Abstract
Vernonia mespilifolia Less. is a shrub of the Asteraceae family used in the South African traditional medicine system for the management of weight loss, hypertension, and heartwater disease. There is a need for scientific evaluation to validate its ethnomedicinal usage. In vitro assays were conducted to evaluate the polyphenolic content, antioxidant and antimicrobial properties of different solvent extracts (acetone, aqueous, and ethanol) of the whole plant of Vernonia mespilifolia spectrophotometric and agar dilution techniques, respectively. The result revealed varying amounts of polyphenolics in the different solvent extracts corresponding to the antioxidant activities. Also, only the acetone and ethanol extracts inhibited the growth of the selected bacteria and fungi. These findings reveal that the extracts have strong bioactive compounds and hence support its ethnomedicinal application.
Keywords: agar dilution, antimicrobial activity, Vernonia mespilifolia, polyphenolic contents, radical scavenging activity
Reactive oxygen species are generated as end-products of cellular metabolism or from exogenous sources such as exposure to ionizing radiation, ultraviolent radiation, and carcinogenic agents.1 These reactive species such as singlet oxygen, superoxide ion, hydroxyl ion, and hydrogen peroxide, are extremely reactive and toxic molecules that cause oxidative damage to important biomolecules such as nucleic acids, proteins, enzymes, and lipids when the antioxidant defense system is overwhelmed, thus resulting in a myriad of chronic and degenerative diseases.2 The use of natural sources of antioxidants from plants has attracted much interest as they have the capability of boosting the antioxidant capacity of the plasma and thus reducing the onset of certain disorders such as cancer, diabetes, obesity, cardiovascular diseases, and neurodegenerative diseases. The alarming trend of drug resistance to synthetic antioxidants as well as antimicrobial agents has resulted in the search for medicinal plants with potent antimicrobial and antioxidant capacity.1 Medicinal plants are sought after because they possess certain bioactive compounds such as flavonoids, alkaloids, tannins, and phenolics with reported antimicrobial as well as other biological activities.3 Herbal medicines are considered to be relatively inexpensive and safer than synthetic drugs. This has prompted the increased use of medicinal plants in the treatment and management of myriad of ailments. In recent times, the amount of polyphenols in plants and antioxidant activities depends on biological factors such as, genotypes/organ used, and environmental conditions, such as water stress, temperature, and light intensity.4,5
Having a wide distribution in South Africa, Vernonia mespilifolia Less. is used for the management of a myriad of ailments. Just like its related species, V amygdalina, V calvaona, and V colorata, which have been shown to exhibit medicinal potentials, such as hypoglycemic and hypolipidemic effects, the whole plant of V mespilifolia is used in folk and traditional medicine for the management of weight loss and hypertension,6 while its stem is used for the management of heartwater disease in goats.7
Owing to the alarming rate of microbial resistance to present-day antibiotics, it is of great importance to search for alternative sources of antimicrobial agents, and medicinal plants could be explored for this purpose. However, there is a dearth of information on the antimicrobial and antioxidant properties of V mespilifolia; hence, this current study is aimed at evaluating the polyphenolic contents and antimicrobial and antioxidant properties of the whole plant of V mespilifolia.
Materials and Methods
The whole plant of V mespilifolia was collected in June 2015 from its natural habitat in the wild at Zihlahleni Village in Eastern Cape, South Africa, which lies at latitude 32° 51′41.846″S and longitude 27°10′59.318″E. The plant was authenticated by Mr Tony Dold of Selmar Schonland Herbarium, Rhodes University, South Africa, and a voucher specimen (Unuofin Med, 2015/1) was prepared and deposited at the Giffen Herbarium, University of Fort Hare.
Preparation of Extracts
The whole plant was rinsed with deionized water and gently blotted with a paper towel to remove the water and subsequently oven-dried (LABOTEC, South Africa) at 40°C for 72 hours until constant weight was achieved. The dried sample was then ground into powder (Polymix PX-MFC 90D, Switzerland) and stored at 4°C until needed for analyses. The ground sample (200 g) was weighed into 3 separate conical flasks containing (2 L) acetone, ethanol, and water, respectively. These were placed on an orbital shaker (Orbital Incubator Shaker, Gallenkamp) for 48 hours. The crude extracts were filtered under pressure using a Buchner funnel and Whatman No. 1 filter paper. The acetone and ethanol extracts were further concentrated to dryness to remove the solvents under reduced pressure using a rotary evaporator (Strike 202 Steroglass, Italy), while the aqueous filtrate obtained was concentrated using a freeze dryer (Vir Tis benchtop K, Vir Tis Co, Gardiner, NY).
Reagents and Chemicals Used
Solvents and chemicals were purchased from Merck and Sigma-Aldrich, Gauteng, South Africa. All the chemicals used in this study were of analytical grade.
Polyphenolic Acid Determination
Phenol content was estimated spectrophotometrically using the Folin-Ciocalteu’s reagent (FCR) method as described by Ghasemzadeh et al8 with some modifications. Briefly, 0.5 mL of the plant extracts (1 mg/mL) and standard gallic acid (0.02 to 0.1 mg/mL) were dispensed in test tubes. To this, 2.5 mL of 10% (v/v) FCR reagent was added and the mixture was vortexed. The reaction was allowed to stand at room temperature for about 5 minutes, after which 2 mL of 7.5% (w/v) anhydrous sodium carbonate was added to the solution, vortexed, and incubated at 40°C for 30 minutes. In the control tube, the extract volume was replaced by methanol. After incubation, the absorbance was measured at 765 nm using a UV-3000 PC spectrophotometer. The phenol content was extrapolated from the gallic acid standard/calibration graph equation—y = 8.7668x + 0.1977, R 2 = 0.9983—and calculated using the following formula:
where C is the total content of phenolic compounds in mg/g GAE or mg GAE/g extract; c is the the concentration of gallic acid established from the calibration curve in mg/mL; V is the volume of extract in mL; and m is the weight of extract used in the assay in grams.
