Abstract
The spatial distribution, biogeochemical cycling and external sources of dissolved iron and dissolved manganese (DFe and DMn) were investigated in Ryder Bay, a small coastal embayment of the West Antarctic Peninsula, during Austral summer (2013 and 2014). Dissolved concentrations were measured throughout the water column at 11 stations within Ryder Bay. The concentration ranges of DFe and DMn were large, between 0.58 and 32.7 nM, and between 0.18 and 26.2 nM, respectively, exhibiting strong gradients from the surface to the bottom. Surface concentrations of DFe and DMn were higher than concentrations reported for the Southern Ocean and coastal Antarctic waters, and extremely high concentrations were detected in deep water. Glacial meltwater and shallow sediments are likely to be the main sources of DFe and DMn in the euphotic zone, while lateral advection associated with local sediment resuspension and vertical mixing are significant sources for intermediate and deep waters. During summer, vertical mixing of intermediate and deep waters and sediment resuspension occurring from Marguerite Trough to Ryder Bay are thought to be amplified by a series of overflows at the sills, enhancing the input of Fe and Mn from bottom sediment and increasing their concentrations up to the euphotic layer.
This article is part of the theme issue ‘The marine system of the West Antarctic Peninsula: status and strategy for progress in a region of rapid change’.
Keywords: iron, manganese, GEOTRACES, trace metals, Rothera, Western Antarctic Peninsula
1. Introduction
The West Antarctic Peninsula (WAP) is a climatically very sensitive region that exhibited rapid warming between the 1950s and late 1990s [1]. These changes have occurred in tandem with glacial retreat and ice shelf thinning, causing major changes in the marine environment. Continental shelves and shelf seas play an important role in the global carbon cycle [2], and future changes in ice shelf melt rates could dramatically influence high-latitude coastal ecosystems [3–7] and the ability of continental shelf waters to sequester atmospheric carbon dioxide. Changing sea-ice cover has already been shown to affect primary production, with a net loss of winter sea ice likely to drive a downward trend in the magnitude of phytoplankton blooms in Ryder Bay, a coastal embayment of the Western Antarctic Peninsula [8]. Changes in sea-ice conditions and glacial melt may also affect trace metal inputs and transport of several coastal areas of high-latitude regions such as the WAP [3–7]. Moreover, changes in the intensities of the sources and sinks of several key trace metals in coastal waters may also have an impact on the structure and the productivity of the more offshore oceanic regions [9]. The input of trace metals associated with the accelerated melting of large ice sheets and glaciers has the potential to increase in magnitude over the coming decades to centuries [3–5].
During recent decades, several studies have described the biological role of trace metals in different coastal regions of Antarctica, including the Amundsen Sea, the Ross Sea and the vicinity of King George Island [10–16]. Studies in the Amundsen Sea near Pine Island Glacier have shown that glacial meltwaters were the predominant source of Fe input to the euphotic zone [13]. In glacially influenced high-latitude fjords, glacial meltwater and sediments can be characterized by extremely high dissolved iron (DFe, µM) and dissolved manganese (DMn, µM) controlled by glacier bedrock mineralogy, and hydrologic and physical processes [5]. The latter study proposes a pattern of iron deposition-recycling that may lead to the delivery of glacially derived iron to the adjacent shelf [5]. Research conducted on the WAP and in Ryder Bay have shown that Fe inputs into the surface layer were strongly correlated with meteoric meltwater and identified a significant source of Fe in surface and deep waters [7,17]. As mentioned by Arrigo et al. [9] for the shelf portion of the WAP, relatively high contents of trace elements such as Fe and Mn near the bottom suggest sedimentary sources. Another study in Ryder Bay showed that bioactive trace metals such as Fe and Mn could vary within a wide range of concentrations in the surface layer (0–75 m below the surface) and that this variability was mainly linked to biological activity during Austral summer [18]. Owing to its nearshore location, the supply of Fe and other bioactive trace metals are thought to be non-limiting for plankton growth in Marguerite Bay [7,18].
Here, we present vertical distributions of DFe and DMn in a relatively shallow coastal embayment near glaciers, islands, drifting icebergs and sea ice. We investigate potential external sources of Fe and Mn such as glacial meltwater, sea ice, deep waters from further offshore, benthic recycling and sediments, and their redistribution through vertical mixing.
2. Methods
(a). Study area
Ryder Bay is a shallow (water depth ≤ ∼520 m) coastal embayment of Marguerite Bay located on Adelaide Island close to the British Antarctic Survey (BAS) research station Rothera (67°34′ S, 68°08′ W, Western Antarctic Peninsula; figure 1). The bay itself is separated from the rest of Marguerite Bay by a sill at approximately 350 m depth. Samples were collected during two field expeditions during austral summer. The first was conducted between January and March 2013, while the second was from late November 2013 to the end of February 2014.
Figure 1.
Map of the Western Antarctic Peninsula (a) and Ryder Bay (b), Rothera Antarctic Time Series sampling site (RATS1) and other sampling sites are marked by an orange star; additional stations for the surface transect are marked by a blue star. (Online version in colour.)
(b). Sample collection
Seawater samples have been collected to investigate the full-depth distributions of Fe and Mn, at 11 sampling sites located along the deepest part of Ryder Bay (B, RATS1 and RATS2; figure 1), A is over the sill, not in the deepest part of the bay. C and D are over the deep parts, E and F near Sheldon glacier, I near Horton and Hurley Glaciers, and J and K in the vicinity of Léonie and Lagoon islands. Most of the full depth casts were performed during the first field season (A, B, RATS2, RATS1, C, D and E sites), while the rest was sampled during the second season (F, I, J, K). In addition, seawater samples were taken at 5 m depth on a transect of 12 sampling locations including A, B, RATS2, RATS1, C, D, E and five extra stations (blue stars on the map given figure 1), with the aim of investigating the impact of glacier and sea-ice melt on trace metal inputs. Ultraclean polyvinylidene fluoride (PVDF) samplers of 4 l volume were used and sampling procedures have been described elsewhere [18]. Briefly, PVDF samplers were flushed by ambient pristine seawater on the downcast and rinsed with MQ water after use. Seawater samples were collected throughout the water column of each sampling location from a small open powerboat equipped with a clean all-titanium winch designed at the Royal Netherlands Institute for Sea Research (NIOZ). The winch comprises 600 m length of metal-free Dyneema 6 mm diameter cable.
Pressure, temperature, salinity and fluorescence were measured with a Sea-Bird conductivity, temperature and depth instrument (CTD, SBE19plusV2, Sea-Bird Electronics, Inc.) attached 10 m above the plastic-enclosed bottom weight at the end of the Dyneema cable. Dissolved oxygen was measured by a polarographic membrane-type dissolved oxygen sensor attached to the CTD (SBE43, Sea-Bird Electronics, Inc.) and the factory calibration was used.
Samples for the determination of dissolved trace elements (DFe and DMn), major nutrients (nitrate, phosphate, silicate) and stable oxygen isotopes were analysed. At most locations (A, RATS2, B, RATS1, C, D and E; figure 1), 12 seawater samples were collected, although 6 to 7 samples were taken at shallower sampling sites (F, I, J and K). After return to the shore, samplers were transported into a trace metal clean overpressurized-container laboratory (class 100).
