Main Text
The structure and interactions between biomolecular assemblies, including biopolymers, filamentous protein networks, lipid membranes, or lipid-biopolymer complexes, have been extensively studied using solution x-ray or neutron scattering and the osmotic stress method (1, 2, 3, 4, 5, 6). Osmotic stress is often applied by an external polymer solution, which is in contact with the investigated solution (or dispersion) through a semipermeable membrane (made of cellulose, for example). The membrane allows passage of water molecules but blocks macromolecules. The chemical potential of water in the polymer solution decreases as the polymer concentration increases. As a result, water molecules from the investigated solution cross the semipermeable membrane and dilute the polymer solution until the chemical potential of water in the investigated solution (or dispersion) is equal to the water chemical potential in the polymer solution.
Solution-scattering data provide structural information and intermolecular distances under varying osmotic pressures and controlled experimental conditions. By measuring the osmotic stress applied by the polymer solution (using an osmometer) and the intermolecular distances (using scattering data), the pressure-distance equation of state is determined. Measured equations of states can then be compared with rigorous theories that reveal the underlying interaction or mechanisms that govern the behavior of macromolecular assemblies. By controlling experimental conditions, the contributions of van der Waals, electrostatic, hydration, or elastic interactions to the intermolecular forces could be isolated and determined (7). Osmotic pressure may induce phase transitions (1, 3, 4, 8), regulate counterion condensation, or ion desorption and therefore surface charge (3, 4). Humidity chambers, which simultaneously control temperature and relative humidity, enable direct force measurements at the limit of high osmotic stresses and provide insight into short-ranged interactions, like hydration forces between DNA molecules (8).
Two-dimensional self-assembled soft interfaces and thin films are widely found in both natural and technological contexts. Detailed insight into the structure and interactions between interfaces is required for understanding the processes mediated by them. Determining the interactions in thin films, however, is more challenging than in bulk solution (5, 6, 9, 10, 11, 12).
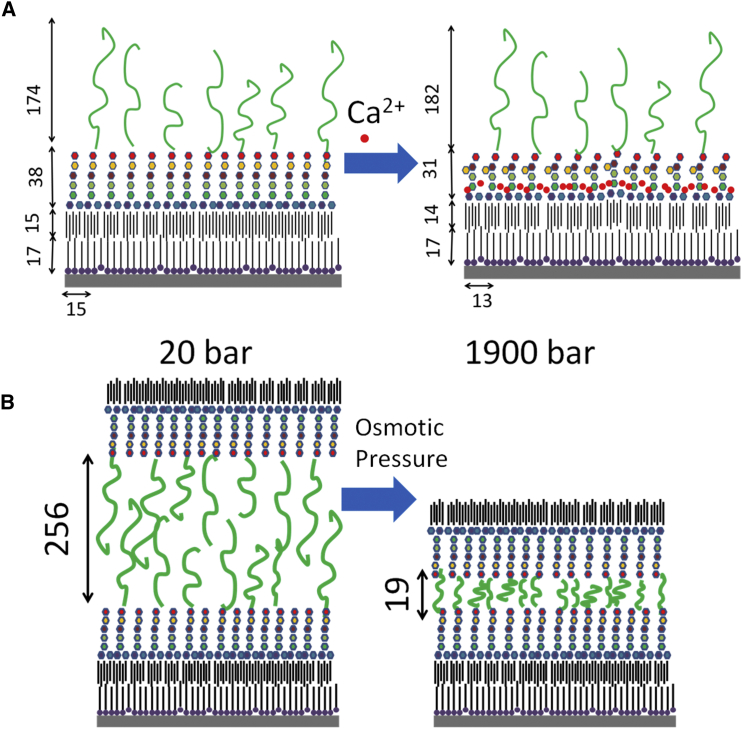
The study by Rodriguez-Loureiro et al. (13) in this issue is a very elegant, detailed, and comprehensive study of the structure and interactions between two planar lipid monolayers, mimicking the outer surfaces of Gram-negative bacteria. The monolayers were made of wild-type lipopolysaccharide molecules from Escherichia coli O55:B5, which expose oligo- and polysaccharides (Fig. 1 A). Lipopolysaccharide chains mediate the interactions between bacteria in colonies or biofilms, have an important structural role, and protect bacteria against harmful molecules (like antimicrobial drugs).
