Abstract
Background.
Renewed interest in vinegar as a glucose-lowering agent led to several small trials in the recent past. However, none of the trials could independently provide sufficient evidence.
Objectives.
Our review aimed to obtain reliable estimates of effects of vinegar on short-term and long-term blood glucose control.
Methods.
Large bibliographic databases were searched from inception to date of search without language and publication date restrictions. All clinical trials evaluating effect of vinegar on diabetes mellitus patients were eligible. Two authors independently extracted data on fasting and 2-hour postprandial blood glucose, insulin, and HbA1c levels at the various time points. MS Excel, SAS® v9.3, and RevMan v5.3 were used for data analysis.
Results.
Small significant reduction in mean HbA1c was observed after 8 to 12 weeks of vinegar administration: −0.39% (95% confidence interval = −0.59, −0.18; I 2 = 0%). Other long-term outcomes favored vinegar but were not significant. Short-term outcomes showed significantly lower pooled mean difference in glucose levels at 30 minutes in the vinegar group. Readings at 60, 90, and 120 minutes were lower in the vinegar group but not statistically significant. Adverse effects profile also favored the vinegar group.
Conclusions.
It is worthwhile to carry out carefully planned large trails to determine the efficacy and effectiveness of vinegar as an adjunct treatment modality.
Keywords: vinegar, acetic acid, diabetes mellitus, control, systematic review, meta-analysis, meta-regression, HbA1c, insulin
Diabetes mellitus (DM) is a major global public health problem, with the prevalence of diabetes increasing from 108 million in 1980 to 422 million in 2014.1 The global prevalence of diabetes has risen from 4.8% in 1980 to 8.5% in 2014. In 2012, an estimated 1.5 million deaths were directly caused by diabetes and another 2.2 million deaths were attributable to high blood glucose.1 The World Health Organization projects that diabetes will be the seventh leading cause of death in 2030.2 Diabetes can be treated and its consequences avoided or delayed with diet, physical activity, medication, and regular screening and treatment for complications.1
Diabetes mellitus is a chronic metabolic disorder resulting in hyperglycemia and disturbances in carbohydrate, fat, and protein metabolism caused by deficient insulin production by pancreas and/or insulin resistance.3 In the long term, poor glycemic control associated with diabetes mellitus results in microvascular and macrovascular complications.3 Postprandial hyperglycemia is an important factor contributing toward glycemic status and in the development of diabetes complications.4,5 Postprandial blood glucose levels can be controlled by modifying diet, for instance, consuming relatively higher proportion of low glycemic index foods or by taking medicines that slow down glucose absorption in the intestines by inhibiting the action of certain carbohydrate-hydrolyzing enzymes, namely, pancreatic α-amylase, and intestinal α-glucosidase and glucose transporters like sodium-glucose transport proteins (SGLT1) and glucose transporter 2 (GLUT 2).6
Vinegar is a widely consumed food ingredient with acetic acid as its main component. It has been shown to have an effect on glucose absorption and metabolism, and hence is an appealing intervention for reducing postprandial glucose excursions. An in vitro study showed that acetic acid suppressed sucrase activity.7 In vivo, apple cider vinegar improved glycated hemoglobin (HbA1c) and serum triglycerides in diabetic rats, and high-density lipoprotein improvement in both normal and diabetic rats.8 In vinegar-fed mice, reduced energy consumption from carbohydrates and increased energy consumption from fats have also been reported.9 Vinegar has also shown to improve pancreatic beta-cell function in diabetic rats.10
Studies on healthy individuals showed delayed gastric emptying when vinegar was added to a starchy meal.11 Taking vinegar with a diet containing polysaccharides reduced postprandial glycemia by 20%; a similar effect could not be elicited with monosaccharides.12 Vinegar resulted in the reduction of acute glycemia and insulinemia when consumed with potatoes.13
In the past decade, several studies evaluated vinegar as an adjunct to the mainstream treatment modalities to improve glycemic control in individuals with diabetes without diabetes mellitus complications.12,14–28 Though promising, the reported effects of vinegar were inconclusive owing to the small sample sizes and inconsistent results from the primary studies. This motivates the need for a systematic review and meta-analysis to evaluate the effectiveness of vinegar for glucose control in individuals with diabetes.
The primary objective of this systematic review is to determine the effect of vinegar on fasting blood/plasma glucose (FPG), postprandial blood/plasma glucose (PPG), or HbA1c. The secondary objectives were the effect on fasting blood/plasma insulin (FPI), postprandial insulin (PPI), and the safety of vinegar.
Methods
Eligibility
Randomized or nonrandomized controlled clinical trials that recruited adults with type 1 or type 2 diabetes, who were treatment naïve or on medication, and who reported at least one of the primary outcomes were eligible. The intervention, vinegar (active ingredient), should have been administered orally in amounts considered effective by the study investigators. The control intervention should be a placebo or no intervention. Studies that recruited patients with advanced diabetic complications (renal failure, retinopathy, amputations) were excluded.
Outcomes
Primary outcomes of effectiveness included FPG, PPG within 120 minutes, and HbA1c levels. FPI and PPI within 120 minutes were considered as secondary outcomes. Safety outcomes included any clinical adverse event and levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urinary pH, and leptin.