For trace metal subsampling, bottles (125, 250 and 500 ml Low-Density Polyethylene, LDPE, Nalgene), Cryo.S cups (2 ml volume) and all other plasticware in contact with sample and/or reagents, were pre-cleaned following a three-step procedure (detergent, 6 M HCl, 3 M HNO3) and then filled with 0.35 M QD-HNO3 (triple subboiled-distilled from 65% reagent grade, J.T. Baker) and stored double-bagged. All rinsing was done with Millipore MilliQ deionized water (referred to as MQ, 18.2 M.Ω.cm−1) and with the sampled seawater prior final sampling. Subsamples of trace metals and nutrients were filtered on 0.2 µm pore size Sartorius Sartobran cartridges under slight overpressure (Air, 0.5 atm overpressure). These filtered trace metal samples were then acidified to pH = 1.8–1.9 with ultrapur HCl (1 ml per half litre, Romil, UpA) and double-bagged. Filtered seawater was collected in pre-rinsed 5 ml polyethylene vials and stored at 4°C for silicate analysis and at −20°C for nitrate and phosphate analyses.
(c). Nutrient analysis
Major nutrient analyses were carried out with a Technicon TRAACS 800 Auto-analyzer at NIOZ in August 2014. The performance of the analyser was monitored by measuring a daily freshly diluted nutrient internal standard (in-house reference material that is monitored consistently) during each run. The precision, calculated as the standard deviation of the ensemble of 48 measurements for phosphate and nitrate (during 8 different runs) and 34 measurements for silicate (during seven different runs), was determined for silicate, phosphate and nitrate as 0.6 µmol l−1, 0.016 µmol l−1 and 0.13 µmol l−1, respectively.
(d). Oxygen isotopes
Unfiltered seawater was collected in 150 ml medical flat bottles with rubber inserts in the caps, which were then further sealed using Parafilm to prevent slippage and protect against sample evaporation. Samples were returned to the UK by dark cool stow (4°C) for analysis at the Natural Environment Research Council Isotope Geosciences Laboratory (NIGL) at the British Geological Survey, Keyworth. This was performed using the equilibration method for oxygen [19], with samples analysed on an Isoprime mass spectrometer. Isotopic ratios are given as ‰ deviations from a reference standard (Vienna Standard Mean Ocean Water 2; VSMOW2), using the delta notation (δ18O). Analytical reproducibility was determined to be better than 0.05‰ based on duplicate analyses.
To determine quantitatively the contributions to the freshwater balance of sea-ice melt and meteoric water (with the latter here representing precipitation and glacial discharge combined), we used a 3-endmember mass balance constrained with salinity and δ18O measurements. The underlying principle is that sea-ice processes have a strong impact on ocean salinity, but have much less impact on δ18O, whereas the injection of meteoric water (which is isotopically very light at high southern latitudes) will strongly affect both tracers. This decoupling allows quantitative recovery of meteoric water concentrations and sea-ice melt concentrations; fuller details are given in Meredith et al. [20].
(e). Trace metal determination
All reagents, standards, samples and blanks were handled in pre-cleaned low-density polyethylene bottles (LDPE) according to GEOTRACES program guidelines for trace elements analysis. The description of the method used for the determination of trace metal concentration in the present study is also given in Bown et al. [18].
All reagents were prepared with deionized water MQ. The buffer reagent was made of clean glacial acetic acid obtained by double sub-boiling quartz distillation of 100% glacial acetic (AnalR Normapur, VWR) and Suprapure ammonium hydroxide 25% (Merck) and was adjusted to pH = 6.50 ± 0.10. Two rinsing solutions were prepared by dilution of QD-HNO3 with MQ (0.01 and 0.02 M) and used for seaFAST-pico cleaning during the pre-concentration procedure. Elution acid (1.5 M) was also obtained by dilution of QD-HNO3 with MQ water and was spiked with an Rh solution to obtain an Rh concentration of 1.5 nM in the to-be-analysed sample that is used as internal standard during the analysis on trace metal.
Both pre-concentration and buffer cleaning columns were cleaned with the following 100 ml solutions prior to use: 1.6 M HCl (Suprapur, Merck), MQ water, acidified seawater (pH = 2), 1.5 M nitric acid and MQ water. Procedure blanks, calibration standards, reference material, laboratory reference samples and samples (411 samples in total) were processed over 10 days, and the buffer cleaning column was cleaned again after 5 days of use. Seawater subsamples were poured in 30 ml LDPE Nalgene® bottles and eluted into 2 ml cups (2 ml pp Cryo.S™; Greiner bio-one) which previously had been cleaned with nitric acid. The procedural blank solution consisting of 0.012 M HCl (Seastar) in MQ water with 0.1% v/v Mediterranean Sea Surface water was freshly prepared every two days in an LDPE 125 ml bottle [21].
Sample pre-concentration was performed in the clean room (class 100) of the NIOZ using the offline mode of the seaFAST-pico pre-concentration flow injection system (Elemental Scientific Inc., ESI, Omaha, NE, USA). Samples are pre-concentrated onto the seaFAST built-in column (V = 200 µl; ESI) containing the NOBIAS PA1 resin. In the offline configuration the automated system buffers 10 ml of acidified seawater sample before loading it onto the NOBIAS PA1 resin. The seawater matrix is then removed and the concentrated sample is eluted with 750 µl of 1.5 M QHNO3 giving a pre-concentration factor of 13.33. In the present study, samples were initially acidified to pH = 1.8–1.9 and subsequently buffered to pH = 6.0 ± 0.1.
The dissolved concentrations of Fe and Mn (DFe and DMn), here defined as less than 0.2 µm, including small colloids and nanoparticulate material as well as aqueous Fe and Mn, were determined by inductively coupled plasma mass spectrometry (ICPMS, Thermo Element 2, Thermo Fisher Scientific) one month after the pre-concentration step. A multi-element stock standard with natural isotopic abundances of Fe and Mn was made in 0.024 M nitric acid from dilution of individual element standards. This multi-element standard was then used to make standard additions to matrix matching seawater collected in the Mediterranean Sea and characterized by relatively low concentrations of the investigated elements. The six produced standards were treated in the same manner as all other samples. A total of five calibration curves were processed by the seaFAST-pico in order to check the reproducibility and the stability of the system during the 10 days of the samples pre-concentration step.
The contributions to the whole seaFAST system, bottles, ICPMS, and its autosampler and reagents such as the buffer solution and the elution acid were quantified by the measurement of procedural blanks. The mean procedural blanks (n = 23) for each metal of interest are reported in table 1 and all the reported values of DFe and DMn of the present study have been corrected from their respective blank value. For Mn the blank is comparable to values presented in other studies using a similar method [21,22]. On the other hand, for Fe, the blank is higher than reported elsewhere; this requires further investigation in the future. Nevertheless, the concentrations of DFe that we measured in the present coastal region of study (lowest DFe = 0.58 nM) are higher than the procedural blanks of Fe (0.21 ± 0.07 nM; table 1). The limit of detection for DFe and DMn was estimated as three times the standard deviation of the blank (table 1). Accuracy and reproducibility have been assessed by measuring an internal laboratory standard (acidified seawater sample from Mediterranean Sea) and SAFe D2 reference material that have been pre-concentrated with the seaFAST in between samples, calibration standard and blanks. Results are given in table 1.
