Abstract
Central nervous system atypical teratoid rhabdoid tumors are very rare aggressive tumor of childhood, primarily occurring at age of less than 3 years old. The prognosis of these tumors is very poor, with a reported median survival of 6–12 months in most cases. Treatment typically consists of aggressive chemotherapy and radiotherapy. We present the case of a 65-year-old man who presented with progressive encephalopathy and change in personality over 3 months period. The patient had further accelerated decline over 3 weeks. The diagnosis of atypical teratoid rhabdoid tumor initially remained elusive despite very extensive workup, but was eventually confirmed via open brain biopsy. To the best of our knowledge, this is the oldest reported case of atypical teratoid rhabdoid tumor in the literature. We further extend the spectrum of this rare disease.
Keywords: Neurology, oncology, primitive neuroectodermal tumor, atypical teratoid rhabdoid tumor, neuro-oncology, leptomeningeal carcinomatosis
Introduction
Central nervous system (CNS) atypical teratoid rhabdoid tumors (AT-RTs) are very rare and highly aggressive tumors of childhood, primarily occurring at age of less than 3 years old.1–5 In the pediatric population, they remain a relatively rare tumor, with an incidence as low as 0.65 per 100,000 person-years.6 As of 2015, there have been less than 55 cases of adult-onset AT-RT, 24 cases of those since 2010, and none with intracranial tumor reported over the age of 61.7–9
AT-RTs are associated with a very poor prognosis, with a reported median survival of 6–12 months in most pediatric case series.3–5,8,9 The literature reports the incidence of these tumors more commonly in the supratentorial region,3–6,10,11 in the cerebral hemispheres or near the pineal region, and often with invasion into adjacent structures.4,5 Less often, they may occur infratentorially, potentially in the cerebellopontine angle, and up to 10% may be multifocal.2,4,8
AT-RTs demonstrate pathological findings that are typical of primitive neuroectodermal tumor (PNET), featuring necrosis with a high rate of mitotic activity and the presence of rhabdoid cells.5,10,12 The majority of AT-RTs also demonstrate a loss of INI-1 nuclear staining, indicative of bi-allelic inactivation of SMARCB1 on chromosome 22.12 A smaller proportion of patients also harbor germline mutations in SMARCB1 or SMARCA4.10,12 Histologically, these tumors are composed of eosinophilic, bizarre looking cells with eccentric nuclei and include features of rhabdoid, neuroectodermal, and mesenchymal cells. They demonstrate active mitotic figures characteristic of highly aggressive malignant tumors.9
As mentioned earlier, in AT-RT, the SMARCB1/INI1 genetic mutation on chromosome 22 (q11.2) is frequently observed.1–3,13 The SMARCB1/INI1 gene is responsible for the production of the SNF5 protein, which is part of the SWI/SNF protein complex, and in turn leads to gene transcription and has a role in chromatin remodeling. When such loss of function occurs, this leads to aggressive tumor genesis. This tumor suppression loss in germline cells leads to AT-RT formation in an estimated 20% of cases.1–4,13,14 This mutation can be either inherited or spontaneous. It is cited that around two-thirds of AT-RTs are located in the supra-tentorial region, the remainder being multifocal, both quite invasive into surrounding tissues.1–4,13,14 Leptomeningeal metastasis within the CNS tends to be observed in up to 30% of cases.15
Interestingly, for the past century, the classification of brain tumors has largely been based on concepts of histopathogenesis, in that tumors can be classified according to their histological putative cell origins and/or levels of cell differentiation.1,8 However, the 2016 World Health Organization presented a major restructuring of the diffuse gliomas, medulloblastomas, and other primitive and embryonal tumors.8 This new concept now incorporates genetics, molecular and histological features, and applies to AT-RTs.1–3,8
Due to the extremely rare incidence of this tumor in adult populations, diagnosis is often delayed given that it falls much lower on the differential diagnosis compared to other more common tumors.7 Between 1987 and 2015, there have only been 54 documented cases of AT-RT per our literature review,9 the eldest being 65 years old, although presenting with signs of cauda equina syndrome rather than an intracranial tumor as in our case.15 This case report appears to be the oldest patient presentation of AT-RT in recent literature.9,15
In this report, we describe the case of a 65-year-old man who presented with progressive encephalopathy and change in personality over 3 months period. The patient had further accelerated decline of cognitive decline, encephalopathy, and stupor over the next 3 weeks.