Search Strategy
A systematic search was carried out in the Medline, Embase, and the Cochrane Register for Clinical Trials (CENTRAL) electronic databases from inception to the date of running the syntax. The initial search was performed in August 2014 and updated in April 2016. The keywords searched were “Diabet*,” “hyperglyc*,” “blood glucose,” “diabetes mellitus,” “vinegar,” and “acetic acid” in any of the search fields. The search was not restricted by date, language, or study design. The search syntax for PubMed and CENTRAL was (diabetes OR hyperglyc* OR “blood glucose”) AND (vinegar OR “acetic acid”) while diabet* AND (‘vinegar’/exp OR ‘acetic acid’/exp) was used for Embase. We also manually scanned bibliographies of the eligible articles and contacted corresponding authors for full texts when only abstracts could be retrieved through electronic search.
Screening and Data Extraction
All identified English language articles were screened independently by 2 authors (FJS and NND), based on information available in the abstracts. Disagreements were resolved by a third author (PNA). The same procedure was employed to confirm eligibility of the full texts.
Two authors (NND and PNA) independently extracted data using a standardized data extraction form specifically developed for this review. In case of disagreement a third author (FJS) was consulted. The extracted data were entered into Excel spreadsheets by RS and reviewed by NND. Data management and imputation were done by RS. Extracted data included subject characteristics, study characteristics (type of study population, number of participants by intervention groups, study design, and duration of follow-up), details of interventions, summary of outcomes by intervention groups, and number of dropouts in each group with corresponding reasons.
Eligible articles in Persian were screened by FJS with the help of a statistician (SES) from Iran. The studies were assessed for eligibility and Cochrane risk of bias. Data was extracted by SES in consultation with FJS.
For both the vinegar and control groups, summary measures extracted for meta-analysis and meta-regression were the number of subjects per group (n), the means (μ), standard deviations (SDs), and standard errors (SEs) for continuous outcomes and number of events (k) in each category for categorical outcomes. All continuous outcomes were analyzed based on change scores, defined as mean endpoint − mean baseline values and corresponding SD. If the SD of a change score was not available but the respective SE and n were available, then the SD was calculated by multiplying the SE with the square root of n.32 Alternatively, if the SD were available for the baseline and endpoint scores, then a pooled SD was calculated as
for independent groups, for example, parallel group randomized controlled trial, and
where ρ is correlation coefficient between groups, for example, crossover trials; CB and CA are the control group before and after intervention, respectively; VB and VA are the vinegar group before and after intervention, respectively; and SD1 and SD2 are standard deviations of group 1 and group 2, respectively.29 Otherwise the SD was imputed by taking the average SD from other relevant studies. Most of the required values were extracted from texts or tables, and the remaining from graphs using Engauge Digitizer software.30 For each graph, all points were mapped 3 times and average values were used. Outcomes were reported in different units across studies so we standardized the units of FPG and PPG to mmol/L, FPI and PPI to pmol/L, and AST, ALT, and ALP to IU/L (U/L) using conversion factors provided by California-based Diagnostic Group of BIORAD Laboratories.31
Risk of Bias Assessment
The Cochrane risk of bias tool was used to assess internal validity with addition of a few domains relevant to special trial designs, for example, crossover design.31,32 Included studies were assessed for the following risk of bias domains: (1) balanced baseline characteristics, (2) uniform patient management, (3) uniform outcome assessment, (4) complete outcome reporting, (5) selective reporting; and other aspects that were likely to introduce bias but not captured by the preceding domains. For parallel-group randomized controlled trials, random sequence generation and allocation concealment methods were sought for balanced baseline characteristics. For crossover trials, in addition, length of washout period was also looked for. Details describing methods to ensure uniform diabetes mellitus management, that is, blinding and follow-up duration, were looked for. Details describing uniformity in outcome assessment throughout the study period and across study arms as well as blinding of outcome assessor were looked for. Completeness of outcome data was checked by comparing the number of individuals with diabetes in the demographic and outcome tables and reviewing the study flow chart. Selective outcome reporting was ascertained by comparing commonly reported outcomes across studies with the ones reported in any particular study. Funding source was looked for potential conflict of interest.
Each domain was judged as having “Low,” “High,” or “Unclear” risk of bias based on the information available in the study. For each domain, if insufficient details were reported then it was judged as having unclear risk of bias. Where adequate details were reported and methods were thought as adequate to minimize bias then the domain was judged as having low risk of bias, otherwise as having high risk of bias.
Data Synthesis and Statistical Analysis
Studies with follow-up periods ≥8 weeks (long term) were analyzed using standard meta-analysis methods, while studies with <3 hours (short term) follow-up period were analyzed using repeated-measures meta-regression. One study that measured outcomes between the above-mentioned 2 time points was not included in the synthesis. For meta-analysis of continuous outcomes, mean differences of change scores were pooled where change scores were calculated as end-point − baseline.
Where SD for the change score was not reported, ρ = 0.5 was used to obtain the value. However, a sensitivity analysis was also conducted using ρ values of 0.3 and 0.8 to evaluate the effect of the assumption on the results.
FPG and FPI were repeatedly measured over time (30, 60, 90, and 120 minutes) for each patient. Differences between vinegar and control groups at each time point were estimated using meta-regression based on repeated-measures mixed-models (repeated ANOVA), which accounts for the dependence among repeated measurements on the same patient. Mixed-models for continuous data was employed, in SAS v9.3 (SAS Institute, Cary, North Carolina; PROC MIXED), with time, group, and time by group interaction as fixed effects, and time as a random effect with an unstructured (general) variance-covariance matrix. The estimation method was based on a residual (restricted) maximum likelihood technique and the variance-covariance matrix of the parameter estimates computed using a sandwich (empirical) estimator.
Assessment of Heterogeneity
Given the nature of the question, clinical and methodological heterogeneity was expected among the studies. Primary sources of clinical heterogeneity were differences between study populations, interventions, and outcomes. For methodological heterogeneity, the primary sources were study designs and assessment of risk of bias. To accommodate anticipated heterogeneity we used a random-effects model to obtain pooled results unless I 2 value was ≥75% when no pooling was done.32,33 Separate analyses were carried out for the short-term and long-term outcomes.
