Abstract
Acute erythroid leukemia, also known as acute myeloid leukemia-M6, may be associated with previous chemotherapy or immunosuppressive therapy. For 10 years, a 69-year-old Japanese female patient with pure erythroid leukemia (or acute myeloid leukemia-M6b) was treated for polymyositis with 50–100 mg/day azathioprine. She complained of dyspnea with low-grade fever and was diagnosed as having pure erythroid leukemia. Chromosomal analysis revealed a complex karyotype abnormality, with the deletion of 5q, -6, -7 and addition of 11q13. No morphological myelodysplastic changes were observed in her bone marrow cells. In this study, azathioprine accumulation was considered to be associated with the patient’s leukemogenesis.
Keywords: Acute myeloid leukemia, pure erythroid leukemia, erythroleukemia, polymyositis, azathioprine, myelodysplastic syndrome
Introduction
Pure erythroid leukemia (PEL), a rare hematological malignancy, is defined as the presence of >80% of proliferating erythroblasts among all nucleated bone marrow cells. According to the classification by the World Health Organization in 2008, PEL is classified as acute myeloid leukemia (AML) not otherwise specified.1 In addition, according to the French–American–British classification, PEL is categorized as AML-M6b. The M6a subtype is simply known as acute erythroleukemia. AML-M6a accounts for 2%–3% of all AML cases, whereas AML-M6b accounts for only 1%.2 Thus, PEL is considered to be rare, with a prevalence of less than 1% of all AML cases. The two erythroleukemia subtypes have similar features but different pathogenesis.3 AML-M6b is considered to have a worse prognosis than AML-6a, with a median survival of approximately 4 months,3 because of its severe resistance to chemotherapy and complex karyotypes that are frequently observed after the progression of myelodysplastic syndrome (MDS) to AML. AML-M6 cases may be associated with chemotherapy or immunosuppressive therapy.4–7 Regardless of whether an antecedent diagnosis of MDS has been established, complex karyotypes are prominent characteristics of AML-M6, particularly of the AML-M6b subtype.3,8 In this study, a patient who developed AML-M6b during long-term azathioprine treatment for polymyositis was treated.
Case presentation
A 69-year-old Japanese female was treated for polymyositis at our hospital. She received 50–100 mg/day azathioprine for 10 years. The total estimated lifetime dose of azathioprine was 297 g. During one of her outpatient clinic visits, which occurred at 3 month intervals, she complained of dyspnea with low-grade fever. Laboratory test results indicated severe anemia (hemoglobin, 7.1 g/dL), leukocytopenia (white blood cell count, 1300/µL), and thrombocytopenia (platelet count, 10.0 × 104/µL). Bone marrow aspiration results revealed prominent proliferation of erythrocytes with 83% proerythroblasts. She was diagnosed with PEL, also known as AML-M6b (Figure 1). Chromosomal analysis revealed a complex karyotype abnormality, with deletion of 5q, -6, -7 and addition of 11q13. The karyotype of the patient’s leukemic cells was as follows:
45, XX, del(5) (q?), -6 add(6) (q21), -7, add(8) (p11.2), add(9) (q13), add(11) (q13), add(11) (q13), -12, add(16) (q12.1), add(16) (q24), der(19) add(19) (p13) add(19) (q13.1), +2 mar
Figure 1.
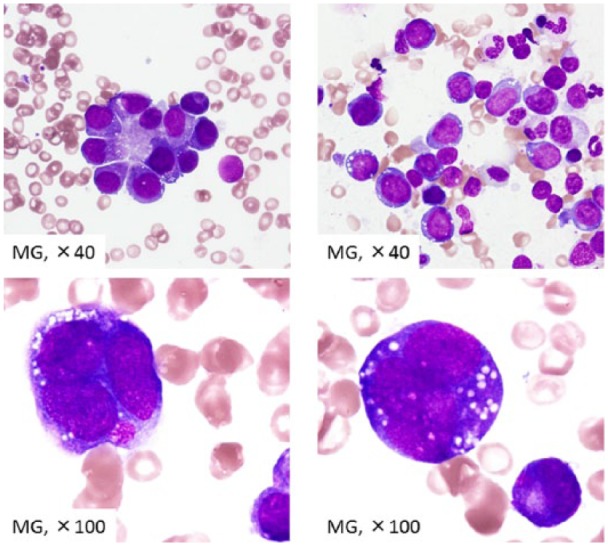
Bone-marrow finding in the patient. A number of nuclear cells were 1.97 × 104/µL. Megakaryocyte were scanty. Blast count was 0.2%. Proerythrocytes increased to 70.0% of all nuclear cells, which were partially positive for PAS and negative for peroxidase, α-naphthyl butyrate esterase, and naphthol AS-D chloroacetate esterase.