Table 1.
Blanks, detection limits and our measured values of SAFe D2 reference sample versus reported consensus values. Latter consensus values of the SAFe D2 reference sample of May 2013 are taken from: http://www.geotraces.org/science/intercalibration/322-standards-and-reference-materials. Average blanks (n = 24) and detection limits (three times the blank standard deviation).
| element | blanks (nmol kg−1) | detection limits (nmol kg−1) |
|---|---|---|
| Fe | 0.210 | 0.201 |
| Mn | 0.008 | 0.011 |
| SAFe D2 (nmol kg−1; n = 7) |
| element | measured | consensus |
|---|---|---|
| dFe | 0.987 ± 0.070 | 0.933 ± 0.023 |
| dMn | 0.44 ± 0.02 | 0.35 ± 0.05 |
(f). Vertical turbulent eddy diffusivity estimates using CTD data
CTD data were obtained at a rate of 4 Hz. The SBE software was used for standard post-processing, which involved, e.g., matching the different time delays of temperature, conductivity and pressure sensors, and corrections for thermal inertia of conductivity cells.
As the CTD profiles were obtained during calm weather only, few depth inversions were apparent in the raw data. Most importantly, the data of the rather slow temperature T sensor were brought forward in time by +0.5 s to align with the pressure P and conductivity C data. In comparison with high-precision SBE911 data, the slow, and somewhat less accurate, T-sensor data of the SBE19 have two effects. (i) They result in smooth T-profiles from which the small-scale turbulent overturns are ‘low-pass filtered’ (taken out). (ii) They are more difficult to match with the C-data, so that the salinity and density anomaly data show spikes at temperature jumps. These artificial spikes are reduced by the post-processing alignment, but can never be completely eliminated.
The corrected temperature, salinity and pressure data were used to compute potential density anomaly data referenced to the surface. Turbulence parameter values were estimated from its profiles with depth using the method originally proposed by Thorpe [23].
The ‘Thorpe scale’ dT is a vertical length scale of turbulent mixing in a stratified flow. It is obtained by rearranging an observed potential density profile, which may contain inversions associated with turbulent overturns, into a stable profile without inversions. The vertical displacement necessary to generate the stable profile is the ‘Thorpe displacement’.
A rather severe threshold of 0.005 kg m−3 had to be used due to the remnant artificial spikes, compared with thresholds of 0.001 kg m−3 employed by others using SBE911 CTDs, e.g. [24]. As an alternative, we additionally computed overturning scales from potential temperature profiles only, after establishing its linear relationship δσθ = αδθ (figure 2). This was found to be tight, but for depths greater than 100 m only: α = +0.17 kg m−3°C−1. As the thermal expansion coefficient is small at temperatures around 0°C, p < 500 dBar, this ‘apparent’ thermal expansion coefficient reflects that potential temperature is increasing with depth and density variations are dominated by salinity. For the SBE19 data both methods are likely to bias turbulence estimates low, the former because of the rather severe threshold, the latter because of the smoothed slow T-sensor data and its applicability well below the near-surface layer.
Figure 2.

The linear relationship between potential density and potential temperature.
Defining dT as the root mean square of the Thorpe displacements within each turbulent overturn, the vertical turbulent eddy diffusivity (m2 s−1) is obtained as
| 2.1 |
where N denotes the buoyancy frequency and the constant 0.128 is derived from an empirical relation with the Ozmidov scale, the largest overturn scale in stratified waters, using a constant mixing efficiency of 0.2, which is typical for shear-induced turbulence [25]. When sufficiently averaged, the method of overturn displacements provides a reasonable estimate of Kz and the turbulence dissipation rate to within a factor of three, as has been established for SBE911 data after comparison with independent estimates using free-falling microstructure data (e.g. [26]). For the present SBE19 data this factor is likely to be about 10 (one order of magnitude).
The raw Kz(z) profiles obtained from 11 CTD profiles (1 per sampling site) were averaged in 30 m vertical bins, comparable with the largest displacement observed, over which mean Kz values were calculated. Overall, because of the relatively large standard error after the necessary averaging, a range of vertical mean Kz values (from the surface to the bottom) was used to calculate vertical fluxes of DFe and DMn.
(g). Glider deployment
Data presented in figure 7 were collected using a deep (1000 m rated) Slocum ocean glider from an FLNTU sensor in an ECO Puck, following the deepest route from the source waters in Marguerite Bay to the RaTS site in Ryder Bay [27]. Data used here are from gliders deployed and recovered at the British Antarctic Survey base at Rothera in the 2012/13 and 2013/14 seasons; further details about data acquisition and processing are available elsewhere [27].
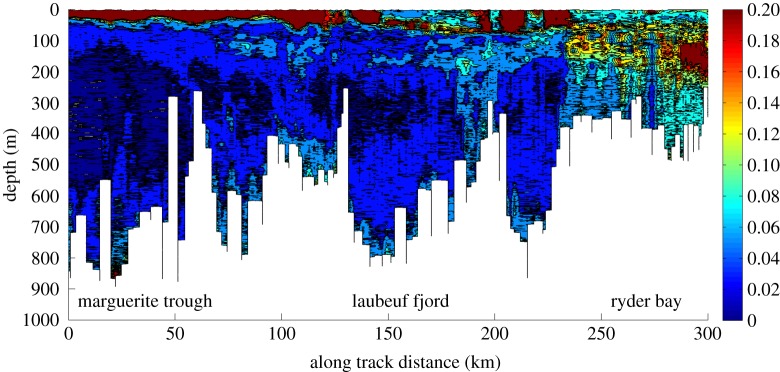
Figure 7.
Turbidity (NTU), from an FLNTU sensor in an ECO Puck on a Slocum glider, following the deepest route from the source waters in Marguerite Bay to the RaTS site in Ryder Bay.
3. Results
(a). Hydrography
At 5 m, along the 12 stations' surface transect that crossed Ryder Bay from Sheldon Glacier to station A, salinity exhibited relatively small variations, the lowest salinity being recorded at station C (S = 32.83) and the highest at station A (S = 33.00, figure 2). Fluorescence was fairly constant between E and RATS stations (approx. 5 mV) and increases to reach its maxima between B and RATS2 station (20 mV) before decreasing to 15 mV at station A (figure 2). δ18O showed scattered but increasing values towards station A (figure 2). The percentage of meteoric water exhibited a small range of values (4.8–5.3%) and only a small decrease at the furthest station from Sheldon Glacier (4.8% at station A).