Results
Study Characteristics and Quality Assessment
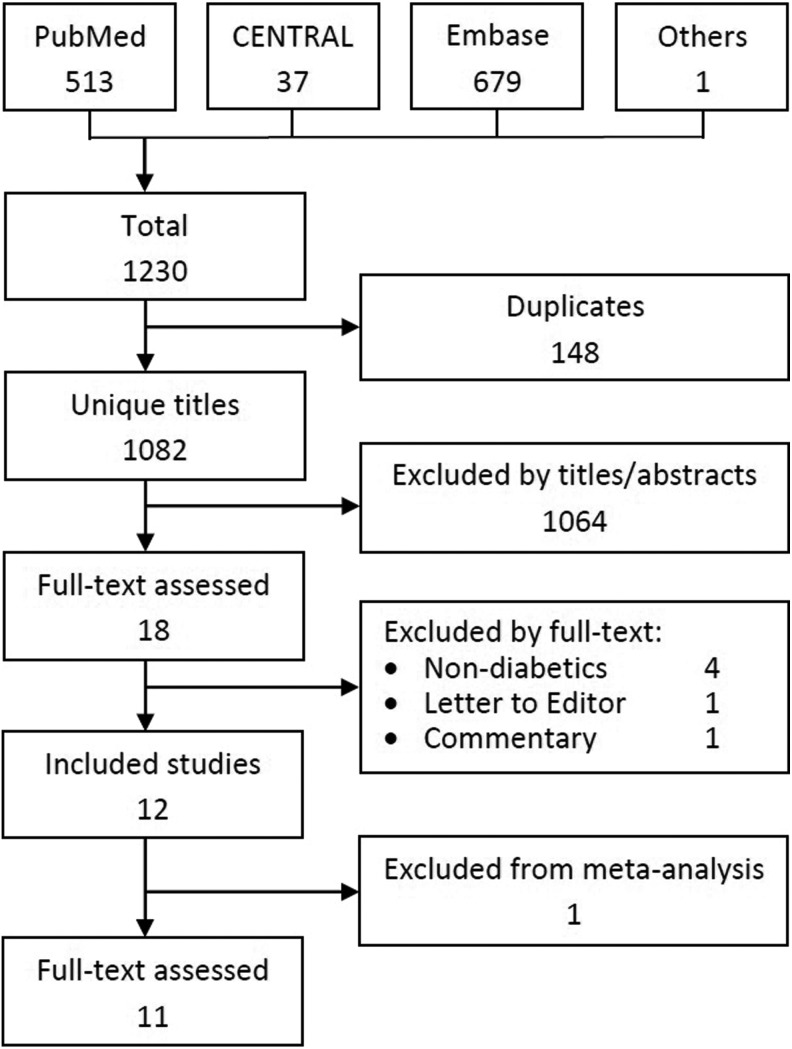
The search of electronic databases and scan of included studies’ bibliographies identified 1230 citations. Of these, 148 were duplicates. After excluding studies conducted on animals, healthy volunteers, and in vitro settings, 18 titles underwent full-text appraisal. Of these, 12 met the inclusion and exclusion criteria. Figure 1 shows the search and selection process.
Figure 1.
Literature search process flow chart.
Twelve articles reporting 11 studies comprising 278 subjects were included in the review. The only 2 non-English articles were in Persian.14,22 Five were parallel group randomized controlled trials,14,17,18,21,24 5 were crossover randomized controlled trials,12,16,22,23,25 1 was a nonrandomized controlled trial,20 and 1 was a single-arm before-and-after study.19 The subjects were treated with diet, oral hypoglycemic agents, or insulin. The study by Yoon et al24 compared 3 different doses of vinegar with placebo. We treated each arm as a separate comparison against control, and the sample size of the placebo arm in each comparison was reduced to one third of the original.34 Table 1 summarizes the characteristics of the included studies.
Table 1.
Characteristics of and Risk of Bias in the Included Studies.