No morphological myelodysplastic changes were observed in the patient’s bone marrow cells. Induction chemotherapy comprising idarubicin and cytosine arabinoside was initiated soon after leukemia was diagnosed. However, initial chemotherapy did not eliminate the leukemic cells but only attenuated the proliferation of proerythroblasts in the bone marrow to 67.0% of all nucleated cells. Following chemotherapy induction, complications such as acute heart failure, multiple intestinal ulcerations, and progressive polymyositis symptoms developed. These complications led to the discontinuation of chemotherapy, and 96 days after diagnosis, the patient died owing to PEL.
The Institutional Review Board (IRB) of Kagawa Medical University approved the case report and submission of medical literature. We obtained the approval from the IRB (IRB approval No. H23-23).
Written informed consent was obtained from the patient for publication of this case series and any accompanying images.
Discussion
We assumed that the patient developed azathioprine-associated leukemogenesis. To date, only one case report of azathioprine-associated acute erythroleukemia has been published,6 in which der(1;7) (q10;p10) was reported to be the sole acquired cytogenetic abnormality. Furthermore, only a single case report has been published regarding azathioprine treatment for Crohn’s disease in a patient who developed AML with multilineage dysplasia (World Health Organization (WHO) classification).5 This case, which was complicated by Crohn’s disease, showed a complex karyotype that included -7 and -13. The authors analyzed the activity of thiopurine S-methyltransferase, which metabolizes azathioprine, and found that the patient was a poor metabolizer. Furthermore, the authors discussed the association between PEL pathogenesis and concurrent immunosuppressive therapy with azathioprine, myelodysplastic changes, and monosomy seven karyotypes. They also demonstrated that poor azathioprine metabolism is associated with a subset of PEL with dysplasia.
In addition to azathioprine metabolism, the leukemogenic mechanism of azathioprine is an important issue. A cytogenetic study of 75 patients with erythroleukemia revealed no specific karyotype abnormality.9 In this study, an association between the subcategory AML-M6a and MDS refractory anemia with excess blasts in transformation was observed. Moreover, the M6b subtype (PEL) harbored t(9;22) (q34;q11), that is, Philadelphia chromosome translocation, in four of seven cases. Other PEL cases revealed that the preexisting risks for PEL were chemoradiotherapy exposure and antecedent MDS.8 An investigation of secondary MDS cases after azathioprine treatment revealed an association of MDS onset with the abnormal karyotype of chromosome 7.10 Kwong et al.11 analyzed three patients with AML who underwent azathioprine treatment for autoimmune diseases; in addition, a comprehensive review discussed the association between azathioprine and AML.12 The carcinogenic potential of azathioprine was introduced and a strong association between therapy-related MDS and chromosomal 7 abnormalities was suggested. Thus, the leukemogenic mechanism of azathioprine, which involved karyotyped events, was hypothesized. In addition, autoimmune diseases increase the risk for hematological malignancies.13 An association between azathioprine and MDS/AML was etiologically determined in a recent observational study,14 which demonstrated that azathioprine increased the risk for myeloid neoplasms (MDS/AML) by 7.05-fold in patients with autoimmune diseases. The study also suggested that the effect of azathioprine on MDS/AML was much stronger than that on autoimmune diseases. In this study, the cytogenetic abnormalities detected were characterized as karyotypes for MDS, including 5q and -7. Although bone marrow cells showed no apparent dysplastic changes, our case could be linked to preexisting MDS. Knipp et al. reported a high frequency of chromosomal aberrancies associated with MDS/AML-M6b. MDS-based leukemogenesis is more closely associated with AML-M6b (PEL) than with AML-M6a.
