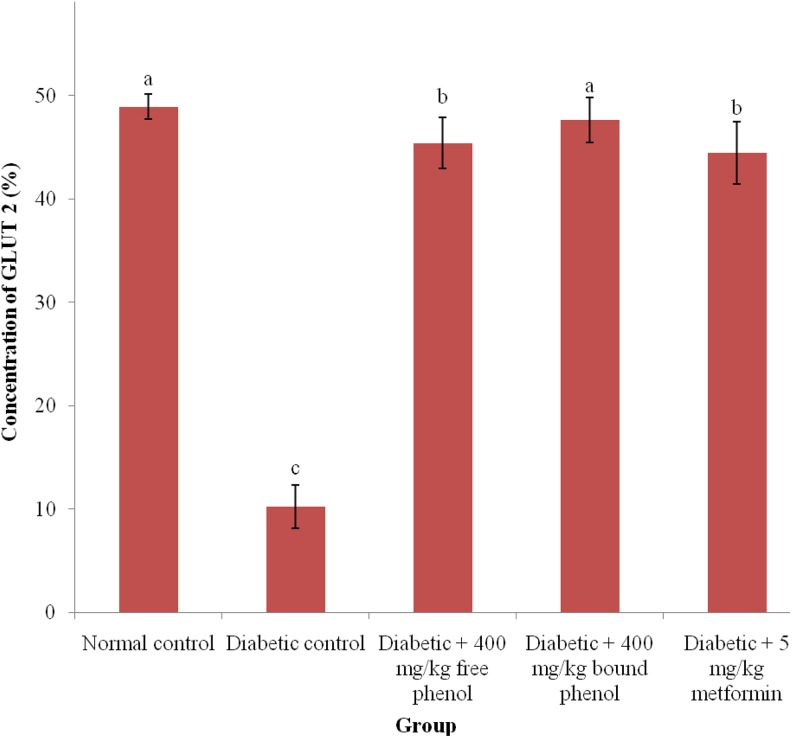
In Figure 5, the activity of GLUT 2 is significantly (P < .05) decreased in the diabetic control rats than in diabetic + 400 mg/kg free phenol, diabetic + 400 mg/kg bound phenol, and diabetic + 5 mg/kg metformin groups, with no significant (P > .05) increase in normal control and diabetic + 400 mg/kg bound phenol; diabetic + 400 mg/kg free phenol, and diabetic + 5 mg/kg metformin groups.
Figure 5.
Glucose transporter 2 level after administration of polyphenolic-rich extract of Syzygium cumini leaves in alloxan-induced diabetic rats for 14 days. Values are represented as means ± standard error mean (SEM) of 8 determinations. Values with different superscripts are significantly different (P < .05).
There was a significant increase (P < .05) in anti-inflammatory activities of polyphenolic-rich extract of S cumini leaves in alloxan-induced diabetic rats (Table 3). IL-1α, TNF-α, and NF-κB activities are significantly (P < .005) increased in diabetic control group compared with diabetic + 400 mg/kg free phenol, diabetic + 400 mg/kg bound phenol, and diabetic + 5 mg/kg metformin groups. Diabetic + 400 mg/kg free phenol group, however, had significant (P < .05) higher anti-inflammatory activity when compared with diabetic + 400 mg/kg bound phenol and diabetic + 5 mg/kg metformin groups.
Table 3.
Anti-Inflammatory Activities in Pancreas of Alloxan-Induced Diabetic Rats After Administration of Polyphenolic-Rich Extract of Syzygium cumini Leaves for 14 Days.*
| Group | IL-1α (pg/mL) | TNF-α (pg/mL) | NF-κB (%) |
|---|---|---|---|
| Normal control | 124.20 ± 6.10a | 384.22 ± 3.10a | 20.14 ± 3.11a |
| Diabetic control | 362.10 ± 4.10b | 1346.04 ± 5.20b | 80.24 ± 2.10c |
| Diabetic + 400 mg/kg free phenol | 130.16 ± 4.22b | 406.2 ± 4.10b | 22.11 ± 5.6b |
| Diabetic + 400 mg/kg bound phenol | 126.24 ± 3.22a | 396.2 ± 6.10a | 19.56 ± 4.11a |
| Diabetic + 5 mg/kg metformin | 132.1 ± 3.09b | 402.22 ± 6.92a | 21.21 ± 3.16b |
Abbreviations: IL-1α, interleukin-1α; TNF-α, tumor necrosis factor–α; NF-κB, nuclear factor–κB.
*Each value is a mean of 8 determinations ± standard error of mean (SEM). Values with different superscripts along the column are significantly different (P < .05).
