Abstract
Objectives:
The initial phase of infection of a foot ulcer in a person with diabetes is often categorized as mild. Clinicians usually treat these infections with antimicrobial therapy, often applied topically. Some experts, however, believe that mild diabetic foot ulcer infections will usually heal with local wound care alone, without antimicrobial therapy or dressings.
Methods:
To evaluate the potential benefit of treatment with a topical antibiotic, we performed a single-center, investigator-blinded pilot study, randomizing (1:1) adult patients with a mild diabetic foot ulcer infection to treatment with a gentamicin–collagen sponge with local care versus local care alone. Systemic antibiotic agents were prohibited.
Results:
We enrolled a total of 22 patients, 11 in the gentamicin–collagen sponge arm and 11 in the control arm. Overall, at end of therapy, 20 (91%) patients were categorized as achieving clinical cure of infection, and 2 (9%) as significant improvement. At the final study visit, only 12 (56%) of all patients achieved microbiological eradication of all pathogens. There was no difference in either clinical or microbiological outcomes in those who did or did not receive the gentamicin–collagen sponge, which was very well tolerated.
Conclusion:
The results of this pilot trial suggest that topical antibiotic therapy with gentamicin–collagen sponge, although very well tolerated, does not appear to improve outcomes in mild diabetic foot ulcer infection.
Keywords: Gentamicin–collagen sponge, mild diabetic foot infections, topical antimicrobial therapy, wound infection, wound outcome, antimicrobial safety, adverse drug events
Introduction
Diabetic foot ulcer infections (DFUIs) are responsible for frequent healthcare visits, severe morbidity, and frequent prescriptions for systemic antibiotic therapy.1,2 While all DFUIs require local wound care, many clinicians also prescribe antimicrobial therapy. Some experts, however, believe that mild wound infections usually resolve with local care alone.3–5 This may have several benefits, including high local antibiotic levels, reduced systemic adverse effects, and possibly enhanced remission of DFUIs.6–8 There is, however, sparse literature concerning the safety and efficacy of topical antimicrobial therapy for DFUIs.9,10 Thus, we examine the potential benefits of treatment of mild DFUI without systemic antibiotic therapy, using only the gentamicin–collagen sponge (and local care) versus local care alone.
Methods
Objectives and sample size considerations
This was a prospective, randomized, controlled, investigator-blinded, pilot single-center study. This pilot study was a side study of a larger similar randomized trial for moderate and severe DFUI involving systemic antibiotics (ClinicalTrials.gov NCT01951768). As we did a pilot and a side study, we decided not to register separately. In this preliminary pilot study, we intended to test (a) whether mild DFUIs can be cured by local professional care alone and (b) whether a topical antibiotic (gentamicin) would enhance wound healing. If there would be a clear difference in favor of gentamicin, a much larger randomized trial would follow as we conduct it for moderate and severe DFUIs.
Because of the pilot nature in a mostly unpublished area of research, the inferior margins were set very largely. Moreover, according to our clinical experience, most patients with moderate DFUI would be hospitalized, whereas many patients with mild DFUIs would be treated in the office of the General Practitioner or at home. Therefore, we expected a smaller recruitment potential for mild DFUIs. We finally estimated, based on clinical experience, that the expected cure rate would be 30% on the placebo arm and 75% in the treatment arm. The necessary sample size for a superiority trial in favor of the treatment arm in 1:1 randomization is 23 patients in each arm (power 80%, alpha 0.05). Using a blinded allocation scheme with unmarked envelopes, we randomized patients 1:1 to either: (a) daily topical application on the wound of a gentamicin sponge (Innocoll Pharmaceuticals Ltd) or (b) covering of the wound with a plain gauze sponge without gentamicin.