| Study ID | Study Design and Follow-up Period | Characteristic of the Participants | Intervention Arm/Control Arm | Outcome(s) | Allocation Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Biases |
|---|---|---|---|---|---|---|---|---|---|---|
| Johnston 2004 | Randomized crossover trial; 1-week washout period Follow-up: 60 minutes | T2DM; n = 10; not on OADs From United States |
|
|
? | ↓ | ↓ | ↓ | ↑ | ↓ |
| Johnston 2008 | RCT Follow-up: 12 weeks | T2DM; n = 27; on OADs; age range = 20-80 years; mean age = 63.0 (±4.1) From United States |
|
|
↓ | ↑ | ↓ | ↓ | ↓ | ↓ |
| Johnston 2009 | RCT Follow-up: 12 weeks | T2DM; n = 27; on OADs; age range = 20-80 years; mean age = 63.0 (±4.1) From United States |
|
|
↓ | ↑ | ↓ | ↓ | ↓ | ↓ |
| Johnston 2010 | Randomized crossover trial; 1-week washout period Follow-up: 2 hours | T2DM; n = 9; on OADs; mean age = 69 (±2) years From United States |
|
|
? | ? | ↓ | ↓ | ↓ | ↓ |
| Liatis 2010 | Before-after (2 groups—low and high GI mixed meal); 1 week (±2 days) washout period Follow-up: 2 hours | T2DM; n = 16; on OADs or diet alone Low GI group: Mean age = 57.4 (±8.0) years; mean duration of DM = 3.6 (±4.0) years High GI group: Mean age = 61.4 (±8.4) years; mean duration of DM = 4.8 (±3.5) years From Greece |
|
|
↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Mahmoodi 2013 | Nonrandomized controlled trial Follow-up: 1 month | T2DM; n = 60; on OADs; age range = 30-60 years From Iran |
|
|
↑ | ↑ | ↓ | ↓ | ↑ | ↑ |
| Mitrou 2010 | RCT Follow-up: 4 hours | T1DM; n = 10; on insulin; mean age = 32 (±3) years; mean HbA1c = 6.7% (±0.2%); mean duration of DM = 14 (±3) years From Greece |
|
|
? | ? | ↓ | ↓ | ↓ | ↓ |
| Van Dijk 2012 | Randomized crossover trial; 1-week washout period Follow-up: 1 week | T2DM; n = 12; on OADs; mean age = 65 (±1) years; mean HbA1c = 6.6% (±0.2%) From Netherlands |
|
|
? | ? | ↓ | ↓ | ? | ↓ |
| White 2007 | Randomized crossover trial; 3-5 days washout period Follow-up: 2 days | T2DM; n = 11; on OADs; age range = 40-72 years; FPG = 7.6 (±0.3) mmol/L; mean HbA1c = 6.2% (±0.2%); mean duration of DM = 4.9 (±1.0) years From United States |
|
|
↑ | ? | ↓ | ↓ | ↑ | ↓ |
| Yoon 2012 | RCT Follow-up: 8 weeks | T2DM; n = 72; not on OADs; mean age = 52.8 (±9.9); FPG of 7.0-15.0 mmol/L and HbA1c of 7.0% to 12.0% From South Korea |
|
|
↓ | ↓ | ↓ | ↓ | ↓ | ↓ |
| Ebrahimi 2009 | RCT Follow-up: 8 weeks | T2DM; n = 65; mean age = 55.8 (±11.4) From Iran |
|
|
? | ↑ | ↓ | ↓ | ? | ↓ |
| Nosrati 2013 | Randomized crossover trial; 1-week washout period Follow-up: 90 minutes | T2DM; n = 32; on OADs; mean age = 47.25 (±16.82) From Iran |
|
|
↓ | ? | ↓ | ↓ | ↓ | ↓ |