Flavonoid Determination
The colorimetric aluminum chloride assay was used to determine the flavonoid content of the plant extract according to the method described by Sen et al9 with some modifications. This method is based on the quantification of the yellow-orange color produced by the interaction of flavonoid with AlCl3. Briefly, 0.5 mL (1 mg/mL) aliquots of the different solvent extracts and different concentrations (0.2 to 1 mg/mL) of quercetin standard were dispensed in different test tubes, and 2 mL of distilled water and 0.15 mL of 5% sodium nitrite was added to the test tubes and the mixture was allowed to stand for 6 minutes. Thereafter, 0.15 mL of 10% AlCl3 was added to the solution, allowed to stand for another 5 minutes, and this was followed by the addition of 1 mL of 1 M sodium hydroxide. The solution was made up to 5 mL with distilled water and the absorbance measured using a spectrometer at 420 nm. A control solution containing 0.5 mL of distilled water instead of the extract/standard served as the blank. The flavonoid content was calculated using the calibration curve equation—y = 1.1734x + 0.1543, R 2 = 0.9698—and expressed as mg of quercetin equivalent (QE)/g using the formula CV/m, as earlier described for total phenol.
Proanthocyanidin (Condensed Tannin)
Determination of proanthocyanidin was as described by Hanen et al.10 The reaction mixture contained 0.5 mL of 1 mg/mL of the extract or standard catechin at different concentrations (0.02 to 1 mg/mL) plus 3 mL of vanillin-methanol (4% w/v) and 1.5 mL of hydrochloric acid. The mixture was vortexed and allowed to stand for 15 minutes at room temperature, while a control solution that had neither an extract nor catechin was used as blank. The absorbance was measured at 500 nm using a UV-3000 PC spectrophotometer. Proanthocyanidin content was calculated from the calibration curve equation—y = 0.9038x + 0.0449, R 2 = 0.9951—and expressed as mg catechin equivalent (CE)/g sample using the formula CV/m, as earlier described for the determination of total phenol.
Determination of Tannins
Tannin was determined as described previously Mbaebie et al.11 Plant extract (0.2 g) was added to 20 mL of 50% methanol. This was mixed thoroughly and placed in a water bath at 80°C for 60 minutes. The extract was filtered into a 100 mL volumetric flask; 20 mL of distilled water was added, followed by 2.5 mL of FCR and 10 mL of 17% Na2CO3. This was thoroughly mixed and made up to 100 mL using distilled water. The mixture was allowed to stand for 20 minutes until a bluish-green color developed. The tannic acid standard concentrations used ranged from 0 to 10 ppm. The absorbance of the tannic acid standard solutions and plant extracts were measured after color development at 760 nm using a UV-3000 PC spectrophotometer. The results were expressed as mg/g of tannic acid equivalent using the calibration curve: Y = 154.45x; R 2 = 0.9585.
Antioxidant Assay
The antioxidant capacities of the different extracts were measured using DPPH radical scavenging activity, ABTS radical scavenging activity, hydrogen peroxide activity, and nitric oxide scavenging activity. These measurements were made against standard antioxidants, including BHT and Rutin.
ABTS (2,2′-Azino-Bis(3-Ethylbenzothiazoline)-6-Sulfonic Acid) Radical Scavenging Activity
The method described by Khan et al12 was adopted for the determination of ABTS activity of the plant extract. Briefly, the working solution was prepared by mixing equal volumes of 2 stock solutions of 7 mM ABTS and 2.45 mM potassium persulfate and allowed to react for 12 hours at room temperature in the dark to release ABTS radicals (ABTS+). The resultant green-colored solution was further diluted by mixing 1 mL of the ABTS+ solution with about 50 mL of methanol to obtain an absorbance of 0.700 ± 0.006 at 734 nm. On obtaining the desired absorbance, 1 mL of the resultant solution was then mixed with 1 mL of the plant extract/or standards at different concentrations (0.005 to 0.08 mg/mL). After 7 minutes, the reduction in absorbance is measured at 734 nm using a spectrophotometer. The percentage inhibition of ABTS+ by the extract or standard was calculated from the following equation:
DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Activity Assay
The method of Kibiti and Afolayan13 was used for the determination of DPPH free radical scavenging activity. Briefly, a solution of 0.135 mM DPPH radical in methanol was prepared. One milliliter of this solution was mixed with 1 mL (0.005 to 0.08 mg/mL) each of the plant fractions/standards antioxidants (BHT, Rutin) at different concentrations. The reaction mixture was thereafter vortexed and left in the dark at room temperature for 30 minutes. The absorbance of the mixture was measured spectrophotometrically at 517 nm. The decrease in absorbance was measured against that of the control. The scavenging ability of the plant extract was then calculated using the following equation:
where Abs control is the absorbance of DPPH + methanol and Abs sample is the absorbance of DPPH radical + sample/standard.