Definitions and study criteria
We enrolled patients based on the criteria of the Infectious Diseases Society of America (IDSA) foot infection guidelines.10 Briefly, a mild DFUI was defined as follows: having ≥2 manifestations of inflammation (erythema, pain, tenderness, warmth, or induration) or purulence; any erythema present extended <2 cm around the ulcer; the local infection was limited to skin or superficial subcutaneous tissues; and, there are no signs or symptoms of systemic infection. A patient was eligible for study participation if he or she met the following criteria: aged ≥18 years; has a diagnosis of diabetes mellitus; has an open wound of ≥1 cm2 located below the malleolus that has findings of infection (defined above); has undergone (or soon will be) any appropriate surgical intervention needed to remove necrotic and infected tissue; and, if female, is non-pregnant and non-lactating. The exclusion criteria were the presence of a DFUI associated with any type of implant or foreign material; peripheral arterial insufficiency requiring revascularization after enrollment; a moderate or severe DFUI2; severe immune-suppression; extensive necrosis requiring amputation; residual osteomyelitis (after any resection); a requirement for any systemic antibiotic therapy; a history of myasthenia gravis or epilepsy, precluding gentamicin use; or recent alcohol or substance abuse.
Study conduct
Innocoll Ltd provided the gentamicin–collagen sponges, which are commercially available in Switzerland under the trade name GARAMYCIN® Sponge. Standard wound care for patients in both study arms included the following: sharp debridement (at presentation, during hospitalization, or at clinic visits); daily dressing changes (0.9% saline for those not treated with the gentamicin–collagen sponge); optimization of glycemic control. If the wound was visually dirty or contaminated by debris, specialized wound nurses cleansed the wound with water before debridement and microbiological tissue sampling. Our podiatry specialist (C.P.) supervised the pressure offloading and professional wound care for all enrolled patients. He chose the offloading device according to his experience and the scheduled compliance and co-morbidities of the patients, before randomization to the study. These various offloading devices were mostly offloading boots and casts, with or without individual adaptation. Enrolled patients could not be treated with any systemic antibiotic agent for any reason; other antiseptics or topical antibiotics (other than the gentamicin sponge) for their DFUI; hyperbaric oxygen therapy; or vacuum-assisted negative-pressure devices. The patients could undergo any needed limb revascularization and partial amputation before they started in the study.
We treated the study patients for 14 days (unless the ulcer completely closed before day 14). Overall, the patients had seven study visits (days 1, 2, 5, 9, 14, 20, 24 (+/− 2 days)) over approximately 24 days, during which they underwent standardized safety and efficacy assessments. The test-of-cure visit was planned to be approximately 10 days after treatment was discontinued. We defined “cure” as the absence of any clinical, laboratory, or imaging evidence of the original infection. We defined “improved” as resolution of most, but not all, of the original findings of infection, but no need for further therapy. We collected soft tissue specimens for cultures from the target DFUI (by curettage or biopsy, but not swab) at baseline and at the final visit (if there was still an open ulcer) and noted whether or not the baseline pathogens were eradicated. We used a custom-made tool (Appendix 1) to describe the wound’s evolution during treatment, which summed up the various elements in a single score. This score (taking into account inflammatory parameters such as induration, pus, and pain) is more suitable to describe the evolution of a wound infection than the mere wound size. At each study visit, the investigators inquired about any adverse events, especially local wound irritation and ototoxicity. The local Ethics Committee approved this study (CER 13-178) and all patients provided informed consent. We also used a custom made scoring system (Consort checklist) available on the Journal’s website. All data are collected in our center.