Discussion
Herbal medicine or phytomedicine is the oldest form of health care known to humankind. This includes uses of a plant’s seeds, berries, roots, leaves, bark (stem), or flowers for medicinal purposes. This has been the main stream in treatment/management and prevention of diseases, especially diabetes mellitus.22 The radical scavenging abilities of the polyphenolic-rich extract of S cumini leaves could be a reference point in the management of diabetes mellitus, since free radicals are intricate in the pathogenesis of diabetes mellitus due to production of reactive oxygen species (ROS), which might lead to autoimmune destruction of the beta cells in the pancreas.23
In this study, alloxan was used to cause selective destruction of β-cells, leads to development of insufficient production of insulin and consequently, the elevation of fasting blood glucose level (Figure 1). However, diabetic rats administered with polyphenolic-rich extracts of S cumini leaves decreased the fasting blood glucose level possibly by increasing insulin synthesis as a result of regeneration of β-cells in the pancreas, releasing the formed insulin from the β-cells of the islet of Langerhans and inducing the sensitivity of cell receptors to insulin showing that the extract possessed normoglycemic properties.24
Glycogen is the major intracellular storage form of glucose, its level in different tissues (most especially the liver) is a direct signal of insulin activity, because insulin enhances intracellular glycogen deposition by stimulating glycogen synthase and inhibiting glycogen phosphorylase.25 There is a reduction in liver glycogen levels of diabetic control rats due to insufficient insulin secretion, since the inflow of glucose into hepatocytes depends on it. But oral administration of polyphenolic-rich extracts of S cumini leaves to diabetic rats, improved hepatic glycogen levels (Figure 2). This might due to the revival of the glycogen synthase system as a result of enhanced insulin secretion.25
Increased nonenzymatic glycosylation is one of the probable mechanism associating hyperglycemia and vascular complications in diabetes mellitus. During diabetes mellitus, the excess glucose present in the blood reacts with hemoglobin to form glycated hemoglobin (HbA1C), which was observed in the present study (Figure 3), indicating their poor glycemic control.26 But administration of polyphenolic-rich extract of S cumini leaves to diabetic rats significantly decreased the level of HbA1C, which might be the result of an improvement in the glucose metabolism.
In addition, diabetes mellitus has been characterized with decrease in insulin secretion due to dysfunction of pancreatic β-cell in response to hyperglycemia as reported by Wang et al.27 In this study, decreased insulin concentration was observed in diabetic control rats (Table 1). However, administration of diabetic rats with polyphenolic-rich extracts of S cumini leaves showed significant improvement in insulin concentration. This could be due to regeneration of damage pancreatic β-cells, supported by the higher HOMA-β index (β-cell function) in the diabetic rats administered with polyphenolic-rich extracts of S cumini leaves compared to the diabetic control group. Also, reduction in HOMA-IR index in diabetic rats administered with polyphenolic-rich extracts of S cumini leaves confirmed the improvement of insulin sensitivity as well as the stimulation of peripheral glucose absorption in these groups.24
Antioxidant enzymes such as SOD, CAT, and GPx are responsible for protecting the biological system from oxidative stress by conserving the physiological concentrations of oxygen and hydrogen peroxide, via increasing the dismutation of oxygen radicals and clearing organic peroxides generated from exposure to alloxan.28 Vincent et al29 documented that SOD is involved in detoxifying superoxide radicals by converting them to H2O2 and O2, both CAT and GPx are involved in the elimination of H2O2. It is has been reported that induction of alloxan to the experiment animals disrupted the activities of hepatic antioxidant enzymes leading to decrease in their activities (Table 2) and this might be due to increase in generation of ROS by alloxan, thereby inactivated the activities of these enzymes.28,29 This may be responsible for the insufficiency of antioxidant enzymes activities in diabetic control rats.30 However, diabetic rats administrated with polyphenolic-rich extract of S cumini leaves reduced the imbalance between the generation of ROS and antioxidant enzyme activities, thereby increased the enzymes activities. This might be as a result of decreased oxidative stress due to free radical scavenging ability of the extract, thus maintain normal levels of antioxidant defense system by preventing ROS from causing further damage to membrane tissue lipids.31
Furthermore, diabetes mellitus has been characterized with increase rate of lipid peroxidation of biological membranes, one of the major mechanisms of cell injury, probably due to persistent hyperglycaemia,32 as also observed in the diabetic control rats of the present study. Conversely, this was ameliorated in diabetic rats administered with polyphenolic-rich extracts of S cumini leaves, which might be attributed to antioxidative nature of the extract.