Figure 1.
Effect of calcium and osmotic pressure on the structure of isolated and interacting lipopolysaccharide layers. The layers were built on Si substrates that were coated by a thin (15–21 Å) SiO2 layer (gray slab). The solid substrates were then hydrophobically functionalized with an octadecyltrichlorosilane monolayer (17 Å thick). LipidA hydrocarbon chains (14–15 Å thick), to which the core oligosaccharide (represented by hexagons) were attached, were than added. 25% of the oligosaccharide units displayed O-side chains (shown in green) consisting of polydispersed pentasaccharide repeats (average of 18 repeats per chain). (A) The effect of added 20 mM calcium ions (shown as red dots) on the structure of lipopolysaccharide monolayers at the solid/water interface is shown. (B) The effect of osmotic pressure on the structure of interacting lipopolysaccharide monolayers formed at the solid/water (bottom monolayer) and air/water interface (top monolayer) in water (no calcium was added) is shown. The dimensions in the figure are in units of Å and are based on Rodriguez-Loureiro et al. (13). To see this figure in color, go online.
The fundamental building block of lipopolysaccharide molecules is LipidA, to which the core oligosaccharide is attached. LipidA contains four to seven hydrocarbon chains and two phosphorylated negatively charged glucosamines. The core oligosaccharide has 8–12 sugar units and additional phosphate and carboxyl that are negatively charged. A small fraction of the lipopolysaccharide molecules contained long O-side chains made of repetitive oligosaccharide units. Fig. 1 A shows the structure of a monolayer formed by lipopolysaccharide molecules. As lipopolysaccharide chains are negatively charged (because of the phosphate and carboxyl groups in the core oligosaccharide unit), calcium or magnesium ions can rigidify the monolayers and suppress the penetration of antimicrobial peptides through them (5).
A double lipopolysaccharide monolayer architecture was established on a solid/water interface and a water/air interface (Fig. 1) (11). The two were independently decorated by the monolayers and were initially separated and structurally characterized using neutron reflectivity with and without calcium ions. The monolayers were then brought into contact and structurally characterized (using neutron reflectivity) under interacting conditions (before calcium was added). The nanometric distance between the two interfaces was measured by ellipsometry and then varied by controlling the relative humidity, which is equivalent to applying osmotic pressure. The electron density profile in the direction normal to the layers was determined for the isolated and interacting surfaces (Fig. 1).
Neutron reflectively showed that the core saccharides formed compact layers near the solid/water or the air/water interfaces, whereas the O-side chains were extended and formed a more dilute region. In the presence of calcium ions, both the LipidA and the core oligosaccharide were closely packed, and the O-side chains assumed more compact conformations than in the calcium-free buffer. Water was excluded from the compact LipidA layer after calcium was added because of denser chain packing. The decrease in the Debye screening length from 7.4 to 6.4 Å after the addition of calcium is insufficient to explain the data. The structural changes were therefore most likely due to formation of calcium bridges between the negatively charged groups (14).
The repulsive pressure-distance curves between two interacting lipopolysaccharide monolayers was measured at the limit of high pressure (20 atm or higher) and followed the Alexander-de Gennes model for interacting polymer brushes. The fitting parameters of the model were consistent with the structural data obtained from the neutron reflectivity curves (Fig. 1).
The structure of hydrated interacting lipopolysaccharide layers under ca. 20 bar was similar to that of the single layers with weak chain interpenetration at the midplane (Fig. 1 B). At higher pressure, chain interpenetration increased, and water release was heterogeneous. Next to the core saccharides, water remained bound to the charged groups and readily released from the neutral O-side chain regions (Fig. 1 B). The surface roughness of lipopolysaccharide layers decreased with pressure.