Abbreviations: T2DM, type 2 diabetes mellitus; OAD, orally administered drug; RCT, randomized controlled drug; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AE, adverse event; AUC, area under the curve; GI, glycemic index; FPG, fasting blood/plasma glucose; BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Overall, the studies were assessed as having a low risk of bias. All studies except one (Johnston et al12) had balanced baseline characteristics in both vinegar and control groups. Baseline characteristics were reported collectively for Johnston et al12 and were hence assessed as “Unclear.” Despite lack of blinding, most studies were judged as having a low risk of performance bias as there was either little opportunity to introduce considerable differences in the treatment arms being compared or investigators attempted to prevent the subjects from changing their practices during the study period. One study required subjects to maintain a dietary record. All primary and secondary outcomes were assessed objectively in all the studies; hence, studies were judged as “Low” risk of bias for outcome assessment. There was no missing outcome data for the short-term studies, and it was negligible for long-term studies. Hence, there was low risk of bias with regard to incompleteness of outcome data.
Data Synthesis
Short-Term Outcomes
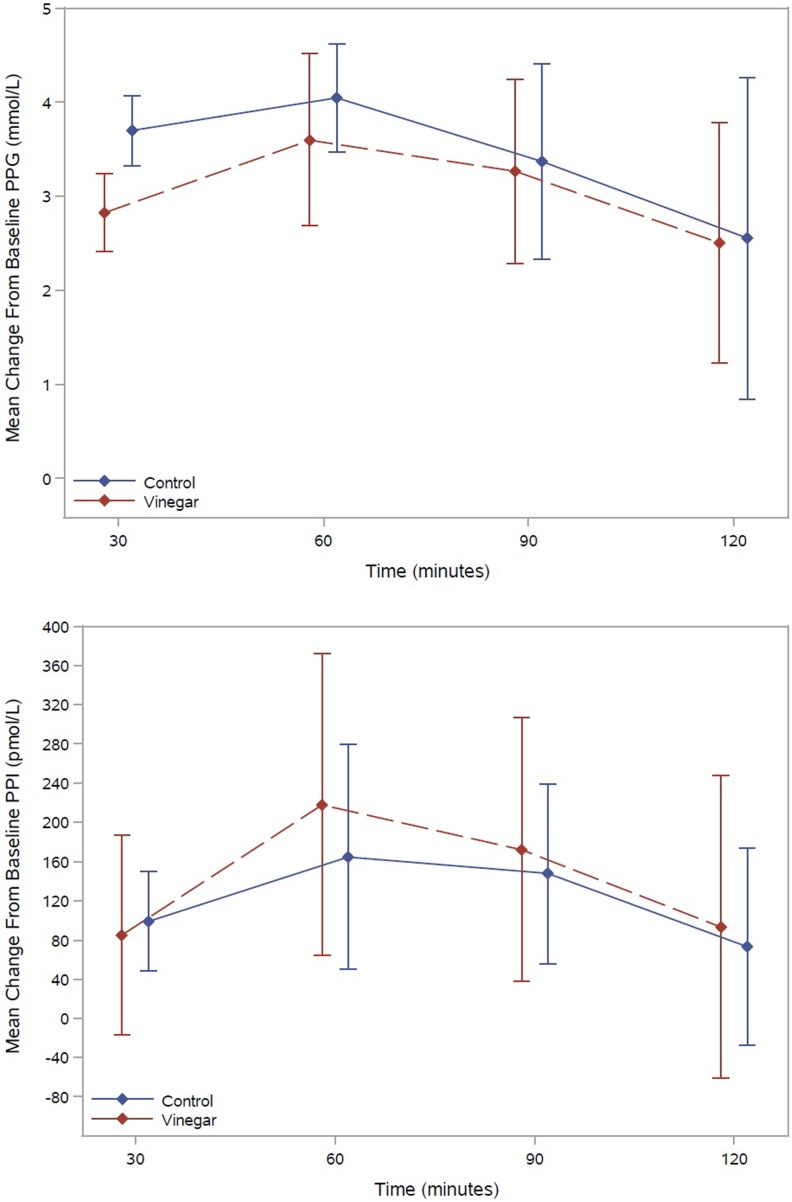
Five studies reported PPG levels at 30 minutes,16,17,19,21,25 4 studies at 60 minutes,16,17,19,25 and 3 studies at 90 and 120 minutes.17,19,25 Pooled mean difference of PPG levels between control and vinegar groups were 0.88 mmol/L (95% confidence interval [CI] = 0.51, 1.25) at 30 minutes (n = 102), 0.45 mmol/L (95% CI = −0.31, 1.21) at 60 minutes (n = 92), 0.10 mmol/L (95% CI = −0.52, 0.73) at 90 minutes (n = 74), and 0.05 mmol/L (95% CI = −1.11, 1.20) at 120 minutes (n = 74). Three studies reported FPI at 30 and 60 minutes17,19,25 and 2 studies at 90 and 120 minutes.19,25 Pooled mean difference of FPI between control and vinegar groups were 13.62 mU/L (95% CI = −63.81, 91.03) at 30 minutes (n = 74), −53.57 mU/L (95% CI = −157.93, 50.80) at 60 minutes (n = 74), −24.77 mU/L (95% CI = −114.07, 64.52) at 90 minutes (n = 56), and −20.06 mU/L (95% CI = −124.02, 83.89) at 120 minutes (n = 56). Figure 2 presents the pooled mean changes from baseline of blood glucose and serum insulin profiles of vinegar and control groups, adjusted for repeated measurements within studies.
Figure 2.
Pooled mean changes from baseline of blood glucose and serum insulin levels in vinegar and control groups, adjusted for repeated measurements within studies.
Long-Term Outcomes
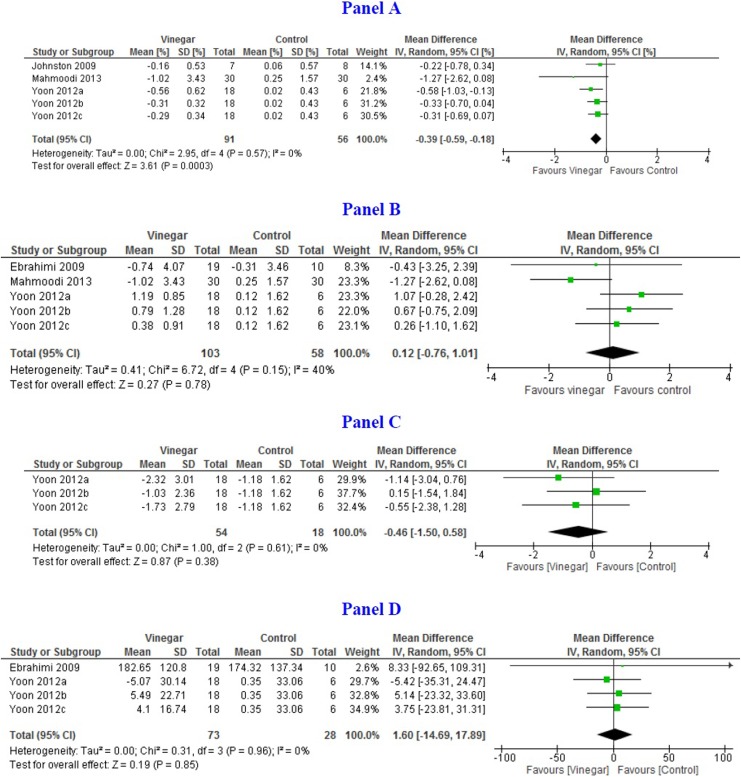
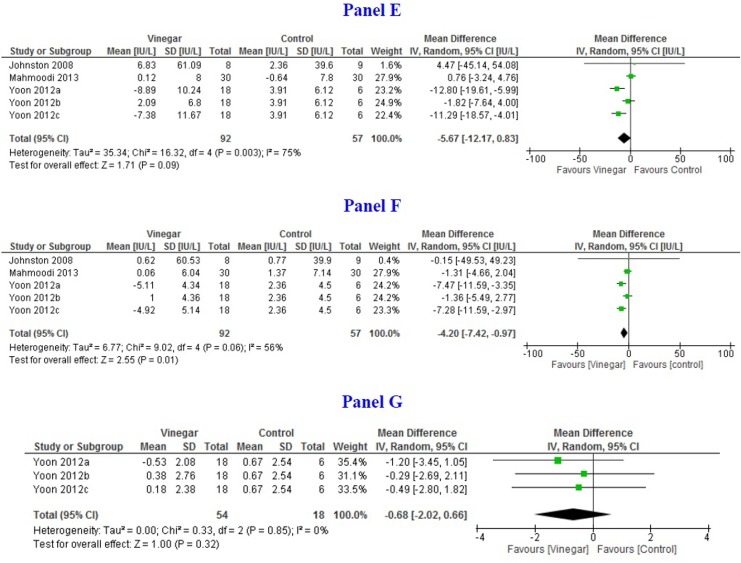
Pooled mean difference of HbA1c measured at 8 weeks or later was −0.39% (95% CI = −0.59, −0.18; I 2 = 0%) based on 3 studies (n = 147).18,20,24 Pooled mean difference of FPG between vinegar and control groups at 8 weeks or later was −0.80 mmol/L (95% CI = −1.47, −0.14; I 2 = 0%) based on 3 studies (n = 161).14,20,24 Pooled mean difference of PPG between vinegar and control groups at 8 weeks was −0.46 (95% CI = −1.50, 0.58; I 2 = 0%) based on 3 arms of one study (n = 72).24 Pooled mean difference of FPI was 1.60 mU/L (95% CI = −14.69, 17.89; I 2 = 0%) based on 2 studies (n = 101).14,24 Figure 3 shows the forest plots for long-term outcomes.