Figures 4 and 5 show the effect of administration of polyphenolic-rich extract of S cumini leaves on the activities of some carbohydrate metabolic enzymes as well as glucose transporter in the liver of alloxan-induced diabetic rats. According to Naik,3 insulin slows down hepatic glucose production by reducing hexokinase and increasing glucose-6-phosphatase activities. Glucose-6-phosphatase is a key enzyme in the last step of gluconeogenesis and glycogenolysis; catalyzes the hydrolysis of glucose-6-phosphate to glucose.3 However, the hepatic gluconeogenic enzyme, glucose-6-phosphatase activity was elevated in diabetic rats. This may be due to increase gluconeogenesis during diabetes mellitus, especially by the liver.33 Also, the activity of hexokinase was reduced in diabetic rats. This abnormal increase and decrease in the activity of the 2 enzymes, respectively, may be due to decrease in insulin secretion. The administration of polyphenolic-rich extract from S cumini leaves to diabetic rats normalizes the activities of the enzymes. Probably through the regulation by cyclic adenosine monophosphate (cAMP) or inhibition of gluconeogenesis by increasing insulin secretion.33,34 The GLUT 2 activity was reduced in diabetic rats. This may be as a result of reduced insulin levels and consequently reduced glucose uptake in diabetic rats. However, the administration of polyphenolic-rich extract of S cumini leaves normalizes its activity.34
In the pathogenesis of diabetes mellitus, pro-inflammatory cytokines such as TNF-α and IL-1α could be produced by immunocytes and adipocytes as reported by Donath.35 Hyperglycemia, elevated levels of ROS, and pro-inflammatory cytokines activates NF-κB, which plays a critical role in mediating inflammatory responses in diabetes mellitus condition. Persistent hyperglycemia increases the levels of ROS led to the activation of inflammatory response in mature adipocytes. In this study, the levels of NF-κB and pro-inflammatory cytokines like TNF-α and IL-1α in pancreatic tissues were increased in diabetic control rats (Table 3). TNF-α and IL-1α were important mediators of insulin resistance, as they could induce serine phosphorylation of insulin receptor substrate through activation of NF-κB pathway according to Gratas-Delamarche et al.36 Therefore, the regulation of NF-κB play a major role in the management of diabetic mellitus complications. NF-κB and cytokines like TNF-α and IL-1α were regulated by the administration of polyphenolic-rich extracts of S cumini leaves in diabetic rats, which is in accordance with the report of Donath.35
Conclusion
Polyphenolic-rich extracts of S cumini leaves possess antidiabetic activity via reducing hyperglycemia, increasing liver glycogen levels, decreasing glycated hemoglobin, increasing insulin sensitivity, ameliorating pancreatic β-ell and β-cell functions, increasing antioxidant enzymes and hexokinase activities, decreasing glucse-6-phsphatase activities and improving GLUT 2. The extract also demonstrated anti-inflammatory activities as shown by reducing inflammation.
Acknowledgments
The authors wish to express their gratitude to Biochemistry Technologist where the experiment was carried out.
Footnotes
Author Contributions: All authors were involved in designed, animal studies, data analysis, and writing of the article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by all the authors.
ORCID iD: Basiru O. Ajiboye  http://orcid.org/0000-0001-5982-2322
http://orcid.org/0000-0001-5982-2322
Ethical Approval: The authors declare that experiments were performed on animals only and ABUAD Ethical Animal Committee approval was obtained.
References
- 1. Kazi S. Use of traditional plants in diabetes mellitus: a review. Int J Pharm. 2014;4:283–289. [Google Scholar]
- 2. Nelson DC, Cox MM. Lehninger Principles of Biochemistry. 4th ed New York: W.H. Freeman and Co, 2010. [Google Scholar]
- 3. Naik P. Biochemistry Textbook. 3rd ed India: Jaypee Brother Medical Publisher, 2011. [Google Scholar]
- 4. Chatterjea MN, Shinde R. Textbook of Medical Biochemistry. Medical Publishers, 7th Edition, 2008. [Google Scholar]
- 5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unnikrishnan P, Suthindhiran K, Jayasri M. Alpha-amylase inhibition and antioxidant activity of marine green algae and its possible role in diabetes management. Pharmacogn Mag. 2015;11(suppl 4):S511–S515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jung HA, Karki S, Ehom NY, Yoon MH, Kim EJ, Choi JS. Anti diabetic and anti-inflammatory effects of green and red kohlrabi cultivars (Brassica oleracea var. gongylodes). Prev Nutr Food Sci. 2014;19:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogbera AO, Ekpebegh C. Diabetes mellitus in Nigeria: the past, present and future. World J Diabetes. 2014;5:905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United Nations Renal Data System. 2012. Atlas of CKD and ESRD. https://www.usrds.org/atlas12.aspx. Accessed March 28, 2018.