Hydrogen Peroxide (H2O2) Scavenging Activity
The H2O2 inhibition activity of the extracts was assessed by the method of Gülçin et al.14 Briefly, a solution of 4 mM H2O2 was prepared in phosphate buffer (0.1 M; pH 7.4) and incubated for 10 minutes. One milliliter of each plant extract at different concentrations (0.025 to 0.4 mg/mL) was added to 0.6 mL of hydrogen peroxide solution. The absorbance of the hydrogen peroxide at 230 nm was determined after 10 minutes against a blank solution containing phosphate buffer solution without hydrogen peroxide. The positive controls used were BHT and vitamin C. The percentage scavenging of hydrogen peroxide by samples was calculated using the formula:
Nitric Oxide Scavenging Activity
A 2 mL aliquot of 10 mM sodium nitroprusside prepared in 0.5 mM phosphate buffer saline (pH 7.4) was mixed with 0.5 mL of plant fractions, vitamin C, and BHT individually at different concentrations (0.025 to 0.4mg/mL). The mixture was incubated at 25°C for 150 minutes, and 0.5 mL of the incubated solution was mixed with 0.5 mL of Griess reagent (1 mL sulfanilic acid reagent [0.33% prepared in 20% glacial acetic acid] and 1 mL of naphthalene diamine dichloride [0.1% w/v] at room temperature for 5 minutes). The mixture was incubated at room temperature for 30 minutes, followed by the measurement of the absorbance at 540 nm. A solution containing water instead of the extract/standard was used as a control. Nitric oxide radical scavenging ability was calculated using the following equation:
where Abs control is the absorbance of NO radicals + methanol and Abs sample is the absorbance of NO radical + extract or standard.
Rationale for the Selection of the Microorganisms
The bacteria and fungi used for this study were selected based on their roles as opportunistic pathogens to humans and animals and their association with obesity and diabetes.15
Microorganism Strains
The selected bacterial strains for this study were 3 gram-positive strains—Actinomyces odontolyticus (ATCC 17929), Lactobacillus sakei (ATCC 15521), Staphylococcus aureus (ATCC 18824)—and 3 gram-negative strains—Enterobacter cloacae (ATCC 13047), Pseudomonas aeruginosa (ATCC 19582), and Bacteriodes thetaiotomicron (ATCC 29741). The fungal strains used for this investigation were Candida albicans (ATCC 10231), Microsporium gypsum (ATCC 24102), Penicillium chrysogenum (ATCC 10106), and Trichophyton tonsurans (ATCC 28942).
Preparation of Inocula
Bacterial Inoculum Preparation
The test bacteria strains that were maintained on nutrient agar slants were recovered in sterile MHB and incubated overnight at 37°C. In order to obtain distinct colonies, the 24-hour-old cultures were diluted 1:100 v/v in fresh sterile MHB and cultured on MHA overnight at 37°C. The colony suspension method of EUCAST16 was used for the preparation of the inoculum. Identical colonies from the culture were suspended in 0.85% sterile saline, adjusted with saline, and compared with 0.5 McFarland standards to obtain a suspension density equivalent to 106 CFU (colony forming unit)/mL. The suspensions were confirmed by spectrophotometric reading at 600 nm. The cell suspensions were finally diluted 1:100 by transferring 0.1 mL of the bacterial suspension into 9.9 mL of sterile broth to give an approximate inoculum of 104 CFU/spot. The inocula suspensions were used for inoculation within 15 minutes.
For the fungal inoculum preparation, the modified method of Therese et al17 was used for the analysis. The fungi strains were freshly subcultured on SDA and incubated at 25°C for 72 hours. About 1 cm2 of 3-day-old spore producing cultures was dropped in sterile distilled water and vortexed for 30 seconds to release the fungal spores. The spore density of each fungus was adjusted with a spectrophotometer at 580 nm to obtain a final concentration of approximately105 spores/mL. Cell suspensions were finally diluted to 104 CFU/spot.18 For the Candida spp, the inocula were prepared by adding 1.0 mL of overnight Candida cultures to 9.0 mL of SDB to yield 104 CFU/spot of the inoculum.19
Agar Dilution Assay
The method described by Wiegand et al19 and the EUCAST,16 which are modifications from the guidelines of the Clinical and Laboratory Standard Institute, were used for this study.
Muller Hinton and Sabouraud Agar were, respectively, prepared according to the manufacturer’s description for antibacterial and antifungi screening. The agar was autoclaved at 121°C for 15 minutes and allowed to cool to 50°C in a water bath. One milliliter from the 2-fold serial dilutions were added to the molten agar (19 mL) in the water bath, swirled, poured into petri dishes, and allowed to cool and solidify. Ten microliters each from both the bacterial and fungal inocula was delivered on individual solidified agar surfaces to give the desired final inoculum of 1 × 104 CFU/spot. The extracts and standard antibiotic concentrations for the antibacterial and antifungal evaluation ranged from 5 mg/mL to 0.1563 mg/mL. The concentration of ciprofloxacin ranged from 64 to 2 μg/mL, while nystatin (antifungal standard) ranged from 16 to 0.5 μg/mL. Bacteria plates were incubated at 37°C for 16 to 24 hours, while fungi plates were incubated at 30°C for 48 hours. The minimum inhibitory concentrations (MICs) were determined as the lowest concentration of extracts inhibiting the visible growth of each organism on the agar plate. The presence of 1 or 2 colonies was disregarded.
Preparation of Extract
A stock solution of 100 mg/mL prepared in little amount of dimethyl sulfoxide and made up with either Muller Hinton or Sabouraud Dextrose Broth for antibacterial and antifungal activities, respectively, was prepared. Two-fold serial dilutions of the (50, 25, 12.5, 6.25, 3.125, 1.5625 mg/mL) were also prepared. Standard antibiotic drugs ciprofloxacin and nystatin for antibacterial and antifungal activities, respectively, were also prepared by 2-fold serial dilutions as described by the Clinical and Laboratory Standard Institute.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD) of 3 replicates. Statistical analysis was performed by ANOVA. Where the data showed significance (P < .05), a mean separation was done using Fischer’s LSD with the aid of MINITAB 17 statistical package.