Case report
A generally healthy sixty-five-year-old Caucasian man with a medical history of mild hypertension developed new onset of headaches 3 months prior to his diagnosis of AT-RT, without a prior history of such headaches. The patient’s headaches were described as throbbing, occipital, and retro-orbital, with occasional neck pain without associated phonophobia or photophobia with the headaches.
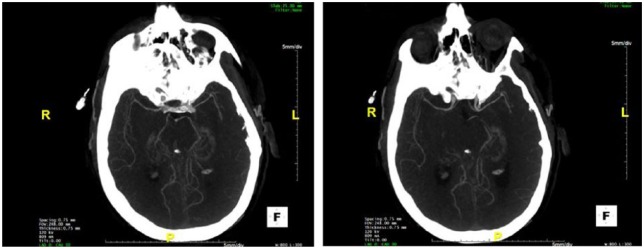
On initial presentation at another institution, the patient had normal vital signs and a normal neurological examination. He was initially thought to have a primary headache disorder and was treated conservatively with over-the-counter medications. This provided little to no symptomatic relief. In the following month, the patient’s symptoms progressed to a persistent severe headache with mild intermittent confusion. As his headaches and cognitive status worsened, he presented to a local emergency department (ER), where a lumbar puncture revealed elevated protein of 58 mg/dL with 38 nucleated cells, 206 RBC, but otherwise normal studies (cytology, viral, and bacterial studies were negative). Opening pressure was within normal range. Computed tomography (CT) of the head without contrast was normal. CT angiography of the head and neck with iodine contrast was however noted for question of diminutive appearance of the distal MCA (middle cerebral artery) and ACA (anterior cerebral artery) bilaterally, although the clinical significance of this was initially uncertain (Figure 1). Subsequently, initial magnetic resonance imaging of the brain, with and without contrast was reported as unremarkable. An MR venogram, without contrast, of the brain was obtained and was normal. Conventional catheter-based cerebral angiography of the brain (digital subtraction angiography) was performed and revealed segmental narrowing of the most distal MCA branches.
Figure 1.
Left/right: Axial (caudal to rostral) CT angiography of the head and neck with iodine contrast, noted for possible diminutive appearance of the distal MCAs (middle cerebral artery) and ACAs (anterior cerebral artery) bilaterally.
Given the above initial evaluation, a working diagnosis of possible vasculitic meningitis was rendered. The patient was treated with high-dose steroids (prednisone 100 mg) and discharged on a gradual prednisone taper. The patient, however, had no relief and his symptoms continued to worsen, including progressive encephalopathy and new gait instability, prompting a second opinion at a large tertiary academic medical center.
Repeat magnetic resonance imaging (MRI) brain with and without gadolinium revealed a new 12 mm focus of mass-like enhancement in the pineal region with adjacent right midbrain T2 signal abnormality and prominent adjacent leptomeningeal enhancement involving parieto-occipital sulci and superior cerebellum (Figure 2). A lumbar spine MRI with and without contrast was obtained and revealed small foci of enhancement around the cauda equina (Figure 3). A lumbar puncture was performed and was significant for RBC 4000, 126 nucleated (lymphocytic predominance), protein 144 mg/dL, and glucose 23 mg/dL. Opening pressure was within normal range. Cerebrospinal fluid (CSF) cultures (bacterial, TB, and fungal) and viral polymerase chain reactions (PCRs) were all negative. CSF cytology, however, was notable for abnormal cells, described as large plasmacytoid cells with frequent cytoplasmic vacuoles. Flow cytometry showed no monoclonal B-cell population.