- 10. Ssenyange CW, Namulindwa A, Oyik B, Ssebuliba J. Plants used to manage type II diabetes mellitus in selected districts of central Uganda. Afr Health Sci. 2015;15:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ayyanar M, Subash-Babu P. Syzygium cumini (L.) skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed. 2012;2:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu YF, Sun J, Wu X, Liu RH. Antioxidant and antiproliferative activities of common vegetables. J Agric Food Chem. 2002;50:6910–6916. [DOI] [PubMed] [Google Scholar]
- 13. Ahmad MS, Pischetsrieder M, Ahmed N. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007;561:32–38. [DOI] [PubMed] [Google Scholar]
- 14. Nayak SS, Pattabiraman TN. A new colorimetric method for the estimation of glycosylated hemoglobin. Clin Chim Acta. 1981;109:267–274. [DOI] [PubMed] [Google Scholar]
- 15. Voller A, Bartlett A, Bidwell D. Enzyme immunoassays with special reference to ELISA techniques. J Clin Pathol. 1978;31:507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Varshney R, Kale R. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733–743. [DOI] [PubMed] [Google Scholar]
- 17. Ádám-Vizi V, Seregi A. Receptor independent stimulatory effect of noradrenalineon Na, K-ATPase in rat brain homogenate: role of lipid peroxidation. Biochem pharmacol. 1982;31:2231–2236. [DOI] [PubMed] [Google Scholar]
- 18. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. [DOI] [PubMed] [Google Scholar]
- 19. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 20. Akinyosoye F, Fawole M, Akinyanju J. Studies on some enzymes of carbohydrate metabolism in Geotrichum candidum . Nig J Microbiol. 1987;7:154–161. [Google Scholar]
- 21. Zar JH. Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall; 1984. [Google Scholar]
- 22. Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290–296. [DOI] [PubMed] [Google Scholar]
- 23. Ademosun A, Oboh G. Inhibition of carbohydrate hydrolyzing enzyme associated with type 2 diabetes and antioxidative properties of some edible seeds in vitro. Int J Diabetes Dev Ctries. 2015;35:516–521. [Google Scholar]
- 24. Ajiboye BO, Ojo OA, Adeyonu O, et al. Inhibitory effect on key enzymes relevant to acute type-2 diabetes and antioxidative activity of ethanolic extract of Artocarpus heterophyllus stem bark. J Acute Dis. 2016;5:423–429. [Google Scholar]
- 25. Grover J, Vats V, Rathi S. Anti-hyperglycemic effect of Eugenia jambolana and Tinospora cordifolia in experimental diabetes and their effects on key metabolic enzymes involved in carbohydrate metabolism. J Ethnopharmacol. 2000;73:461–470. [DOI] [PubMed] [Google Scholar]
- 26. Gandhi GR, Sasikumar P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed. 2012;2:281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang HJ, Jin YX, Shen W, et al. Low dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expression. Asia Pac J Clin Nutr. 2007;16(suppl 1):412–417. [PubMed] [Google Scholar]
- 28. Pari L, Latha M. Antidiabetic effect of Scoparia dulcis: effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005;24:13–26. [PubMed] [Google Scholar]
- 29. Vincent AM, Russell JW, Low P, Feldma EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612–628. [DOI] [PubMed] [Google Scholar]
- 30. Ravi K, Ramachandran B, Subramanian S. Protective effect of Eugenia jambolana seed kernel on tissue antioxidants in streptozotocin-induced diabetic rats. Biol Pharm Bull. 2004;27:1212–1217. [DOI] [PubMed] [Google Scholar]
- 31. Szkudelski T, Kandulska K, Okulicz M. Alloxan in vivo does not only exert deleterious effects on pancreatic B cells. Physiol Res. 1998;47:343–346. [PubMed] [Google Scholar]
- 32. Oboh G, Rocha JBT. Polyphenols in red pepper [Capsicum annuum var. aviculare (Tepin)] and their protective effect on some pro-oxidants induced lipid peroxidation in brain and liver. Eur Food Res Technol. 2007;225:239–247. [Google Scholar]
- 33. Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 34. Kazeem MI, Akanji MA, Yakubu MT, Ashafa AOT. Protective effect of free and bound polyphenol extracts from ginger (Zingiber officinale Roscoe) on the hepatic antioxidant and some carbohydrate metabolizing enzymes of streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2013;2013:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Donath M. Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab. 2013;15(suppl 3):193–196. [DOI] [PubMed] [Google Scholar]
- 36. Gratas-Delamarche A, Derbré F, Vincent S, Cillard J. Physical inactivity, insulin resistance, and the oxidative-inflammatory loop. Free Radic Res. 2014;48:93–108. [DOI] [PubMed] [Google Scholar]