The θ–S relationship of full depth data and the vertical distribution of dissolved oxygen at each sampling location are shown in figures 3 and 4, respectively. The hydrography of the RATS1 site has been described in several studies [8,27,28]. In the present study, every sampling location exhibited less saline surface waters due to freshwater inputs (salinity approximately 32.6–33.5), with the lowest salinities being recorded next to the glaciers (F, E and I stations; figure 3). During austral summer, surface waters are warmed by solar radiation (up to 2.8°C; figure 3) which leads to the formation of Antarctic Surface Water, AASW [7]. The underlying Winter Water (WW) core was located between 70 and 110 m. It was identified by a potential temperature minimum ranging from −1.39 to −0.87°C (figure 3). Deeper waters were marked by increasing salinity (S > 34; figure 3) and potential temperature (greater than 0.5°C; figure 3) and the core of modified circumpolar deep water (mCDW) was found at approximately 300–330 m at the deepest sites (A, RATS1). It was characterized by a relative minimum in dissolved oxygen concentration (approx. 156–165 µM; figure 4). At shallower sites (I, F, E, K), the oxygen minimum was found at the bottom, with values ranging from 158 (I site) to 245 µM (K; figure 4).
Figure 3.

Potential temperature (°C) versus salinity of each sampling site.
Figure 4.

Dissolved O2 (μmol kg−1) versus depth (m).
In Ryder Bay, Kz ranged from 3 × 10−5 to 3 × 10−4 m2 s−1, with a (statistically insignificant) tendency for larger values towards station A. These relatively large values are typical for near-surface convection-driven turbulence by wind and (night time) cooling. The values are comparable to values observed on the shelves near Pine Island Glacier [13] and in the Ross Sea [29].
(b). Trace metal distribution
Along the 5 m depth transect, the concentrations of DFe and DMn showed a systematic decrease towards station A, from 5 to 2 nM and from 3 to 1.5 nM, respectively (figure 5).
Figure 5.
Surface (5 m depth samples) transect plots of DFe (dark red dots), DMn (purple dots); salinity (blue line) and fluorescence (green line, mV); δ18O (‰) and glacier and sea-ice melt percentage (%).
The full depth vertical distribution showed that dissolved concentrations of Fe and Mn both displayed monotonic increases from the surface to the bottom at each sampling site with concentrations ranging from 0.58 to 35 nM and 0.18 to 26 nM, respectively (figure 6). The largest concentration gradients throughout the whole water column were observed at the shallower sites (D, E, F and I), while strongest concentration increases were observed at RATS1, D and B for DFe and RATS1 and D for DMn close to the bottom (table 2). The concentrations of dissolved iron and manganese were linearly related according to the following equation:
| 3.1 |
Figure 6.
The concentration of DFe and DMn vertical distributions (nM). (Online version in colour.)
Table 2.
DFe and DMn fluxes associated to vertical mixing. Vertical gradient of DFe and DMn at the different sampling locations are determined by the slope of the linear regression line DFe = f(z) and DMn = f(z). n = Number of data used in the calculation of d(DFe)/dz and d(DMn)/dz. R2: Coefficient of correlation.
| location | depth range (m) | dDFe/dz (nM m−1) | R2 | dDMn/dz (nM m−1) | R2 | n | vertical supply DFe (nmol m−2 d−1) | vertical supply DMn (nmol m−2 d−1) |
|---|---|---|---|---|---|---|---|---|
| A | 2–300 | 20 | 0.93 | 35 | 0.93 | 12 | 52–520 | 91–910 |
| RATS-2 | 2–325 | 29 | 0.90 | 30 | 0.94 | 12 | 75–750 | 78–780 |
| B | 2–450 | 36 | 0.76 | 28 | 0.99 | 12 | 93–930 | 73–730 |
| RATS-1 | 2–500 | 39 | 0.75 | 36 | 0.90 | 12 | 101–1010 | 93–930 |
| C | 2–350 | 29 | 0.95 | 25 | 0.94 | 12 | 75–750 | 65–650 |
| D | 2–250 | 60 | 0.67 | 40 | 0.60 | 11 | 155–1550 | 104–1040 |
| E | 2–200 | 64 | 0.93 | 63 | 0.96 | 11 | 166–1660 | 163–1630 |
| F | 2–160 | 88 | 0.86 | 73 | 0.97 | 6 | 228–2280 | 189–1890 |
| I | 2–170 | 65 | 0.93 | 55 | 0.98 | 6 | 168–1680 | 143–1430 |
| J | 2–270 | 46 | 0.96 | 46 | 0.96 | 7 | 119–1190 | 120–1200 |
| K | 2–75 | 42 | 0.90 | 10 | 0.69 | 6 | 109–1090 | 26–260 |
| global | 37 | 0.74 | 34 | 0.82 | 107 | 122–1220 | 104–1040 |
4. Discussion
Ryder Bay is surrounded by mountains, glaciers and islands (figure 1), and such geographical configuration indicates numerous potential sources for trace metals such as atmospheric inputs, glacial and sea-ice melt, upwelling of trace metal-replete deeper water masses, release of terrigenous material from drifting icebergs and suboxic shelf sediments [7,9]. Among these sources, a focus has been made on those that could have a direct impact on the trace metal concentrations in the euphotic zone, and thus on phytoplankton growth. The observed full-depth vertical distributions of DFe and DMn presented here show relatively small spatial variability in terms of concentrations and profile shapes when similar water column depths are compared (figure 6).
(a). The vertical distribution of DFe and DMn
DFe and DMn showed a very similar vertical structure, with a monotonically increasing concentration from the surface to near-bottom waters. The concentrations of DFe and DMn displayed a strong linear relationship indicating that their biogeochemical cycling might be linked in the area of study. The absolute concentration ranges of DFe and DMn are extremely broad and ranged from approximately 0.10 to greater than 30 nM (figure 6). Biological uptake of Fe and Mn accounts for the low concentrations measured in the euphotic zone (DFe and DMn = 0.1 nM–0.2 nM; [18]). The ranges of DFe and DMn in surface waters are similar to those found previously in the Southern Ocean [30–33] and in the local region of study [7]. In the present study, the highest concentrations of DFe and DMn were detected close to the bottom. Such profile shapes are similar to those observed in the Amundsen Sea [13] and the Ross Sea [29], although concentration values are much higher here in Ryder Bay. Such profiles suggest the bottom of Ryder Bay to be a strong local source of DFe and DMn. Concentrations are indeed much higher than those measured in Marguerite Bay and on the edge of Marguerite Trough which is in line with the hypothesis of strong local sources (DFe and DMn ranging from 0.1 to 2.5 nM; Sherrell et al. [34]). Different mechanisms could be involved to sustain such extreme concentrations in the dissolved phase. The complexation of iron with organic matter including organic ligands and colloids might play a key role to prevent abiotic and biotic scavenging of DFe. In the Southern Ocean, both DFe and DMn have similar sources ([31]; their figure 9) but their biological scavenging occurs at different timescales. In Ryder Bay, the strong correlation observed between DFe and DMn (above equation (3.1)) could indicate that biotic/abiotic scavenging of DFe and DMn have similar timescales. In Amundsen Polynyas, DFe was much lower than in Ryder Bay (less than 1.5 nM), but during summer, phytoplankton blooms produce fresh organic material and enhance the abundance of relatively unsaturated organic ligands capable of stabilizing additional Fe supplied from glacial melt [35]. Primary production could also drive microbial processes in sediments and play a critical role in sustaining DFe by remineralization. Particulate iron (greater than 0.45 µM) showed high concentrations and caused large concentration gradients along the relatively shallow water column continental shelf of the Amundsen Sea (1–66 nM from 0 to 300 m; [36]). Sediment resuspension can also generate the particulate Fe inputs which can also be a source of DFe by redissolution.