How the use of azathioprine promotes the onset of leukemia is a major concern. Among azathioprine-associated MDS cases, the median treatment duration was 83 months (range, 24–168 months), and the median cumulative azathioprine dose was 253 g (range, 27–765 g).10 In this study, azathioprine (accumulated dose of 297 g over 10 years) was sufficient to contribute to the onset of PEL.10 However, another study reported no significant association between the duration of exposure to azathioprine and the onset of MDS/AML;14 the accumulated drug exposure was similar between MDS/AML cases and controls. Therefore, the risk threshold for the onset of leukemia was unknown. As a result, azathioprine metabolism vulnerability in each patient should be considered as a contributor to the onset of MDS/AML.5
In conclusion, because case reports of PEL after azathioprine treatment remain scarce, we hope that our case report will contribute to the discussion of azathioprine as a potential etiology of PEL.
Footnotes
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5): 937–951. [DOI] [PubMed] [Google Scholar]
- 2. Liu W, Hasserjian RP, Hu Y, et al. Pure erythroid leukemia: a reassessment of the entity using the 2008 World Health Organization classification. Mod Pathol 2011; 24(3): 375–383. [DOI] [PubMed] [Google Scholar]
- 3. Santos FP, Faderl S, Garcia-Manero G, et al. Adult acute erythroleukemia: an analysis of 91 patients treated at a single institution. Leukemia 2009; 23(12): 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sadrzadeh H, Hasserjian R, Fathi AT. Pure erythroid leukemia evolving from a therapy-related myelodysplastic syndrome secondary to treatment for chronic lymphocytic leukemia. Am J Hematol 2013; 88(3): 240–241. [DOI] [PubMed] [Google Scholar]
- 5. Yenson PR, Forrest D, Schmiegelow K, et al. Azathioprine-associated acute myeloid leukemia in a patient with Crohn’s disease and thiopurine S-methyltransferase deficiency. Am J Hematol 2008; 83(1): 80–83. [DOI] [PubMed] [Google Scholar]
- 6. Park TS, Cheong JW, Song J, et al. Acute erythroleukemia with der(1;7)(q10; p10) as a sole acquired abnormality after treatment with azathioprine. Cancer Genet Cytogenet 2008; 186(1): 58–60. [DOI] [PubMed] [Google Scholar]
- 7. Gupta SK, Kumar R, Chharchhodawala T, et al. Secondary pure erythroid leukaemia in relapsed acute lymphoblastic leukaemia: lineage switch or chemotherapy effect? BMJ Case Rep. Epub ahead of print 19 May 2014. DOI: 10.1136/bcr-2013-201724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong E, Ling V, Westerman D, et al. How unique is pure erythroid leukaemia? A retrospective analysis of seven cases and review of the literature. J Clin Pathol 2015; 68(4): 301–305. [DOI] [PubMed] [Google Scholar]
- 9. Lessard M, Struski S, Leymarie V, et al. ; Groupe Francophone de Cytogénétique Hématologique (GFCH). Cytogenetic study of 75 erythroleukemias. Cancer Genet Cytogenet 2005; 163(2): 113–122. [DOI] [PubMed] [Google Scholar]
- 10. Knipp S, Hildebrandt B, Richter J, et al. Secondary myelodysplastic syndromes following treatment with azathioprine are associated with aberrations of chromosome 7. Haematologica 2005; 90(5): 691–693. [PubMed] [Google Scholar]
- 11. Kwong YL, Au WY, Liang RH. Acute myeloid leukemia after azathioprine treatment for autoimmune diseases: association with -7/7q-. Cancer Genet Cytogenet 1998; 104(2): 94–97. [DOI] [PubMed] [Google Scholar]
- 12. Kwong YL. Azathioprine: association with therapy-related myelodysplastic syndrome and acute myeloid leukemia. J Rheumatol 2010; 37(3): 485–490. [DOI] [PubMed] [Google Scholar]
- 13. Anderson LA, Pfeiffer RM, Landgren O, et al. Risks of myeloid malignancies in patients with autoimmune conditions. Br J Cancer 2009; 100(5): 822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ertz-Archambault N, Kosiorek H, Taylor GE, et al. Association of therapy for autoimmune disease with myelodysplastic syndromes and acute myeloid leukemia. JAMA Oncol 2017; 3(7): 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]