Figure 2.
(a and b) Axial T1-post-contrast gadolinium MRI of the brain, which reveals a 12 mm focus of mass-like enhancement in the pineal region abutting the tectum (green arrow). (c) Sagittal view, with area of post-contrast enhancement around the tectum and occipital lobe.
Figure 3.
Left: Sagittal T1 lumbar spine MRI, right: sagittal T1-post contrast, with noted small focus of enhancement among the cauda equina, particularly at the right posterior lateral aspect of the thecal sac at the level of L4.
An EVD (external ventricular drain) was placed due to concern of developing communicating hydrocephalus, which was confirmed by this intervention. Brain biopsy of the right pineal gland confirmed the diagnosis of AT-RT (World Health Organization (WHO) grade IV). Fluorescence in situ hybridization (FISH) testing did not show rearrangement of the EWSR1 (22q12) gene region. Patient continued to clinically decline due to encephalopathy and stupor leading to poor functional status and family elected to rescind any further therapies given his rapidly worsening quality of life and the confirmed diagnosis of a terminal disease. The patient expired after 2 weeks of his hospitalization. The prospect of autopsy was not desired by the family.
Discussion
We describe the first case report of intracranial AT-RT in a 65-year-old patient. To the best of our knowledge, there are no described reports of intracranial AT-RTs occurring in individuals older than 61 years of age,8,9,14 the exception in this age group being a 65-year-old reported with spinal tumor. Thus, we extend the age spectrum of this very rare and highly aggressive malignancy.
In this patient, the AT-RT diagnosis was confirmed by surgical pathology and was admittedly unclear for some time, despite the clinician team entertaining a broad differential diagnosis due to its general rarity, in addition to rarity in this age group. This is often the case in the medical literature, given that AT-RTs fall much lower on the differential diagnosis list than other more statistically common adult brain tumors. Upon admission, this patient’s symptoms were suspected to be directly attributed to a high-grade malignancy, resulting in vasculitis and communicating hydrocephalus. A vasculitic process was suspected on the basis of conventional cerebral angiogram (digital subtraction angiography), and in direct part, linked to cerebral vascular reactivity of malignant tumor spread. Communicating hydrocephalus was thought to be directly related to the inflammatory process leading to decreased CSF absorption. The vacuolated “plasmacytoid cells” on pathology were similar in description to tumor cells of AT-RT often described in the literature as appearing in this way and also indicating metastatic spread.15
As noted, these tumors are considered to be some of the most aggressive of all PNET malignancies. Patients with familial mutations tend to do worse compared to those with sporadic incidence.12 Treatment typically consists of gross surgical resection coupled with focal radiotherapy with and without concurrent chemotherapy, those on combination therapies tended to do better generally. Aggressive chemotherapy regimens have been described in the literature with varied agents such as methotrexate, high-dose chemotherapy (HDC) with agent combinations such as cyclophosphamide/topotecan, carboplatin/thiotepa, or cyclophosphamide/carboplatin/thiotepa.16 All of the aforementioned strategies have demonstrated marginal success in increasing patient survival. Radiotherapy treatment typically consists of focal craniospinal radiation with/without concurrent chemotherapy, those on combination therapies tended to do better globally in relative terms. Intrathecal chemotherapy can also be pursued in patients too young to receive radiation therapies (i.e. <3 years of age) or those with contraindications to radiotherapy but, once again, is not seen as effective as chemotherapy/radiotherapy combination.16,17,18 Thus, given the rarity and complexity of these tumors, transfer to highly specialized centers and a multidisciplinary approach is encouraged. Targeted therapies and numbers of early-phase trials are currently ongoing.6,7,11,12