(b). Potential sources for surface waters
(i). Atmospheric inputs
There is potential for some local atmospheric inputs of trace metals in the area of study as Ryder Bay is surrounded by mountains with exposed rock. As far as we are aware, there are no data reported for dust deposition flux estimates in Ryder Bay and thus it is not currently possible to assess the potential impact on DFe and DMn distributions. A recent study showed that atmospheric input is unlikely to contribute significantly to Fe supply to the WAP [37]. Using an estimated dust deposition ratio from Wagener et al. [38], an average crustal Fe content and a high Fe solubility estimate of 10%, an estimate of the annual DFe accumulation due to atmospheric inputs has been calculated, and is in the range of 5–10 pmol kg−1 [37]. The solubility of Fe could vary on a broad range according to the literature (ranging from 0.001% to up to 80%). Raiswell et al. [39] estimate dust fractional solubility of 0.5% using a highly specific chemical leach, while Tagliabue et al. [40] assume fractional solubility of 0.5% (with continued slower dissolution in the water column), which would contribute to a much lower input than the estimated range from Annett et al. [37].
(ii). Glacial meltwater and sea-ice melt
To understand how glacial meltwater and sea-ice melt would affect the surface concentration, we measured DFe and DMn concentrations at 5 m with δ18O and salinity along a transect from Sheldon Glacier to A station. DFe and DMn both exhibited decreasing concentrations, from 5.0 to 2.0 nM and from 3.0 to 1.5 nM, respectively, towards station A. The salinity measured along this transect suggests that freshwater inputs are of similar magnitude at 5 m, which does not confirm that glacial meltwater is more pronounced closer to Sheldon Glacier. However, in addition to glacial discharge, changing concentrations of sea-ice melt also affect the salinity of the water, and this leads to δ18O having great utility as a tracer of glacial inputs in polar waters. Here, δ18O showed higher values towards the outermost end of the transect, with corresponding decreases in the percentage of meteoric water towards station A.
On the 5 m surface transect fluorescence is increasing towards station A, which would suggest less biological activity nearby Sheldon Glacier. DFe and DMn have been shown to be used by phytoplankton cells in Ryder Bay [18], so the observed decrease of their concentrations is probably due to both biological utilization and the increasing distance from the Sheldon Glacier. The inputs of local glacial meltwater could also be overshadowed by numerous icebergs of unknown origin that were entering and leaving Ryder Bay, driven by surface currents and winds. This made the glacier and sea-ice conditions different for each location that have been sampled to study the input of Fe and Mn at 5 m, and this could partially explain why the highest proportion of meteoric water appears to occur around Station C. Studies on high-latitude fjords showed that only a limited component of Fe is from ice melt, with the majority probably from subglacial chemical weathering of finely ground rock [3,4,41,42]. Improved sampling of the trace metal composition of meltwater discharge from the glaciers surrounding Ryder Bay would help better constrain their rates of input and fate.
Studies also report that DFe and DMn concentrations in sea-ice could be high and that sea-ice could be a significant source of Fe [9,41–43]. The latter study demonstrates largest effects near fronts associated with the melting. The percentage of sea-ice melt calculated from oxygen isotopes is mostly negative, which indicates that net sea-ice formation had occurred prior to the sampling of the surface transect. The derived sea-ice melt has a temporal aspect, reflecting both the magnitude of the previous winter's sea-ice formation and the magnitude of the subsequent spring/summer melt; it is the relative balance between these that control the magnitude of the quantity calculated. Given the relatively small amount of sea-ice melt, it seems likely that sea ice was a negligible Fe and Mn source during the time of the present study. In addition, results from the analysis of trace metal fluxes from melting sea ice suggests that seasonal ice melt may not contribute significantly to the supply of trace metals other than Fe [41,42].
(c). Potential sources for deep waters
(i). From CDW to mCDW
Within the Antarctic circumpolar current (ACC), DFe concentration in the CDW layer is relatively high (up to 0.5 nM; [30]), but this remains far below the extremely high DFe and DMn concentrations measured in the mCDW in the present study (DFe and DMn greater than 10 nM; figure 6) and in a previous study conducted at the RATS1 site where DFe= 10 nM at a 200 m depth [7]. Intermediate and deeper water concentration levels for both DFe and DMn exhibit strong enrichment compared to the Southern Ocean deep waters. CDW flows onto the WAP shelf in deep glacially carved channels such as Marguerite Trough. Then CDW becomes modified en route, flowing through a series of overflows at the sills, including into Ryder Bay, with considerable downward flow of water after each sill, with entrainment of water above and resuspension of sediment [27]. This is in line with both the increase of the turbidity and the concentration of DFe and DMn observed in intermediate and deep waters between Marguerite Trough and Ryder Bay (figure 7). Thus the enrichment of CDW as it flows from Marguerite Bay to Ryder Bay could be a relevant source of DFe and DMn. Moreover, the ‘sill effect’ described above is strongest in Ryder Bay but also happens at sills further offshore, and the T/S modification above the sills extends to around 100 m, so it could also mix DFe and DMn up to near-surface water, under favourable wind conditions and/or frontal passages.
(ii). Enrichment by local sediments
In addition to the enrichment of CDW as it flows on the WAP shelf and becomes mCDW, local Ryder Bay sediments could also generate inputs of DFe and DMn. The observation of the strong increase of DMn and DFe concentrations towards the bottom suggests that Ryder Bay shelf sediments are a significant local source of these two metals. We have partial direct evidence of sediment resuspension (turbidity data are not available for the whole sampling time period of the present study) but it seems that turbidity is generally higher throughout the water column of Ryder Bay, compared to deeper water in Marguerite Trough for instance (figure 7). The turbidity peak around a 150 m depth is a recurring feature either of biological origin or being swept off a shallow area in the surroundings of Ryder Bay (figure 7). In Ryder Bay, due to the presence of four glaciers (Sheldon, Horton, Hurley and Turner glaciers), glacial physical erosion of bedrock and export of finely ground reactive flour via subglacial meltwater pathways could be an important source of Fe- and Mn-enriched sediments. Following the latter hypothesis, the subsurface turbidity plume in figure 7 could be indicative of flour-rich subglacial discharge delivered at depth below the glacier calving front [44].
Also, redissolution remains a possibility given the sharp increase of DFe and DMn near the bottom of stations RATS1 and D (from 15 nM to 35 nM at RATS1; figure 6). Further, Hatta et al. [14] suggest that the origin of DFe and DMn enrichment near King George and Edward Islands appears to be the result of shelf sediment resuspension occurring when the ACC waters reach the shelf regions of the South Shetland Islands. In Ryder Bay, the extremely high DFe and DMn concentrations (up to 30 nM) detected near the bottom could be due to the release of pore waters from anoxic sediments. Around King George Island, the redox condition in coastal surface sediments can exhibit high spatial variability [45]. In shallow waters, sulphate reduction can be the major pathway of organic matter mineralization. By contrast, metal oxide reduction seems to be prevailing in the newly ice-free areas and the deeper troughs, where concentrations of dissolved iron of up to 700 µM were found [45].