Results
Patients
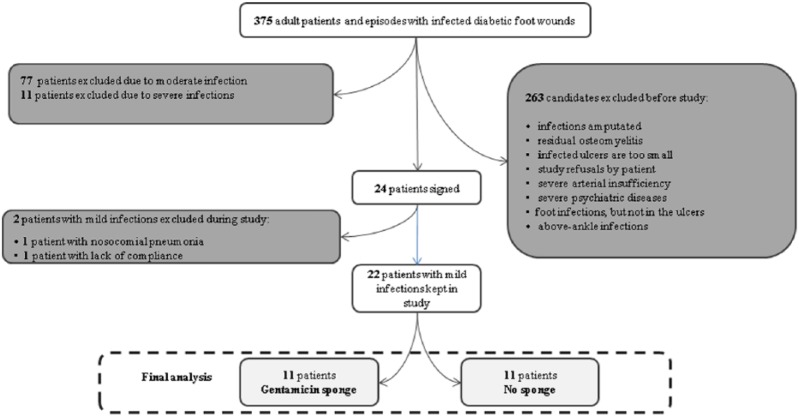
Between August 2014 and June 2015, we screened patients with 375 DFUI episodes. Of these, we excluded 287 based on them meeting at least one of our exclusion criteria, including 88 cases that had a moderate DFUI (Figure 1). We enrolled the remaining 22 episodes of mild DFUI, which occurred in 22 different patients in the study; all completed the required visits and were followed-up for a median of 1 month. The enrolled patients’ baseline characteristics were similar in the two treatment arms (Table 1). At enrollment, the patients median age was70 years, glycosylated hemoglobulin level was 7 mmol/L, body mass index 31.7 kg/m2, blood pressure 145/85 mmHg, heart rate 78 beats/min, respiratory rate 12/min, and axillary temperature 36.5°C. Overall, 11 patients were receiving insulin therapy, 7 had evidence of peripheral arterial disease (with a median ankle–brachial index 1.0) and 14 (64%) were males. The location of the DFUI was the hindfoot in 5 cases (23%), the midfoot in 5 (23%), and the toes in 12 episodes (54%). The three most frequently isolated pathogens from the wound tissue were Staphylococcus aureus (n = 8), Pseudomonas aeruginosa (4), and S. epidermidis (3). Cultures demonstrated polymicrobial infection in eight patients (36%).
Figure 1.
Study flowchart (patients included and excluded).
Table 1.
Comparison of patients with mild diabetic foot ulcers infections, who were or were not treated with gentamicin-collagen sponges.
| n = 22 | No sponges (control arm) |
p valuea | Sponges (investigational arm) |
|---|---|---|---|
| n = 11 | n = 11 | ||
| Female sex | 4 (36%) | 1.00 | 4 (36%) |
| Median age | 73 years | 0.87 | 69 years |
| Median body mass index | 31.7 kg/m2 | 0.62 | 31.6 kg/m2 |
| Median leukocyte count at inclusion | 8.9 G/L | 0.82 | 7.6 G/L |
| Median serum glycosylated hemoglobulin | 7.0 mmol/L | 0.41 | 6.8 mmol/L |
| Those on insulin therapy | 6 (55%) | 0.67 | 5 (45%) |
| Clinical arterial insufficiency | 5 (45%) | 0.17 | 2 (18%) |
| Median ankle–brachial index | 0.95 | 0.43 | 1.05 |
| Median serum creatinine level at inclusion | 92 μmol/L | 0.83 | 83 μmol/L |
| Total with cure or significant improvementb | 11 (100%) | 1.00 | 11 (100%) |
| Adverse events related to topical dressing | 0 (0%) | 1.00 | 0 (0%) |
| Total with pathogen eradication | 6 (55%) | 1.0 | 6 (55%) |
Pearson’s χ2 test, Fisher’s exact test, or Wilcoxon’s rank sum test.
See text for definition.
Outcomes
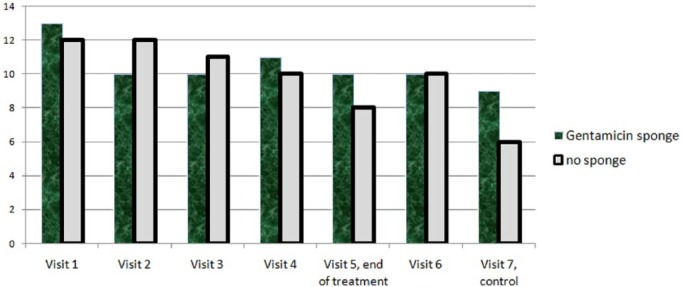
Among the 22 episodes, at the test of cure, 20 (91%) met criteria for being cured and 2 (9%) were improved. There was no failure regarding infection (Table 1). Equally, the number of finally completely healed ulcers (independently of infection or pathogen eradication) was equal. In the sponge arm, 10/11 (91%) of all ulcers were healed at the last control visit versus 10/11 (91%) in the control arm (Fisher’s exact test; p = 1.00). Likewise, there was no difference in the rate of pathogen eradication in patients treated with or without the gentamicin–collagen sponges (6/11 and 6/11, Pearson’s χ2 test; p = 1.0). All patients remained afebrile throughout the study and the median leukocyte count decreased from 9.9 G/L on enrollment to 7.8 G/L at the test of cure (ANOVA test; p = 0.84). The wound score (Appendix 1) decreased from a median of 13 points at enrollment to 7 points at the last visit, with no significant differences between the two study arms on up to the first 7 weeks (Figure 2). Both the saline-moistened dressings and the gentamicin–collagen sponges were well tolerated, with no adverse events detected in either group. The median serum creatinine levels for those treated with the gentamicin–collagen sponges were 83 mmol/L at inclusion and 91 mmol/L at the end (ANOVA test; p = 0.18).