At the limit of high pressure that was applied by Rodriguez-Loureiro et al. (13), the pressure-distance curve is insensitive to the exact chain conformation, but it is sensitive to their volume fraction (Fig. 1 B). It would therefore be of much interest to continue and study the limit of low pressure, in which the chain architecture of each layer should play a role. In that limit, combining the neutron reflectivity data, which provide the exact volume faction along the normal direction to the layer plane, with rigorous theories that require the volume fraction as an input (15) will provide beautiful additional insight into the physical interactions across lipopolysaccharide-bearing surfaces under conditions that have relevance to biology. Adding additional extracellular biofilm components, like lectins, will be another relevant step toward a deeper understanding of interacting bacteria surfaces.
Acknowledgments
Daniel Harries is acknowledged for helpful discussions.
Editor: Georg Pabst.
References
- 1.Danino D., Kesselman E., Harries D. Osmotically induced reversible transitions in lipid-DNA mesophases. Biophys. J. 2009;96:L43–L45. doi: 10.1016/j.bpj.2008.12.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szekely P., Asor R., Raviv U. Effect of temperature on the interactions between dipolar membranes. J. Phys. Chem. B. 2012;116:3519–3524. doi: 10.1021/jp209157y. [DOI] [PubMed] [Google Scholar]
- 3.Dvir T., Fink L., Raviv U. Charged membranes under confinement induced by polymer-, salt-, or ionic liquid solutions. Soft Matter. 2013;9:10640–10649. [Google Scholar]
- 4.Fink L., Feitelson J., Raviv U. Osmotic stress induced desorption of calcium ions from dipolar lipid membranes. Langmuir. 2017;33:5636–5641. doi: 10.1021/acs.langmuir.7b00596. [DOI] [PubMed] [Google Scholar]
- 5.Abraham T., Schooling S.R., Katsaras J. Monolayer film behavior of lipopolysaccharide from Pseudomonas aeruginosa at the air-water interface. Biomacromolecules. 2008;9:2799–2804. doi: 10.1021/bm800562r. [DOI] [PubMed] [Google Scholar]
- 6.Abraham T., Schooling S.R., Katsaras J. Neutron diffraction study of Pseudomonas aeruginosa lipopolysaccharide bilayers. J. Phys. Chem. B. 2007;111:2477–2483. doi: 10.1021/jp066012+. [DOI] [PubMed] [Google Scholar]
- 7.Louzon D., Ginsburg A., Raviv U. Structure and intermolecular interactions between L-type straight flagellar filaments. Biophys. J. 2017;112:2184–2195. doi: 10.1016/j.bpj.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case R., Schollmeyer H., Li Y. Hydration forces between aligned DNA helices undergoing B to A conformational change: in-situ X-ray fiber diffraction studies in a humidity and temperature controlled environment. J. Struct. Biol. 2017;200:283–292. doi: 10.1016/j.jsb.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Kucerka N., Liu Y., Nagle J.F. Structure of fully hydrated fluid phase DMPC and DLPC lipid bilayers using X-ray scattering from oriented multilamellar arrays and from unilamellar vesicles. Biophys. J. 2005;88:2626–2637. doi: 10.1529/biophysj.104.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrache H.I., Gouliaev N., Nagle J.F. Interbilayer interactions from high-resolution x-ray scattering. Phys. Rev. E. 1998;57:7014–7024. [Google Scholar]
- 11.Rodriguez-Loureiro I., Scoppola E., Schneck E. Neutron reflectometry yields distance-dependent structures of nanometric polymer brushes interacting across water. Soft Matter. 2017;13:5767–5777. doi: 10.1039/c7sm01066d. [DOI] [PubMed] [Google Scholar]
- 12.Kanduč M., Schlaich A., Schneck E. Tight cohesion between glycolipid membranes results from balanced water-headgroup interactions. Nat. Commun. 2017;8:14899. doi: 10.1038/ncomms14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Loureiro I., Latza V.M., Schneck E. Conformation of single and interacting lipopolysaccharide surfaces bearing O-side chains. Biophys. J. 2018;114:1624–1635. doi: 10.1016/j.bpj.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asor R., Ben-nun-Shaul O., Raviv U. Crystallization, reentrant melting, and resolubilization of virus nanoparticles. ACS Nano. 2017;11:9814–9824. doi: 10.1021/acsnano.7b03131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsh D. Scaling and mean-field theories applied to polymer brushes. Biophys. J. 2004;86:2630–2633. doi: 10.1016/S0006-3495(04)74317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]