The relatively strong turbulent mixing in the near-surface 200 m reflects the monotonic increase of DFe and DMn concentrations and their transport into the euphotic zone. A long residence time of deep waters would be a factor that would help to maintain high concentrations of DFe and DMn in the deep reservoir. The seasonal variation of temperature at 450 m is not strong, but it is quite gradual, suggesting a continual supply of ‘new’ water that has been previously mixed with shallower water. Overall, this suggests that the residence time of Ryder Bay water masses below the sill depth is relatively short, but that there are multiple opportunities for resuspension.
(iii). Vertical mixing
Vertical turbulent mixing may cause the DFe and DMn from sediments to be delivered to the mixed surface layer and represent a key source of bioavailable iron and manganese as previously shown by Lam & Bishop [46] for the HNLC western Subarctic Pacific. The lowest DFe and DMn gradients were calculated at the deepest site with values ranging from 20 to 40 nM m−1 (A, RATS1, RATS2, B), while stronger DFe and DMn gradients were generally observed at shallower sites (D, E and I, bottom depth less than 300 m). The strongest DFe and DMn gradients, 88 and 73 nM m−1, respectively, were determined for station F, located nearby Sheldon Glacier which is in line with the importance of benthic recycling of DFe and DMn and inputs of Fe and Mn from subsurface glacial meltwater delivery [5,44]. These gradients are much higher than the gradients calculated for Pine Island Glacier, Bellingshausen Sea, the South Shetland Islands (SSI) and Kerguelen Plateau regions [12–14,47]. In the present study, the range of the estimated Kz values is broad, between 3 × 10−5 to 3 × 10−4 m2 s−1. The lower part of this Kz range is close to the Kz determined in the Bellingshausen Sea and in the SSI regions [12,14], whereas the upper limit is one order of magnitude higher. This range of Kz values is similar to those estimated using shear data from a moored Long Ranger Acoustic Doppler Currrent Profiler [48]. This latter dataset calculated mean summertime values between depths of 100 and 200 m between 2005 and 2007 of 4.5 × 10−5 m2 s−1, though the authors acknowledge significant uncertainties in the fine-scale method used. Howard et al. [49], using wintertime shear data in Marguerite Bay, estimated a value of 1 × 10−5 m2 s−1. Such variability has been observed in Pine Island Glacier Polynya with Kz varying over 1.5 orders of magnitude but at 10 times higher values in the upper 300 m [13]. The variability from station to station is typical for near-surface ocean turbulence [50].
Based on the Kz range, the DFe and DMn fluxes associated with vertical mixing were estimated for each sampling location and are presented in table 2. In Ryder Bay, the fluxes of DFe and DMn associated to vertical mixing are ranging from 26 and 2280 mmol m−2 d−1 and are generally higher than fluxes reported for the Kerguelen Plateau (31 nmol m−2 d−1; [47]) and the Bellingshausen Sea (0.1–18 nmol m−2 d−1; [12]).
5. Conclusion
The present study reports extremely high concentrations of DFe and DMn within Ryder Bay, a small coastal embayment on the Western Antarctic Peninsula. Our results indicate that, among numerous potential sources, the major sources to the euphotic zone are likely to be benthic recycling from shallow sediments and glacier ice melt, while benthic recycling of sediments is the most significant source of DFe and DMn to intermediate and deep waters. We also describe a mechanism under which CDW becomes modified as it flows through a series of sills from Marguerite Trough to Ryder Bay. The considerable downward flow of water after each sill, with entrainment of water above and resuspension of sediment, results in an increase of the concentration of DFe and DMn in intermediate and deep waters. The relatively strong turbulent mixing calculated in Ryder Bay is thought to be an important pathway for DFe and DMn transport into the euphotic zone.
Ryder Bay is surrounded by four glaciers that may provide an important but poorly constrained source of iron and manganese. The analyses of iron and manganese solid-phase speciation in pore waters and sediments interpreted in the light of the sulfur cycle would provide new insights into iron and manganese biogeochemistry in the region of study.
Supplementary Material
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the British Antarctic Survey, the Royal Netherlands Institute for Sea Research (NIOZ) and the Netherlands Organisation for Scientific Research (NWO) for the opportunity to conduct fieldwork at the Dirck Gerritsz and Bonner laboratories at Rothera. Particular thanks are expressed to our colleagues Amber Annett, Mairi Fenton and Elisabeth Jones for their wonderful help during samples collection. We also would like to thank Rothera's boat officers Timothy Fox and Paul Samways, and Rothera Technical Services for their assistance. Support was gratefully received from Sven Ober (NIOZ), for CTD maintenance and data calibration.
Data accessibility
Two .xls files comprising all data of dissolved Fe and Mn and the ancillary data of depth, salinity and so forth, for these samples are given in the electronic supplementary material.
Authors' contributions
J.B. participated in the design of the study, did the samples collections, carried out trace metal laboratory work, led the data analysis and wrote the first version of the manuscript. M.P.M. carried out oxygen isotopes analysis and data interpretation. H.v.H. and J.A.B. carried out calculation and interpretation of vertical turbulent eddy diffusivity estimates using CTD data. H.J.V. collected turbidity data. P.L. was involved in sample collection and carried out trace metal laboratory work. H.J.W.d.B. conceived and designed the study, initiated the construction of clean equipment and laboratories, wrote the research grant proposal and did overall coordination. All the authors contributed considerably to make the second and third versions of the manuscript. All the authors gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work is part of postdoctoral research (J. Bown) at NIOZ under the research programme 866.20.031, which was financed by the Netherlands Organisation for Scientific Research (NWO).