Figure 2.
Median wound scores over 7 weeks, stratified by study arm.
Discussion
The results of our randomized controlled trial showed no differences between those patients randomized to receive the sponge and those who were not, in rates of clinical cure, wound healing (as assessed by wound score), or pathogen eradication. The published literature on the use of topical antimicrobial agents for infected ulcers is limited, and of generally low quality.7–9 The lack of a strong evidence base for treatment decisions is supported by the results of a recent Cochrane systematic review and meta-analysis that concluded that evaluations of topical antimicrobials for diabetic foot ulcers were limited to a few poorly designed trials.8 Landsman et al.6 conducted a three-arm randomized trial of the efficacy of topical Microcyn Rx® irrigation (Oculus Innovative Sciences, Inc, a superoxidized solution) versus oral levofloxacin versus levofloxacin plus Microcyn Rx in mild DFUIs. The clinical success was higher in the Microcyn Rx alone group (75%) than in the levofloxacin (57%) or in the combined (64%) groups, suggesting that treatment with a topical agent might be similar (or even superior) to a systemic antibiotic.6 In a prospective trial, Chu et al.4 randomized patients with a diabetic foot infection whose signs and symptoms had resolved into one of two groups–either continuing with or discontinuing systemic antibiotic treatment. For mild infections, they found that the outcome was similar, suggesting a less than expected efficacy of systemic antibiotics. Of note, in these studies neither treatment with a systemic antibiotic nor a topical antimicrobial agent compared to local wound care alone, or a placebo.
Our study has two important limitations. First, the sample size is small. We planned to enroll more patients, but terminated this pilot study early (after enrolling a total of 24 episodes instead of the planned 46) because it was more difficult than we expected to find eligible cases that met our enrollment criteria. We performed an interim analysis, which suggested the two treatments were equivalent; thus, continuation of the study with further enrollment was unlikely to demonstrate a significant difference between the study groups. We found, as have others,10 that catching patients with a mild DFUI is difficult, as this represents a relatively brief moment in time in the transition from clinically uninfected to moderate or severe infection. Second, we specifically wished to enroll only patients with a mild DFUI, as this is the group that might be treated with topical antimicrobial therapy alone, without the need for systemic antibiotics. This contrasts with our previous study, in which we enrolled patients with moderate DFUI, where the gentamicin sponge combined with systemic antibiotic therapy revealed a higher proportion of cure and pathogen eradication.7 To further assess our findings, we are currently performing a second study in which we are combining a gentamicin-collagen sponge with systemic antibiotic therapy for moderate to severely infected diabetic foot ulcers.
In conclusion, our small, but randomized prospective pilot trial appears to reveal two important messages that are new in the literature. First, mildly infected diabetic foot ulcers may be successfully treated by local care alone. Secondly, adding topical treatment with gentamicin, an antibiotic with a large spectrum activity against many pathogens, may not improve the healing of mildly infected diabetic foot ulcers. We believe, however, that a larger trial would be useful and feasible. If the results of the current trial are similar to those in other trials, these data might lead to withholding topical gentamicin; this could help avoid ineffective use of an antibiotic for a ubiquitous and burdensome health problem. Based on our results, we decided to stop the use of topical antibiotics for all types of infected wounds for patients treated in our service.