Results
The total polyphenol, flavonoid, proanthocyanidin, and tannin concentrations in V mespilifolia leaf and stem extracts are shown in Table 1.
Table 1.
Polyphenolic Content of Various Solvent Extracts of Vernonia mespilifolia.
| Solvent | Total phenol (mg GAE/g) | Proanthocyanidin (mg CE/g) | Flavonoids (mg QE/g) | Tannins (mg TAE/g) |
|---|---|---|---|---|
| Aqueous | 39.07 ± 0.01a | 34.55 ± 0.00c | 171.58 ± 0.02c | 0.36 ± 0.01c |
| Acetone | 99.04 ± 0.00b | 132.10 ± 0.00a | 542.87 ± 0.01a | 0.984 ± 0.00b |
| Ethanol | 106.99 ± 0.01c | 83.90 ± 0.00b | 464.75 ± 0.02b | 0.932 ± 0.02a |
Abbreviations: mg GAE/g, milligram gallic acid equivalent per gram of extract; mg QE/g, milligram quercetin equivalent per gram of extract; mg TAE/g, milligram tannic acid equivalent per gram of extract; mg CE/g, milligram catechin equivalent per gram of extract.
a Values are expressed as mean ± standard deviation of 3 replicates.
All the superscrits indicate significant difference (P<0.005) between the means. Values within the same column, with different superscript are significantly different.
The flavonoid, proanthocyanidin, and tannin concentrations were higher in the acetone extracts when compared with the aqueous and ethanol extracts. Also, the amount of phenolic content of plant materials depend on the polarity of the solvent used for extraction.5 The acetone extract had the highest concentrations of flavonoid, proanthocyanidin, and tannins, while the aqueous extract had the lowest concentration of these polyphenolics. The total phenol concentration ranged from 39.07 to 106.99 mg GAE/g. Flavonoid concentration ranged from 171.58 to 542.87 mg QE/g. Proanthocyanidin concentration ranged from 34.55 to 89.90 mg CE/g, while the concentration of tannin ranged from 0.36 to 0.984 mg TAE/g.
Antioxidant Activities of the Extracts
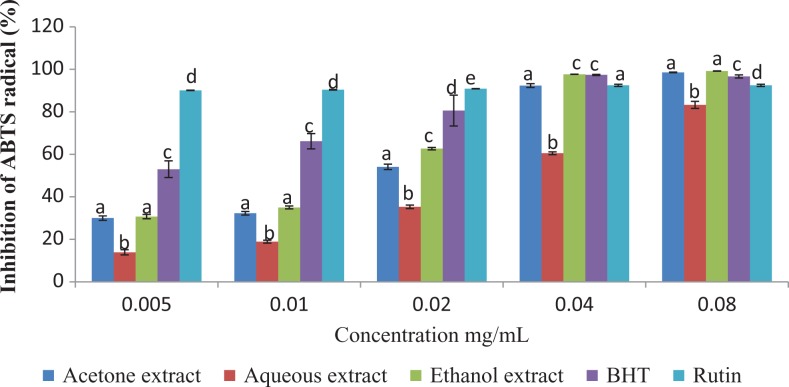
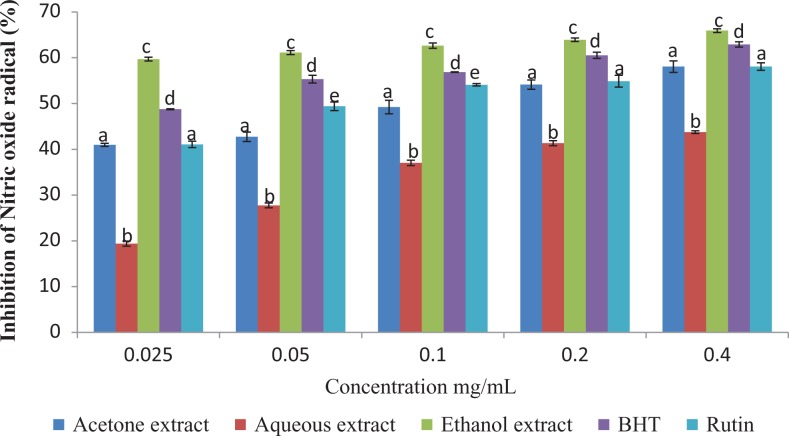
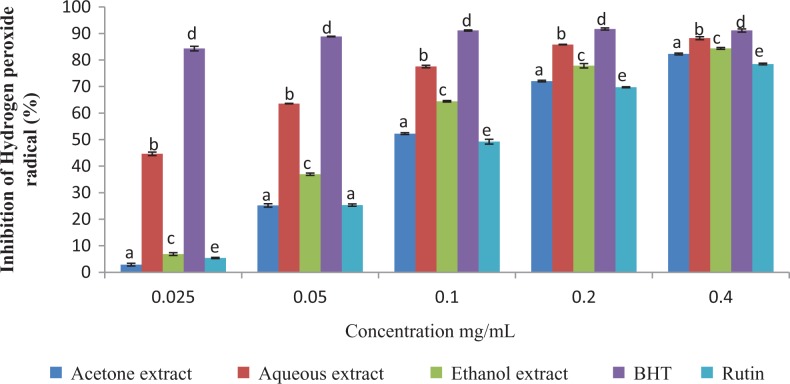
The results of this study showed that the antioxidant activities of the plant extracts compared favorably with those of the standard antioxidants (BHT and Rutin) employed in the antioxidant assays (Figures 2 –4).