Figure 3.
Forest plots depicting pooled estimates for the long-term outcomes and adverse effects comparing means of the vinegar and control groups. (Panel A) Hba1C% at 8 to 12 weeks; (Panel B) Fasting plasma glucose (mmol/L); (Panel C) Postprandial blood glucose (mmol/L); (Panel D) Plasma insulin (pmol/L); (Panel E) Alanine aminotransferase at 12 weeks (IU/L); (Panel F) Aspartate aminotransferase (IU/L); and (Panel G) Leptin (pg/mL).
Pooled mean difference of ALT and AST was −5.67IU/L (95% CI = −12.17, 0.83; I 2 = 75%) and −4.20 (95% CI = −7.42, −0.97; I 2 = 56%), respectively, between 8 and 12 weeks (n = 149).17,20,24 One study assessed urinary pH whereas another measured serum leptin levels.24 No difference was found between vinegar and control groups.
Discussion
Vinegar has been used as medicine since the time of Hippocrates.33,35 However, in recent times there is a renewed interest in finding new indications using modern technologies and research methods. In 2014, Petsiou et al published a systematic review on the effects of vinegar on glucose and lipid metabolism. The review described the research done until the time of publication and shed light on possible mechanisms of action of vinegar.34,36 Our review takes this work further in diabetic populations by searching more databases, including non-English articles, and conducting a meta-analysis. Although most of the included studies were small, conducted in diverse settings, using various definitions for diagnosis of diabetes, and using vinegar from different sources and in various forms, the results were consistent across studies with little variation in outcomes (I 2 values close to zero).
A thorough search of the medical literature revealed that considerable research has been done to evaluate the effects of vinegar on carbohydrate metabolism both in vitro and in vivo. The in vivo effects of vinegar have been assessed in animal models, healthy volunteers, and people with type 1 and type 2 diabetes. Although the precise mechanism of action of vinegar is yet to be known, vinegar has been shown to act at various “points” in the carbohydrate metabolism. Vinegar has been shown to slow gastric emptying and inhibit sucrase and other related enzymes, thereby slowing digestion of complex carbohydrates and consequently flattening the peak of postmeal blood glucose.7,11 It has been shown to decrease hepatic neoglucogenesis and improve pancreatic insulin secretion.23,37 Vinegar intake has also been shown to improve uptake of glucose by skeletal muscles.37
This signifies the potential of vinegar as an adjuvant to the main diabetes mellitus treatment modalities. Studies conducted on individuals with diabetes were chosen specifically in order to evaluate the effects of vinegar on known proxy biochemical markers of diabetes mellitus irrespective of the pathways involved.
The most important proxy indicator of long-term blood glucose control is HbA1c. Studies that evaluated the effect of vinegar intake from 8 to 12 weeks showed a reduction in HbA1c by at least 0.14 percentage points. This is despite the fact that one of the studies measured HbA1c earlier than the standard practice of 12 weeks. Extended use of vinegar might have produced greater reductions as suggested by results of short-term outcomes. Despite differences among the studies that measured HbA1c, results were quite consistent (I 2 = 0%).
For short-term outcomes, regression analysis showed that the vinegar group had PPG values almost 1 mmol/L lower than that of the control group at 30 minutes. At the later time points the statistical significance was lost but the mean PPG levels consistently remained lower until the 120 minutes time point. The flattened peak may be due to the fact that vinegar has been shown to delay gastric emptying in both healthy individuals and type 1 diabetes patients with gastroparesis, and gastric emptying is a significant determinant of 30-minute PPG values in individuals with normal glucose tolerance or impaired glucose tolerance and in patients with overt diabetes.11,15,38
Although lower glucose values in the vinegar group were observed, confidence intervals were wide, due to the fact that there were differences among the studies especially in use of meals with varying carbohydrate contents both in terms of glycemic load and glycemic index, which are known to affect postprandial glucose especially at 120 minutes.39 However, as vinegar preferentially works in high glycemic load diets and in high glycemic index diets to reduce 120 minutes postprandial hyperglycemia, on average blood glucose levels remained modest.16,19 A recent meta-analysis that comprised of data from individuals with and without diabetes also showed reduced postprandial blood glucose.40
Regression analysis of PPI levels corroborated the aforementioned finding by showing higher levels of insulin in the vinegar group at 30 minutes but lower values at subsequent time points. The delayed response in the secretion of the insulin could be due to the delay in the absorption of the glucose through the gut due to the action of vinegar as suggested by various studies. The confidence intervals for mean PPI were very wide, thus precluding any strong conclusions.
Dose of vinegar may also have an influence on FPG levels when used for longer periods.24 It appears that increasing the dose of acetic acid, administered as ginsam, has diminishing effect on the benefit. However, more research is needed.