References
- 1.Turner J, et al. 2016. Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 535, 411–415. ( 10.1038/nature18645) [DOI] [PubMed] [Google Scholar]
- 2.Charette MA, et al. 2016. Coastal ocean and shelf-sea biogeochemical cycling of trace elements and isotopes: lessons learned from GEOTRACES. Phil. Trans. R. Soc. A 374, 20160076 ( 10.1098/rsta.2016.0076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia MP, Kujawinski EB, Das SB, Breier CF, Henderson PB, Charette MA. 2013. Greenland meltwater as a significant and potentially bioavailable source of iron to the ocean. Nat. Geo. 6, 274–278. ( 10.1038/ngeo1746) [DOI] [Google Scholar]
- 4.Hawkings JR, et al. 2014. Ice sheets as a significant source of highly reactive nanoparticulate iron to the oceans. Nat. Comm. 5, 3929 ( 10.1038/ncomms4929) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehrmann LM, Formolo MJ, Owens JD, Raiswell R, Ferdelman TG, Riedinger N, Lyons TW. 2014. Iron and manganese speciation and cycling in glacially influenced high-latitude fjord sediments (West Spitsbergen, Svalbard): evidence for a benthic recycling-transport mechanism. Geochim. Cosmochim. Acta 141, 628–655. ( 10.1016/j.gca.2014.06.007) [DOI] [Google Scholar]
- 6.Aciego SM, Stevenson EI, Arendt CA. 2015. Climate versus geological controls on glacial meltwater micronutrient production in southern Greenland. Earth Planet. Sci. Lett. 424, 51–58. ( 10.1016/j.epsl.2015.05.017) [DOI] [Google Scholar]
- 7.Annett AL, Skiba M, Henley SF, Venables HJ, Meredith MP, Statham PJ, Ganeshram RS. 2015. Comparative roles of upwelling and glacial iron sources to Ryder Bay, coastal Western Antarctic Peninsula. Mar. Chem. 176, 21–33. ( 10.1016/j.marchem.2015.06.017) [DOI] [Google Scholar]
- 8.Venables HJ, Clarke A, Meredith MP. 2013. Wintertime controls on summer stratification and productivity at the western Antarctic Peninsula. Limn. Oceano. 58, 1035–1047. ( 10.4319/lo.2013.58.3.1035) [DOI] [Google Scholar]
- 9.Arrigo KR, et al. 2017. Early spring phytoplankton dynamics in the Western Antarctic Peninsula. J. Geophys. Res. 122, 9350–9369. [Google Scholar]
- 10.Hendry KR, Rickaby REM, Weston K, de Hoog JC, Rehkamper M. 2008. Cadmium and phosphate in coastal Antarctic seawater: implications for Southern Ocean nutrient cycling. Mar. Chem. 112, 149–157. ( 10.1016/j.marchem.2008.09.004) [DOI] [Google Scholar]
- 11.Saito MA, Goepfert TJ, Noble AE, Bertrand EM, Sedwick PN, DiTullio GR. 2010. A seasonal study of dissolved cobalt in the Ross Sea, Antarctica: micronutrient behavior, absence of scavenging, and relationships with Zn, Cd, and P. Biogeosciences 7, 4059–4082. ( 10.5194/bg-7-4059-2010) [DOI] [Google Scholar]
- 12.De Jong JTM, Stammerjohn SE, Ackley SF, Tison J-L, Mattielli N, Schoemann V. 2015. Sources and fluxes of dissolved iron in the Bellingshausen Sea (West Antarctica): The importance of sea ice, icebergs and the continental margin. Mar. Chem. 177, 518–535. ( 10.1016/j.marchem.2015.08.004) [DOI] [Google Scholar]
- 13.Gerringa LJA, Alderkamp A-C, Laan P, Thuroczy C-E, De Baar HJW, Mills MM, van Dijken GL, van Haren H, Arrigo KR. 2012. Iron from melting glaciers fuels the phytoplankton blooms in Amundsen Sea (Southern Ocean): iron biogeochemistry. Deep-Sea Res. II 71–76, 16–31. ( 10.1016/j.dsr2.2012.03.007) [DOI] [Google Scholar]
- 14.Hatta M, Measures CI, Selph KE, Zhou M, Hiscock WT. 2013. Iron fluxes from the shelf regions near the South Shetland Islands in the Drake Passage during the austral-winter 2006. Deep-Sea Res. II 90, 89–101. ( 10.1016/j.dsr2.2012.11.003) [DOI] [Google Scholar]
- 15.Noble AE, Moran DM, Allen AE, Saito MA. 2013. Dissolved and particulate trace metal micronutrients under the McMurdo Sound seasonal sea ice: basal sea ice communities as a capacitor for iron. Front. Chem. 1, 25 ( 10.3389/fchem.2013.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherrell RM, Lagerström ME, Forsch KO, Stammerjohn SE, Yager PL. 2015. Dynamics of dissolved iron and other bioactive trace metals (Mn, Ni, Cu, Zn) in the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anthr. 3, 71 ( 10.12952/journal.elementa.000071) [DOI] [Google Scholar]
- 17.Eveleth R, Cassar N, Sherrell RM, Ducklow H, Meredith MP, Venables HJ, Lin Y, Li Z. 2017. Ice melt influence on summertime net community production along the Western Antarctic Peninsula. Deep Sea Res. II 139, 89–102. ( 10.1016/j.dsr2.2016.07.016) [DOI] [Google Scholar]
- 18.Bown J, Laan P, Ossebaar S, Bakker K, Rozema P, de Baar HJW. 2017. Bioactive trace metal time series during Austral summer in Ryder Bay, Western Antarctic Peninsula. Deep-Sea Res. II 139, 103–119. ( 10.1016/j.dsr2.2016.07.004) [DOI] [Google Scholar]
- 19.Epstein S, Mayeda TK. 1953. Variation of O content of waters from natural sources. Geochim. Cosmochim. Acta 4, 213–224. ( 10.1016/0016-7037(53)90051-9) [DOI] [Google Scholar]
- 20.Meredith MP, et al. 2017. Changing distributions of sea ice melt and meteoric water west of the Antarctic Peninsula. Deep-Sea Res. II 139, 40–57. [Google Scholar]
- 21.Lagerström ME, Field MP, Seguret M, Fischer L, Hann S, Sherrell RM. 2013. Automated on-line flow-injection ICP-MS determination of trace metals (Mn, Fe, Co, Ni, Cu and Zn) in open ocean seawater: Application to the GEOTRACES program. Mar. Chem. 155, 71–80. ( 10.1016/j.marchem.2013.06.001) [DOI] [Google Scholar]
- 22.Quéroué F, Townsend A, van der Merwe P, Lannuzel D, Sarthou G, Bucciarelli E, Bowie A. 2014. Advances in the offline trace metal extraction of Mn, Co, Ni, Cu, Cd, and Pb from open ocean seawater samples with determination by sector field ICP-MS analysis. Anal. Methods 6, 2837–2847. ( 10.1039/C3AY41312H) [DOI] [Google Scholar]
- 23.Thorpe SA. 1977. Turbulence and mixing in a Scottish Loch. Phil. Trans. R. Soc. Lond. A 286, 125–181. ( 10.1098/rsta.1977.0112) [DOI] [Google Scholar]
- 24.Gargett A, Garner T. 2008. Determining Thorpe scales from ship-lowered CTD density profiles. J. Atmos. Ocean. Tech 25, 1657–1670. ( 10.1175/2008JTECHO541.1) [DOI] [Google Scholar]
- 25.Dillon TM. 1982. Vertical overturns: a comparison of Thorpe and Ozmidov length scales. J. Geophys. Res. 87, 9601–9613. ( 10.1029/JC087iC12p09601) [DOI] [Google Scholar]