Figure 2.
ABTS radical scavenging activity of the different extracts of Vernonia mespilifolia. Values are mean ± SD of 3 replications. Set of bars with the same letter are not significantly different (P < .05).
Figure 3.
Nitric oxide radical scavenging activity of the different extracts of Vernonia mespilifolia. Values are mean ± SD of 3 replications. Set of bars with the same letter are not significantly different (P < .05).
Figure 4.
Hydrogen peroxide scavenging activity of the different extracts of Vernonia mespilifolia. Values are mean ± SD of 3 replications. Set of bars with the same letter are not significantly different (P < .05).
DPPH Free Radical Scavenging Assay
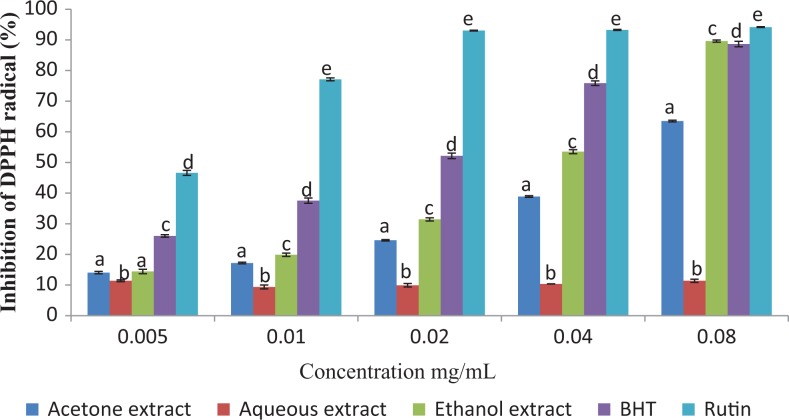
The DPPH radical scavenging activities of the extracts and standards are presented in Figure 1. At the maximum concentration tested (0.08 mg/mL), Rutin exhibited the highest DPPH scavenging activity (94.15%), followed by the ethanol extract (89.60%), BHT (88.63%), acetone (63.50%), and the aqueous extract (11.37%). The IC50 values of the tested extracts/standards were in the following order: Rutin > BHT > ethanol extract > acetone extract > aqueous extract (Table 2).
Figure 1.
DPPH radical scavenging activity of the different extracts of Vernonia mespilifolia. Values are mean ± SD of 3 replications. Set of bars with the same letter are not significantly different (P < .05).
Table 2.
IC50 Values of the Various Extracts of Vernonia mespilifolia Whole Plant and Standard Drugsa.
| Extracts/Standard | DPPH | ABTS | Nitric Oxide | H2O2 | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 | R 2 | IC50 | R 2 | IC50 | R 2 | IC50 | R 2 | |
| Aqueous | 1.87 | 0.9291 | 0.039 | 0.9527 | 0.6033 | 0.9646 | 0.0249 | 0.9176 |
| Acetone | 0.06 | 0.9985 | 0.013 | 0.9210 | 0.1158 | 0.9792 | 0.1108 | 0.9786 |
| Ethanol | 0.039 | 0.9378 | 0.012 | 0.9233 | 0.0003 | 0.9747 | 0.0865 | 0.9332 |
| BHT | 0.015 | 0.9857 | 0.003 | 0.9418 | 0.0294 | 0.9897 | <<0.025b | 0.7246 |
| Rutin | 0.003 | 0.7427 | 0.996 | 0.9059 | 0.0768 | 0.8933 | 0.1172 | 0.9805 |
Abbreviations: IC50, the concentration (mg/mL) required to scavenge/inhibit 50% of the radical; R 2, coefficient of determination.
a Values obtained from regression lines with 95% confidence level.
b Far less than 0.025.
ABTS Radical Scavenging Assay
The results of this assay, as shown in Figure 2, revealed that all the extracts and standards possessed considerable ABTS radical scavenging activities. The percentage inhibition of ABTS by the plant and the standards were concentration-dependent. At the highest concentration tested (0.08 mg/mL), the values for acetone, aqueous, and ethanol extracts were 98.55%, 83.29%, and 99.23%, respectively, while the standards (BHT and Rutin) produced inhibition as high as 96.68% and 92.49%, respectively. The IC50 values of the tested extracts/standards were in the following order: BHT > ethanol extract > acetone extract > aqueous extract > Rutin. The results are presented in Table 2.
Nitric Oxide Scavenging Assay
The percentage nitric oxide inhibitory activities of the extracts and standards (Figure 3) were in the following order: ethanol extract > BHT > Rutin > acetone extract > aqueous extract, at the highest concentration (0.40 mg/mL). The IC50 values of the tested extracts/standards were in the following order: ethanol extract > BHT > Rutin > acetone extract > aqueous extract (Table 2) with ethanol extract possessing the lowest IC50, indicating its strong nitric oxide scavenging ability.
Hydrogen Peroxide Scavenging Assay
The scavenging ability of the plant extracts against hydrogen peroxide is shown in Figure 4. The scavenging activity of the plant in different solvents were in the following order: BHT > aqueous extract > ethanol extract > acetone extract > Rutin (Table 2). The percentage inhibition of hydrogen peroxide by the different solvents were concentration-dependent and significantly different (P < .05). The aqueous extract had highest radical scavenging potential among the extracts used but did not scavenge as much as BHT.