Supplemental Material
Supplemental material, Appendix_1 for A randomized controlled trial of the safety and efficacy of a topical gentamicin–collagen sponge in diabetic patients with a mild foot ulcer infection by Ilker Uçkay , Benjamin Kressmann, Sébastien Di Tommaso, Marina Portela, Heba Alwan, Hubert Vuagnat, Sophie Maître, Christophe Paoli and Benjamin A Lipsky in International Journal of Music Education
Supplementary Material
Acknowledgments
The authors are indebted to Mrs Lisa Hemsen and Dr David Prior from Innocoll Pharmaceuticals Ltd for their invaluable help. Lisa Hemsen monitored this study and visited our study site. She furthermore enabled the shipping of the gentamicin sponges and helped to set up the study. David Prior helped to monitor and advised to set up the study. I.U. and B.K. equally contributed as first authors.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The Geneva Ethical Committee approved conduct of this study (Number CER 13-178).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Innocoll Pharmaceuticals Ltd. made an unrestricted donation to support this pilot study, with no conditions regarding the investigators conduct of the study. Innocoll Pharmaceuticals Ltd. is an Ireland-based pharmaceutical company engaged in many products for (postoperative) wound care. It also operates in the sale and marketing of topical gentamicin for the prevention and treatment of (postoperative) infections and wounds.
Informed consent: Written informed consent was obtained from all subjects before the study.
Supplementary material: Supplementary material for this article, including CONSORT-2010-Checklist, is available online.
Trial registration: This was a prospective, randomized, controlled, investigator-blinded, pilot single-center study for mild diabetic foot infections. It was a side study of a larger similar randomized trial for moderate and severe diabetic foot infections involving systemic antibiotics (ClinicalTrials.gov NCT01951768). As we did a pilot and side study, we decided not to register internationally.
ORCID iD: Ilker Uçkay  https://orcid.org/0000-0002-5552-0973
https://orcid.org/0000-0002-5552-0973
References
- 1. Lebowitz D, Gariani K, Kressmann B, et al. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int J Infect Dis 2017; 59: 61–64. [DOI] [PubMed] [Google Scholar]
- 2. Lipsky BA, Aragón-Sánchez J, Diggle M, et al. ; International Working Group on the Diabetic Foot. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev 2016; 32: 45–74. [DOI] [PubMed] [Google Scholar]
- 3. Chahine EB, Sucher AJ. Skin and soft tissue infections. PSAP 2015; 1: 1–27. [DOI] [PubMed] [Google Scholar]
- 4. Chu Y, Wang C, Zhang J, et al. Can we stop antibiotic therapy when signs and symptoms have resolved in diabetic foot infection patients? Int J Low Extrem Wounds 2015; 14: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weledji EP, Fokam P. Treatment of the diabetic foot—to amputate or not? BMC Surg 2014; 14: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landsman A, Blume PA, Jordan DA, Jr, et al. An open-label, three-arm pilot study of the safety and efficacy of topical Microcyn Rx wound care versus oral levofloxacin versus combined therapy for mild diabetic foot infections. J Am Podiatr Med Assoc 2011; 101: 484–496. [DOI] [PubMed] [Google Scholar]
- 7. Lipsky BA, Kuss M, Edmonds M, et al. Topical application of a gentamicin-collagen sponge combined with systemic antibiotic therapy for the treatment of diabetic foot infections of moderate severity: a randomized, controlled, multicenter clinical trial. J Am Podiatr Med Assoc 2012; 102: 223–232. [DOI] [PubMed] [Google Scholar]
- 8. Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother 2015; 16: 821–832. [DOI] [PubMed] [Google Scholar]
- 9. Dumville JC, Lipsky BA, Hoey C, et al. Topical antimicrobial agents for treating foot ulcers in people with diabetes. Cochrane Database Syst Rev 2017; 6: CD011038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pexiganan versus placebo control for the treatment of mild infections of diabetic foot ulcers (OneStep-2). ClinicalTrials.gov Identifier: NCT01594762, https://clinicaltrials.gov/ct2/show/NCT01594762?cond=pexiganan+diabetic+foot&rank=1 (accessed 19 September 2017).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1 for A randomized controlled trial of the safety and efficacy of a topical gentamicin–collagen sponge in diabetic patients with a mild foot ulcer infection by Ilker Uçkay , Benjamin Kressmann, Sébastien Di Tommaso, Marina Portela, Heba Alwan, Hubert Vuagnat, Sophie Maître, Christophe Paoli and Benjamin A Lipsky in International Journal of Music Education