- 26.Hosegood P, van Haren H, Veth C. 2005. Mixing within the interior of the Faeroe-Shetland Channel. J. Mar. Res. 63, 529–561. ( 10.1357/0022240054307902) [DOI] [Google Scholar]
- 27.Venables HJ, Meredith MP, Brearley JA. 2017. Modification of deep waters in Marguerite Bay, western Antarctic Peninsula, caused by topographic overflows. Deep-Sea Res. II 139, 9–17. ( 10.1016/j.dsr2.2016.09.005) [DOI] [Google Scholar]
- 28.Clarke A, Meredith MP, Wallace MI, Brandon MA, Thomas DN. 2008. Seasonal and interannual variability in temperature, chlorophyll and macronutrients in northern Marguerite Bay, Antarctica. Deep-Sea Res. II 55, 1988–2006. [Google Scholar]
- 29.Gerringa LJ.A, Laan P, van Dijken GL, van Haren H, de Baar HJW, Arrigo KR, Alderkamp A-K. 2015. Sources of iron in the Ross Sea polynya in early summer. Mar. Chem. 177, 447–459. ( 10.1016/j.marchem.2015.06.002) [DOI] [Google Scholar]
- 30.Klunder MB, Laan P, Middag R, de Baar HJW, van Ooijen JC. 2011. Dissolved Fe in the Southern Ocean (Atlantic sector). Deep-Sea Res. II 58, 2678–2694. ( 10.1016/j.dsr2.2010.10.042) [DOI] [Google Scholar]
- 31.Klunder MB, Laan P, de Baar HJ.W, Middag R, Neven I, Van Ooijen J. 2014. Dissolved Fe across the Weddell Sea and Drake Passage: impact of DFe on nutrient uptake. Biogeosciences 11, 651–669. ( 10.5194/bg-11-651-2014) [DOI] [Google Scholar]
- 32.Middag R, de Baar HJW, Laan P, Cai PH, van Ooijen JC. 2011. Dissolved manganese in the Atlantic sector of the Southern Ocean. Deep-Sea Res. II 58, 25–26. [Google Scholar]
- 33.Middag R, de Baar HJW, Klunder MB, Laan P. 2013. Fluxes of dissolved aluminum and manganese to the Weddell Sea and indications for manganese co-limitation. Limn. Oceano. 58, 287–300. ( 10.4319/lo.2013.58.1.0287) [DOI] [Google Scholar]
- 34.Sherrell RM, Annett AL, Fitzsimmons JN, Roccanova VJ, Meredith MP. 2018. A ‘shallow bathtub ring’ of local sedimentary iron input maintains the Palmer Deep biological hotspot on the West Antarctic Peninsula shelf. Phil. Trans. R. Soc. A 376, 20170171 ( 10.1098/rsta.2017.0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thuróczy CE, Alderkamp AC, Laan P, Gerringa LJA, Mills MM, van Dijken GL, de Baar HJW, Arrigo KR. 2012. Key role of organic complexation of iron in sustaining phytoplankton blooms in the Pine Island and Amundsen Polynyas (Southern Ocean). Deep-Sea Res. II 71–76, 49–60. ( 10.1016/j.dsr2.2012.03.009) [DOI] [Google Scholar]
- 36.Planquette H, Sherrell RM, Stammerjohn S, Field MP.. 2013. Particulate iron delivery to the water column of the Amundsen Sea, Antarctica. Mar. Chem. 153, 15–30. ( 10.1016/j.marchem.2013.04.006) [DOI] [Google Scholar]
- 37.Annett AL, Fitzsimmons JN, Séguret MJM, Lagerström M, Meredith MP, Schofield O, Sherrell RM. 2017. Controls on dissolved and particulate iron distributions in surface waters of the Western Antarctic Peninsula shelf. Mar. Chem. 196, 81–97. [Google Scholar]
- 38.Wagener T, Guieu C, Losno R, Bonnet S, Mahowald N. 2008. Revisiting atmospheric dust export to the Southern Hemisphere ocean: biogeochemical implications. Glob. Biogeochem. Cycles 22 ( 10.1029/2007GB002984) [DOI] [Google Scholar]
- 39.Raiswell R, et al. 2016. Potentially bioavailable iron delivery by iceberg-hosted sediments and atmospheric dust to the polar oceans. Biogeosciences 13, 3887–3900. ( 10.5194/bg-13-3887-2016) [DOI] [Google Scholar]
- 40.Tagliabue A, Bopp L, Aumont O. 2009. Evaluating the importance of atmospheric and sedimentary iron sources to Southern Ocean biogeochemistry. Geo. Res. Lett. 36, L13601 ( 10.1029/2009GL038914) [DOI] [Google Scholar]
- 41.Hodson A, Nowak A, Sabacka M, Jungblut A, Navarro F, Pearce D, Ávila-Jiménez ML, Convey P, Vieira G. 2017. Climatically sensitive transfer of iron to maritime Antarctic ecosystems by surface runoff. Nat. Comm. 8, 14499 ( 10.1038/ncomms14499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lannuzel D, Bowie AR, van der Merwe PC, Townsend AT, Schoemann V. 2011. Distribution of dissolved and particulate metals in Antarctic sea ice. Mar. Chem. 124, 134–146. ( 10.1016/j.marchem.2011.01.004) [DOI] [Google Scholar]
- 43.Lannuzel D, Vancoppenolle M, van der Merwe P, de Jong J, Meiners KM, Grotti M. 2016. Iron in sea ice: review and new insights. Elem Sci. Anth. 4, 130 ( 10.12952/journal.elementa.000130) [DOI] [Google Scholar]
- 44.Meire L, Mortensen J, Meire P, Juul-Pedersen T, Sejr MK, Rysgaard S, Nygaard R, Huybrechts P, Meysman FJR. 2017. Marine-terminating glaciers sustain high productivity in Greenland fjords. Glob. Change Biol. 23, 5344–5357. ( 10.1111/gcb.13801) [DOI] [PubMed] [Google Scholar]
- 45.Monien P, Lettmann KA, Monien D, Asendorf S, Wölfl A-C, Lim CH, Thal J, Schnetger B, Brumsack H-J. 2014. Redox conditions and trace metal cycling in coastal sediments from the maritime Antarctic. Geo. & Cosmo. Acta 141, 26–44. ( 10.1016/j.gca.2014.06.003) [DOI] [Google Scholar]
- 46.Lam PJ, Bishop JK. 2008. The continental margin is a key source of iron to the HNLC North Pacific Ocean. Geophys. Res. Lett. 35, L07608. [Google Scholar]
- 47.Blain S, Sarthou G, Laan P. 2008. Distribution of dissolved iron during the natural iron fertilisation experiment KEOPS (Kerguelen Plateau, Southern Ocean). Deep-Sea Res. II 55, 594–605. ( 10.1016/j.dsr2.2007.12.028) [DOI] [Google Scholar]
- 48.Brearley JA, Meredith MP, Naveira Garabato AC, Venables HJ, Inall ME. 2017. Controls on turbulent mixing on the West Antarctic Peninsula shelf. Deep-Sea Res. II 139, 18–30. ( 10.1016/j.dsr2.2017.02.011) [DOI] [Google Scholar]
- 49.Howard SL, Hyatt J, Padman L. 2004. Mixing in the pycnocline over the western Antarctic Peninsula shelf during Southern Ocean GLOBEC. Deep Sea Res. Part II 51, 1965–1979. ( 10.1016/j.dsr2.2004.08.002) [DOI] [Google Scholar]
- 50.Gregg MC. 1989. Scaling turbulent dissipation in the thermocline. J. Geophys. Res. 94, 9686–9698. ( 10.1029/JC094iC07p09686) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Two .xls files comprising all data of dissolved Fe and Mn and the ancillary data of depth, salinity and so forth, for these samples are given in the electronic supplementary material.