The results of the antibacterial MICs using agar dilution is shown in Table 3. The result revealed that both the gram-positive and gram-negative bacteria tested were susceptible to the various extracts of V mespilifolia. The MIC values ranged from 2.5 to 5 mg/mL for both gram-positive and gram-negative bacteria except for P aeruginosa and S aureus (acetone extract), which had MIC values greater than 5 mg/mL. The aqueous extract had the lowest activity against both gram-positive and gram-negative bacteria with MIC values greater than 5 mg/mL. The standard drug (ciprofloxacin) showed higher antibacterial activity with MIC value ranging from 2 to 32 μg/mL.
Table 3.
Minimum Inhibitory Concentrations (MICs) of the Different Solvent Extracts of Vernonia mespilifolia on Selected Gram Negative and Gram Positive Bacteria Using the Agar Dilution Assay.
| Bacteria | Gram | Extract (mg/mL) | Ciprofloxacin (μg/mL) | ||
|---|---|---|---|---|---|
| Acetone | Aqueous | Ethanol | |||
| Bacteriodes thetaiotomicron | − | 2.5 | >5 | 5 | 2 |
| Actinomyces odontolyticus | + | 2.5 | >5 | 2.5 | 2 |
| Lactobacillus sakei | + | 2.5 | >5 | 5 | 2 |
| Enterobacter cloacae | − | 5 | >5 | 5 | 2 |
| Pseudomonas aeruginosa | − | >5 | >5 | 2.5 | 2 |
| Staphylococcus aureus | + | >5 | >5 | 5 | 32 |
The antifungal activities of the extracts of V mespilifolia on some selected fungi associated with obesity are shown in Table 4. The MICs were taken after 3 days of incubation. The acetone extract was more active against the 4 fungi tested. Its MICs ranged from 0.3125 to 2.5 mg/mL, except for T tonsurans, with MIC greater than 5 mg/mL. The ethanol extract was also active against the various fungi tested but not as much as the acetone extract. Its MIC ranged from 1.25 to 5 mg/mL. The aqueous extract had the least activity of all the extracts tested, with an MIC value greater than 5 mg/mL for all the fungi used for the study.
Table 4.
Minimum Inhibitory Concentrations (MICs) of the Different Solvent Extracts of Vernonia mespilifolia on Selected Human Pathogenic Fungi.
| Fungi | Extract (mg/mL) | Nystatin (μg/mL) | ||
|---|---|---|---|---|
| Acetone | Aqueous | Ethanol | ||
| Microsporium gypsum | 2.5 | >5 | 2.5 | 8 |
| Penicillium chrysogenum | 0.3125 | >5 | 1.25 | 4 |
| Trichophyton tonsurans | >5 | >5 | >5 | 4 |
| Candida albicans | 0.3125 | >5 | 5 | >16 |
Discussion
In this study, flavonoid, total phenol, proanthocyanidins, tannin concentrations, antioxidant activity, and antimicrobial activity were determined for different extracts of V mespilifolia. These parameters usually depend on the type and polarity of solvent.20,21 In the present study, acetone extract of V mespilifolia had the highest concentration of flavonoids, proanthocyanidins, and tannins. The ethanol extract had the highest concentration of polyphenol. This is similar to results of Sharifi-Rad et al21 obtained using Chrozaphora tinctoria, where the acetone extract gave the highest yield of total polyphenols, flavonoids, and tannins. In recent times, various scientists have been appraising the effects of extraction solvents on natural products. The polarity of solvent used for extraction has a great impact on the yield of different phytochemical classes present in the plant.21,22 According to Eloff,23 acetone extract contains more bioactive components with better antimicrobial potency.
According to Farasat et al,24 the highest antioxidant activity in plants is attributed to polyphenols among other secondary metabolites. Phenolics have the ability of oxidizing a broad spectrum of free radicals to their stable radical intermediates.25 They also serve as electron donors, metal chelators, and singlet and triplet oxygen quenchers.26 Tannins (a polyphenol) bind and precipitate microbial proteins, thus starving bacteria of major nutrients.27
Babaa and Malik28 stated that flavonoids such as flavones, flavanols, and condensed tannins possess antioxidant potentials as a result of their free OH groups and thus they can suppress the formation of reactive oxygen species, mast cell histamine release, and antimicrobial activities. Antioxidants scavenge free radicals by different modes of action, which include using transition metal chelation, singlet oxygen quenchers, free radical scavenger (donate H), and peroxide stabilizers.29 Because of these numerous modes of action of scavenging free radicals, we decided to assay for 4 different antioxidant assays. DPPH radical shows a maximum absorption at 517 nm. On encountering proton radical scavengers, its purple color fades rapidly. Antioxidants with the capacity of donating a hydrogen atom or electrons can quench DPPH free radicals, thus converting them to colorless bleached products, which brings about the reduction in absorbance.30 The antioxidant activity was confirmed by a decrease in absorbance band on increasing concentrations of V mespilifolia. The ethanol extract had the highest DPPH scavenging potential when compared with other extracts used, and this may be due to its high phenolic content, which contribute to their electron transfer/hydrogen donating ability.
The ABTS radical assay measures the relative antioxidant ability to scavenge the radical. It is an excellent tool for determining the antioxidant capacity of hydrogen-donating antioxidants.31 According to Heim et al,32 flavonoids and phenolic acids exhibit antiradical and antioxidant activities. The ethanol extract thus may have scavenged best due to its high phenolic content because of its high phenolic acid content.