Unfortunately, and despite advance in the multidisciplinary care and treatment of brain tumors, AT-RTs remain extremely malignant with a very high rate of associated morbidity and mortality. It should be considered as a rare, but possible differential diagnosis in adults with brain tumors.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors were responsible for the neurologic care and management of the patient described in this manuscript. All authors agree to the conditions outlined in the Authorship and Contributorship section of the Information for authors. No statistical analysis was performed. M.-A.B., P.F., M.L., Y.A.O., and O.P. report no disclosures.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
References
- 1. Woehrer A, Slavc I, Waldhoer T, et al. Incidence of atypical teratoid/rhabdoid tumors in children: a population-based study by the Austrian brain tumor registry, 1996–2006. Cancer 2010; 116: 5725–5732. [DOI] [PubMed] [Google Scholar]
- 2. Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 1996; 85: 56–65. [DOI] [PubMed] [Google Scholar]
- 3. Buscariollo DL, Park HS, Roberts KB, et al. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a surveillance, epidemiology, and end results analysis. Cancer 2012; 118: 4212–4219. [DOI] [PubMed] [Google Scholar]
- 4. Athale UH, Duckworth J, Odame I, et al. Childhood atypical teratoid rhabdoid tumor of the central nervous system: a meta-analysis of observational studies. J Pediat Hematol Onc 2009; 31: 651–653. [DOI] [PubMed] [Google Scholar]
- 5. Schrey D, Lechón FC, Malietzis G, et al. Multimodal therapy in children and adolescents with newly diagnosed atypical teratoid rhabdoid tumor: individual pooled data analysis and review of the literature. J Neurooncol 2016; 126: 81–90. [DOI] [PubMed] [Google Scholar]
- 6. Park HG, Yoon JH, Kim SH, et al. Adult-onset sellar and suprasellar atypical teratoid rhabdoid tumor treated with a multimodal approach: a case report. Brain Tumor Res Treat 2014; 2(2): 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst 2001; 17(9): 503–511. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 2016; 131(6): 803–820. [DOI] [PubMed] [Google Scholar]
- 9. Wu WW, Bi WL, Kang YJ, et al. Adult atypical teratoid/rhabdoid tumors. World Neurosurg 2016; 85: 197–204. [DOI] [PubMed] [Google Scholar]
- 10. Versteege I, Sévenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998; 394: 203–206. [DOI] [PubMed] [Google Scholar]
- 11. Eaton KW, Tooke LS, Wainwright LM, et al. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 2011; 56: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bruggers CS, Bleyl SB, Pysher T, et al. Clinicopathologic comparison of familial versus sporadic atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. Pediatr Blood Cancer 2011; 56: 1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winn RH. Atypical teratoid/rhabdoid tumor. In: Winn RH. (ed.) Youmans and Winn neurological surgery. 7th ed. Philadelphia PA: Elsevier, pp. 1650–1658.e3, 2017. [Google Scholar]
- 14. Chheda MD, Wen PY. Uncommon brain tumors: UpToDate. Waltham, MA: UpToDate Inc., http://www.uptodate.com (accessed 17 September 2017). [Google Scholar]
- 15. Yang M, Chen X, Wang N, et al. Primary atypical teratoid/rhabdoid tumor of central nervous system in children: a clinicopathological analysis and review of literature in China. Int J Clin Exp Pathol 2014; 7(5): 2411–2420. [PMC free article] [PubMed] [Google Scholar]
- 16. Ginn FK, Galjar A. Atypical teratoid rhabdoid tumor: current therapy and future directions. Frontiers Oncol. Epub ahead of print 12 September 201. DOI: 10.3389/fonc.2012.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sinha P, Ahmad M, Varghese A, et al. Atypical teratoid rhabdoid tumour of the spine: report of a case and literature review. Eur Spine J 2015; 24 (Suppl. 4): S472–484. [DOI] [PubMed] [Google Scholar]
- 18. Dardis C, Yeo J, Muilton K, et al. Atypical teratoid rhabdoid tumor: two case reports and an analysis of adult cases with implications for pathophysiology and treatment. Front Neurol 2017; 8:247. [DOI] [PMC free article] [PubMed] [Google Scholar]