Some of the studies also evaluated the effects of vinegar on other serum biomarkers including serum ALT and AST. Precision of ALT was sensitive to the analysis model employed. However, AST levels were statistically significantly lower in the vinegar group. Urinary pH and leptin levels were also measured in 2 separate studies but no differences were observed.
At the moment the quantity and quality of evidence is insufficient to provide definitive answers about the effectiveness and safety of vinegar for a very diverse group of individuals with diabetes. This is also suggested by a recent narrative review.41 Nevertheless, current evidence strongly supports the fact that vinegar does have favorable effect on carbohydrate metabolism that could be exploited in the management of diabetes mellitus.
Conclusion
This review highlights that vinegar is a promising candidate and should be thoroughly evaluated for its possible incorporation as an adjuvant in diabetes mellitus management. It highlights the following directions for future research: (1) studies of long-term effectiveness and safety of vinegar; (2) larger studies in more diverse settings; (3) other patient important outcomes need to be studied including reduction in oral hypoglycemic agents or injected insulin use; (4) the appropriate dosage of vinegar needs to be established; (5) the effects of different types of vinegar; and (6) the effect of different modes of administration.
Acknowledgments
We acknowledge the help of our colleague Syed Ehsan Saffari of Center for Quantitative Medicine, Duke-NUS Medical School, Singapore, for helping in extracting data from the articles published in Persian and communicating with authors in Iran. We also are grateful to the authors of studies published in Iran for providing clarifications. We also thank Mr Javaid Umar Siddiqui, a type 2 diabetes mellitus patient, who asked for the advice on effect on vinegar on blood glucose levels as reported in lay press. His query led to this work.
Footnotes
Author Contributions: Fahad Javaid Siddiqui conceived the idea.
Fahad Javaid Siddiqui, Pryseley Nkouibert Assam, and Nurun Nisa de Souza conducted literature search, risk of bias assessment, and data extraction.
Pryseley Nkouibert Assam, Rehena Sultana, and Nurun Nisa de Souza did data analysis.
Fahad Javaid Siddiqui, Pryseley Nkouibert Assam, Rehena Sultana, and Nurun Nisa de Souza developed the draft.
Fahad Javaid Siddiqui, Rinkoo Dalan, Edwin Chan, and Pryseley Nkouibert Assam interpreted the results.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Centre for Quantitative Medicine, Duke-NUS and Singapore Clinical Research Institute by allowing staff to conduct this study. No direct funding was used to carry out this study.
ORCID iD: Fahad Javaid Siddiqui, MSc  http://orcid.org/0000-0002-9046-5105
http://orcid.org/0000-0002-9046-5105
Ethics Approval: As the study used all anonymous, publicly available published data, no ethics approval was sought.
References
- 1. World Health Organization. Global report on diabetes. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf. Published 2016. Accessed December 26, 2017.
- 2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 4. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes Importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280–285. [DOI] [PubMed] [Google Scholar]
- 5. O’Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100:899–904. [DOI] [PubMed] [Google Scholar]
- 6. O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249–255. [DOI] [PubMed] [Google Scholar]
- 7. Ogawa N, Satsu H, Watanabe H, et al. Acetic acid suppresses the increase in disaccharidase activity that occurs during culture of caco-2 cells. J Nutr. 2000;130:507–513. [DOI] [PubMed] [Google Scholar]
- 8. Shishehbor F, Mansoori A, Sarkaki AR, Jalali MT, Latifi SM. Apple cider vinegar attenuates lipid profile in normal and diabetic rats. Pak J Biol Sci. 2008;11:2634–2638. [DOI] [PubMed] [Google Scholar]
- 9. Ichikawa M, Ohta M, Kanai S, et al. Bitter melon malt vinegar increases daily energy turnover in rats. J Nutr Sci Vitaminol (Tokyo). 2003;49:428–433. [DOI] [PubMed] [Google Scholar]
- 10. Gu X, Zhao HL, Sui Y, Guan J, Chan JC, Tong PC. White rice vinegar improves pancreatic beta-cell function and fatty liver in streptozotocin-induced diabetic rats. Acta Diabetol. 2012;49:185–191. [DOI] [PubMed] [Google Scholar]
- 11. Liljeberg H, Björck I. Delayed gastric emptying rate may explain improved glycaemia in healthy subjects to a starchy meal with added vinegar. Eur J Clin Nutr. 1998;52:368–371. [DOI] [PubMed] [Google Scholar]
- 12. Johnston CS, Steplewska I, Long CA, Harris LN, Ryals RH. Examination of the antiglycemic properties of vinegar in healthy adults. Ann Nutr Metab. 2010;56:74–79. [DOI] [PubMed] [Google Scholar]
- 13. Leeman M, Ostman E, Björck I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr. 2005;59:1266–1271. [DOI] [PubMed] [Google Scholar]
- 14. Ebrahimi-Mamaghani M, Arefhosseini S, Golzarand M, Aliasgarzadeh A, Vahed-Jabbary M. Long-term effects of processed Berberis vulgaris on some metabolic syndrome components. Iranian J Endocrinol Metab. 2009;11:41–47. [Google Scholar]