Nitric oxide is classified as a free radical because of its unpaired electron and displays important reactivity with certain types of proteins and other free radicals. Its toxic effect becomes adverse when it reacts with superoxide radical, forming a highly reactive peroxynitrite anion (ONOO−).33 Our finding suggests that the polyphenolics present in the V mespilifolia could be responsible for nitric oxide scavenging effect as ethanolic extract of V mespilifolia had the highest inhibitory effect on nitric oxide production.
Hydrogen peroxide itself is not very reactive, but when it becomes hydroxyl radical in the cells its toxic nature is revealed. Thus, the removal of H2O2 as well as O2 −·is very important for antioxidant defense in cell or food systems.34 The various extracts of V mespilifolia scavenged H2O2 and this may be attributed to the phenolics, which can donate electrons to H2O2, thereby neutralizing it into water. The aqueous extract had the highest inhibitory effect on hydrogen peroxide radical.
Antimicrobial activity is one of the important properties of polyphenolic compounds.21 The antimicrobial activity of phenolics is due to their partially hydrophobic nature. They inhibit the hydrolytic enzymes (proteases), microbial adhesion, as well as cell envelope transport proteins, and so on.35 Additionally, flavonols can bind with extracellular and soluble proteins and with bacterial cell walls. Most antibacterial medicinal plants are effective on gram-positive bacteria, but few against gram-negative bacteria; this is due to the presence of lipopolysaccharide barrier on the outer membrane of gram-negative bacteria, which is absent on gram-positive bacteria.36
The antibacterial and antifungal activities of the acetone, aqueous, and ethanol extracts of V mespilifolia were investigated in this study. In this study, acetone and ethanol extracts of V mespilifolia showed the greatest antibacterial and antifungal activities, while the aqueous extract had the lowest (Tables 3 and 4).
The disparity in the activities of the crude extracts and standard antimicrobial drugs could be attributed to the combination of bioactive components inherent in the crude extracts as compared to standard drugs, which are pure compounds.18,37 However, the active components in the crude extracts could act in synergy to bring about the antimicrobial effects.23 The weak activity of the aqueous extract in this current study could be due to a very low concentration of the compounds present in the crude extracts that are active against the various organisms. The weak activity of the aqueous extract against all bacterial strains investigated in this study is in agreement with previous reports.18,38 According to Saglam and Arar,38 medicinal plant extracts and pure compounds have displayed both antibacterial and fungicidal potentials both in vitro and in vivo. Therefore, plant extracts with renowned antimicrobial properties could be employed for therapeutic purposes39 and as such these would boost the use of herbal remedies for microbial infections.18 The results from this study are in line with several reports on antimicrobial activities of many medicinal plants.40,41
Conclusion
This study reveals that Vernonia mespilifolia has high amount of polyphenolic compounds. The ethanol extract had the highest polyphenolic content, which could have contributed to the high antioxidant activities observed. Also, the acetone extract was the most promising against gram-positive and gram-negative bacteria and also exhibited higher antifungal ability.
Acknowledgments
Authors wish to acknowledge the financial support of Govan Mbeki Research and Development Centre, University of Fort Hare, Eastern Cape, South Africa (Grant Number C 127).
Footnotes
Author Contributions: JOU, GAO, and AJA conceived and designed the study. JOU performed the experiments and wrote the draft manuscript. GAO and AJA coordinated and helped revise the manuscript. JOU, GAO, and AJA read and approved the final manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Govan Mbeki Research and Development Centre, University of Fort Hare, Eastern Cape, South Africa (Grant Number C127).
ORCID iD: Jeremiah Oshiomame Unuofin  http://orcid.org/0000-0002-7460-9594
http://orcid.org/0000-0002-7460-9594
Gloria Aderonke Otunola  http://orcid.org/0000-0003-2613-2741
http://orcid.org/0000-0003-2613-2741
Ethical Approval: Ethical approval is not required for this article as it does not contain any studies involving human or animal participants.
References
- 1. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shukla V, Mishra SK, Pant HC. Oxidative stress in neurodegeneration. Adv Pharmacol Sci. 2011;2011:572634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohamed AA, Ali SI, El-Baz FK. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS One. 2013;8:e60269 doi:10.1371/journal.pone.0060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. del Baño MJ, Lorente J, Casstillo J, et al. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems and roots of Rosmarinus officinalis antioxidant activity. J Agric Food Chem. 2003;51:4247–4253. [DOI] [PubMed] [Google Scholar]
- 5. Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–660. [Google Scholar]
- 6. Afolayan AJ, Mbaebie BO. Ethnobotanical study of medicinal plants used as anti-obesity remedies in Nkonkobe Municipality of South Africa. Pharmacognosy J. 2010;2:368–373. [Google Scholar]
- 7. Dold AP, Cocks ML. Traditional veterinary medicine in the Alice district of the Eastern Cape Province, South Africa. S Afr J Sci. 2001;97:375–379. [Google Scholar]
- 8. Ghasemzadeh A, Jaafar HA, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules. 2010;15:4324–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sen S, De B, Devanna N, Chakraborty R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb, an Indian medicinal plant. Chin J Nat Med. 2013;11:149–157. [DOI] [PubMed] [Google Scholar]
- 10. Hanen F, Riadh K, Samia O, Sylvain G, Christian M, Chedly A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem Toxicol. 2009;47:2308–2313. [DOI] [PubMed] [Google Scholar]
- 11. Mbaebie BO, Edego HO, Afolayan AJ. Phytochemical analysis and antioxidants activities of aqueous of bark extract of Schofia latifolia Jacq. Asian Pac J Trop Biomed. 2012;2:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan RA, Khan MR, Sahreen S, Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L) Hill. Chem Cent J. 2012;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kibiti CM, Afolayan AJ. Preliminary phytochemical screening and biological activities of Bulbine abyssinica used in the folk medicine in the Eastern Cape Province, South Africa. Evid Based Complement Alternat Med. 2015;2015:617607 doi:10.1155/2015/617607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gülçin I, Alici HA, Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem Pharm Bull (Tokyo). 2005;53:281–285. [DOI] [PubMed] [Google Scholar]
- 15. Vajro P, Paolella G, Fasano A. Microbiota and gut-liver axis: their influences on obesity and obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2013;56:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. European Committee on Antimicrobial Susceptibility Testing. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin Microbiol Infect. 2003;9:1–7.12691538 [Google Scholar]
- 17. Therese KL, Bagyalakshmi R, Madhavan HN, Deepa P. In-vitro susceptibility testing by agar dilution method to determine the minimum inhibitory concentrations of amphotericin B, fluconazole and ketoconazole against ocular fungal isolates. Indian J Med Microbiol. 2006;24:273–279. [DOI] [PubMed] [Google Scholar]
- 18. Olajuyigbe OO, Afolayan AJ. Antimicrobial potency of the ethanolic crude bark extract of Ziziphus mucronata Willd. subsp. mucronata Willd. Afr J Pharm Pharmacol. 2012;6:717–723. [Google Scholar]
- 19. Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175. [DOI] [PubMed] [Google Scholar]
- 20. Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum . J Taibah Univ Sci. 2014;8:216–224. [Google Scholar]
- 21. Sharifi-Rad J, Hoseini-Alfatemi SM, Miri A, et al. Phytochemical analysis, antioxidant and antibacterial activities of various extracts from leaves and stems of Chrozaphora tinctoria . Environ Exp Biol. 2015;13:169–175. [Google Scholar]
- 22. Daoud A, Malika D, Bakari S, et al. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of date palm pollen (DPP) from two Tunisian cultivars [published online August 1, 2015]. Arab J Chem. doi:10.1016/j.arabjc.2015.07.014. [Google Scholar]
- 23. Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1998;60:1–8. [DOI] [PubMed] [Google Scholar]
- 24. Farasat M, Khavari-Nejad RA, Nabavi SM, Namjooyan F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran J Pharm Res. 2014;13:163–170. [PMC free article] [PubMed] [Google Scholar]
- 25. Koolen HF, da Silva FMA, Gozzo FC, de Souza AQL, de Souza ADL. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L f) by UPLC–ESI-MS/MS. Food Res Int. 2013;51:467–473. [Google Scholar]
- 26. Shawkatul IB, Shahriar M, Akhter R, Bhuiyan MA. In vitro antioxidant activities of the whole plant extract of Chrozophora prostrata (Dalz). Ann Biol Res. 2015;6:19–26. [Google Scholar]
- 27. Funatogawa K, Hayashi S, Shimomura H, et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori . Microbiol Immunol. 2004;48:251–261. [DOI] [PubMed] [Google Scholar]
- 28. Babaa SA, Malik SA. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J Taibah Univ Sci. 2015;9:449–454. [Google Scholar]
- 29. Cioffi G, Auria MD, Braca A, et al. Antioxidant and free radical scavenging activity of constituents of the leaves of Tachigalia paniculata . J Nat Prod. 2002;65:1526–1529. [DOI] [PubMed] [Google Scholar]
- 30. Illavarasan R, Mallika M, Venkataraman S. Anti-inflammatory and antioxidant activities of Cassia fistula Linn bark extracts. Afr J Tradit Complement Altern Med. 2005;2:70–85. [Google Scholar]
- 31. Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2004;44:198–206. [DOI] [PubMed] [Google Scholar]
- 32. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. [DOI] [PubMed] [Google Scholar]
- 33. Nagmoti DM, Khatri DK, Juvekar PR, Juvekar AR. Antioxidant activity and free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radic Antioxidants. 2011;2:37–43. [Google Scholar]
- 34. Gülcin I, Küfrevioglu OI, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L). J Ethnopharmacol. 2004;90:205–215. [DOI] [PubMed] [Google Scholar]
- 35. Wei L, Zhang W, Yin L, Yan F, Xu Y, Chen F. Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron J Biotechnol. 2015;18:88–95. [Google Scholar]
- 36. Gatsing D, Nkeugoauapi CFN, Nkah BFN, Kuiate JR, Tchouanguep FM. Antibacterial activity, bioavailability and acute toxicity evaluation of the leaf extract of Alchornea cordifolia (Euphorbiaceae). Int J Pharmacol. 2010;6:173–182. [Google Scholar]
- 37. Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn). Afr J Pharm Pharmacol. 2009;3:58–62. [Google Scholar]
- 38. Saglam H, Arar G. Cytotoxic activity and quality control determinations on Chelidonium majus . Fitoterapia. 2003;74:127–129. [DOI] [PubMed] [Google Scholar]
- 39. Rojas JJ, Ochoa VJ, Ocampo SA, Muñoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC Complement Altern Med. 2006;6:2 doi:10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Govindappa M, Bharath N, Shruthi HB, Sadananda TS, Sharanappa P. Antimicrobial, antioxidant and in vitro anti-inflammatory activity and phytochemical screening of Crotalaria pallida Aiton. Afr J Pharm Pharmacol. 2011;5:2359–2371. [Google Scholar]
- 41. Afolayan AJ, Grierson DS, Kambizi L, Madomombe I, Masika PJ. In vitro antifungal activity of some South African medicinal plants. S Afr J Bot. 2002;68:72–76. [Google Scholar]