- 15. Hlebowicz J, Darwiche G, Bjcrgell O, Almér LO. Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: a pilot study. BMC Gastroenterol. 2007;7:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston CS, Kim CM, Buller AJ. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004;27:281–282. [DOI] [PubMed] [Google Scholar]
- 17. Johnston CS, White AM, Kent SM. A preliminary evaluation of the safety and tolerance of medicinally ingested vinegar in individuals with type 2 diabetes. J Med Food. 2008;11:179–183. [DOI] [PubMed] [Google Scholar]
- 18. Johnston CS, White AM, Kent SM. Preliminary evidence that regular vinegar ingestion favorably influences hemoglobin A1c values in individuals with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;84:e15–e17. [DOI] [PubMed] [Google Scholar]
- 19. Liatis S, Grammatikou S, Poulia KA, et al. Vinegar reduces postprandial hyperglycaemia in patients with type II diabetes when added to a high, but not to a low, glycaemic index meal. Eur J Clin Nutr. 2010;64:727–732. [DOI] [PubMed] [Google Scholar]
- 20. Mahmoodi M, Seyed-Mostafa HZ, Gholamhossein H, et al. The effect of white vinegar on some blood biochemical factors in type 2 diabetic patients. J Diabetes Endocrinol. 2013;4:1–5. [Google Scholar]
- 21. Mitrou P, Raptis AE, Lambadiari V, et al. Vinegar decreases postprandial hyperglycemia in patients with type 1 diabetes. Diabetes Care. 2010;33:e27. [DOI] [PubMed] [Google Scholar]
- 22. Nosrati HR, Mousavi SE, Sajjadi P, Firoozjahi AR, Moazezi Z. Effect of apple cider vinegar on postprandial blood glucose in type 2 diabetic patients treated with hypoglycemic agents [in Persian]. J Babol Univ Med Sci. 2013;15:7–11. [Google Scholar]
- 23. White AM, Johnston CS. Vinegar ingestion at bedtime moderates waking glucose concentrations in adults with well-controlled type 2 diabetes. Diabetes Care. 2007;30:2814–2815. [DOI] [PubMed] [Google Scholar]
- 24. Yoon JW, Kang SM, Vassy JL, et al. Efficacy and safety of ginsam, a vinegar extract from Panax ginseng, in type 2 diabetic patients: results of a double-blind, placebo-controlled study. J Diabetes Investig. 2012;3:309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Dijk JW, Tummers K, Hamer HM, van Loon LJ. Vinegar co-ingestion does not improve oral glucose tolerance in patients with type 2 diabetes. J Diabetes Complications. 2012;26:460–461. [DOI] [PubMed] [Google Scholar]
- 26. Heljić B, Velija-Ašimi Z, Bureković A, Karlović V, Avdagić A, Ćemalović M. The role of natural supplement of apple vinegar and syrup in the management of type 2 diabetes mellitus. J Health Sci. 2014;4:176–180. [Google Scholar]
- 27. Johnston CS, Buller AJ. Vinegar and peanut products as complementary foods to reduce postprandial glycemia. J Am Diet Assoc. 2005;105:1939–1942. [DOI] [PubMed] [Google Scholar]
- 28. Ostman E, Granfeldt Y, Persson L, Björck I. Vinegar supplementation lowers glucose and insulin responses and increases satiety after a bread meal in healthy subjects. Eur J Clin Nutr. 2005;59:983–988. [DOI] [PubMed] [Google Scholar]
- 29. Rongwei F, Benjamin WV, Tatyana AS, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 30. Mitchell M. Engauge Digitizer [software]. http://markummitchell.github.io/engauge-digitizer/ . Accessed December 26, 2017.
- 31. Table de conversion–immunoessais. Conversietabel–immunoassays. http://www.qcnet.com/Portals/50/PDFs/eenheden/Conversietabel-Immunoassay.pdf. Accessed December 26, 2017.
- 32. Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies In: Higgins JPT, Sally G, ed. Cochrane Handbook for Systematic Reviews of Interventions Versions 5.1.0. London, England: Cochrane Collaboration; 2011. http://handbook-5-1.cochrane.org/. Accessed December 26, 2017. [Google Scholar]
- 33. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ginosar Y, Shapira SC. The role of an anaesthetist in a field hospital during the cholera epidemic among Rwandan refugees in Goma. Br J Anaesth. 1995;75:810–816. [DOI] [PubMed] [Google Scholar]
- 35. Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. MedGenMed. 2006;8:61. [PMC free article] [PubMed] [Google Scholar]
- 36. Petsiou EI, Mitrou PI, Raptis SA, Dimitriadis GD. Effect and mechanisms of action of vinegar on glucose metabolism, lipid profile, and body weight. Nutr Rev. 2014;72:651–661. [DOI] [PubMed] [Google Scholar]
- 37. Mitrou P, Petsiou E, Papakonstantinou E, et al. Vinegar consumption increases insulin-stimulated glucose uptake by the forearm muscle in humans with type 2 diabetes. J Diabetes Res. 2015;2015:175204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marathe CS, Horowitz M, Trahair LG, et al. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. J Clin Endocrinol Metab. 2015;100:3565–3571. [DOI] [PubMed] [Google Scholar]
- 39. Katsilambros N, Liatis S, Makrilakis K. Critical review of the international guidelines: what is agreed upon—what is not? Nestle Nutr Workshop Ser Clin Perform Programme. 2006;11:207–218. [DOI] [PubMed] [Google Scholar]
- 40. Shishehbor F, Mansoori A, Shirani F. Vinegar consumption can attenuate postprandial glucose and insulin responses; a systematic review and meta-analysis of clinical trials. Diabetes Res Clin Pract. 2017;127:1–9. [DOI] [PubMed] [Google Scholar]
- 41. Lim J, Henry CJ, Haldar S. Vinegar as a functional ingredient to improve postprandial glycaemic control - the human intervention findings and the molecular mechanisms. Mol Nutr Food Res. 2016;60:1837–1849. [DOI] [PubMed] [Google Scholar]