Abstract
This study aims to investigate the effect of oral administration and the direct action of ginger extract or [6]-gingerol on small intestinal contractility. The direct effect of 10 minutes preincubation of ginger ethanolic extract (10, 100 and 300 μg/mL) or [6]-gingerol (1, 30, and 100 μM) on 0.01 to 30 μM ACh-induced contractions of all parts of the small intestine isolated from normal rats was investigated using the organ bath technique. For in vivo study, the rats were orally administered with extract (10, 20, and 100 mg/kg/d) or [6]-gingerol (2 mg/kg/d) for 7 days, followed by determining the contractile responses to ACh of rat isolated duodenum, jejunum, and ileum and their histology were assessed. Direct application of the extract or [6]-gingerol attenuated ACh-induced contractions in each small intestinal segment, E max was reduced by 40% to 80%, while EC50 increased 3- to 8-fold from control. Similarly, in the in vivo study ACh-induced contractions were reduced in all parts of the small intestine isolated from rats orally treated with ginger extract (20 and 100 mg/kg/d) or [6]-gingerol (2 mg/kg/d). E max decreased 15% to 30%, while EC50 increased 1- to 3-fold compared to control. No discernable changes in the histology of intestinal segments were detectable. Thus, the results support the clinical application of ginger for disorders of gastrointestinal motility.
Keywords: ginger, [6]-gingerol, small intestine, contraction
Ginger (Zingiber officinale Roscoe, Zingiberaceae) is well established around the world as a food spice and a traditional medicine. The ginger rhizome contains many biologically active constituents, of which [6]-gingerol is the most common.1 Ginger and its active constituents have been widely studied for its pharmacological properties for antioxidant,2 antimicrobial,3 antinausea and vomiting,4 and antidiarrheal activities.5 Ginger is marketed for treating diseases of the gastrointestinal tract, including dyspepsia,6 diarrhea, and nausea and vomiting. Clinical studies have demonstrated that ginger can prevent postoperative nausea and vomiting7 and reduced nausea and vomiting in pregnancy,8,9 motion sickness,10 and in patients receiving chemotherapy.11 The anti-emetic action of ginger arises from the digestive tract, by promoting pyloric emptying into the intestine by its anticholinergic and antiserotonergic actions. Ginger stimulates antral anterograde contractions toward the pylorus, thereby promoting gastric emptying in healthy humans12 and in patients with functional dyspepsia.6 These actions also explain anecdotal reports of dyspepsia, nausea, and vomiting where the gastric hypotonia is relieved by ginger. Ginger also prevents cisplatin-induced emesis in dogs13 and promotes gastric emptying in cisplatin-treated rats,14 suggesting a role in human cancer chemotherapy. Other chemotherapeutic agents share this induction of nausea and vomiting via the medullary chemoreceptor trigger zone, gastrointestinal tract, and vagal afferents and the vomiting center.15 In the gastrointestinal tract, chemotherapeutics stimulate 5-hydroxytryptamine (5-HT) from enterochromaffin cells of small intestine and activate 5HT3 receptor on vagal visceral neurons leading to visceral afferent vagus nerve activation15–18 and neurally released ACh,19 acting on muscarinic (M3) receptors of the intestinal smooth muscle,20,21 culminating in nausea and vomiting. However, the role of ginger on the gastrointestinal tract is not well understood because most mechanistic studies were conducted on animal intestine in vitro. These studies showed that ginger extracts inhibited contractions induced by electrical field stimulation or exogenously applied ACh in the isolated rat ileum.22 Moreover, ginger and its constituents, [6]-gingerol, [6]-shogaol, and zingerone, inhibited 5-HT3-evoked inward (depolarizing) currents in rat nodose ganglionic neurons.23 Also, ginger and [6]-gingerol blocked 5-HT3 and M3 receptors, thus blocking both serotonin- and carbachol-induced contractions of the isolated guinea-pig ileum.24–26 However, the extent of current knowledge is incomplete in several important respects: (1) studies were confined to the ileum, (2) there are no studies on tissues from orally treated animals, and (3) there is no information about possible adverse effects on intestinal integrity. Therefore, to answer these questions, animals orally treated with [6]-gingerol were used to study contractility along different segments of the small intestine, and the corresponding structural changes.
Materials and Methods
Preparation of the Plant Extract
Fresh rhizomes of ginger (Z. officinale Roscoe) were collected from Lom Sak district, Phetchabun province, Thailand. The voucher specimen (No. 004330) was identified by Dr. Pranee Nangngam, Department of Biology, Faculty of Sciences, Naresuan University, and kept at Faculty of Sciences, Naresuan University, Phitsanulok, Thailand. The fresh rhizomes of ginger (100 kg) were cut into pieces (0.4 cm) and dried at 50°C for 24 hours. The dried material was ground with a roller grinding machine to powder. The dried ginger rhizome powder (7.6 kg) was extracted with 95% ethanol (5 L) for 10 days. Then, it was filtered and evaporated under vacuum until dryness at 50°C on a rotary evaporator to give the crude ethanolic extract with 6.84% yield. The ginger extract was analyzed by high-performance liquid chromatography (HPLC) for quality control of its active compounds, [6]-gingerol (11.91%) and [6]-shogaol (0.92%), and stored at 4°C until used.
Animals
Male Wistar rats (200-250 g) were obtained from the National Laboratory Animal Centre, Mahidol University, Salaya, Thailand. Experiments were ethically approved by Naresuan University Animal Care and Use Committee (NUACUC, Naresuan University, Phitsanulok, Thailand; Ethic Number: NU-AE590715) for the care and use of animals for scientific purposes. The rats were maintained in plastic cages at 22 ± 1°C with a 12-12 hour light–dark cycle, fed with standard rodent diet (082G) and tap water in the Center for Animal Research, Naresuan University, Phitsanulok, Thailand.
Tissue Preparation and Experimental Protocol for In Vitro Study
The rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally). The small intestine was divided into 3 parts corresponding to the duodenum, jejunum, and ileum, which were flushed through, cleaned, and surface fat removed. Each intestinal section was subdivided into 1 cm in length and suspended in 20 mL organ bath containing Krebs’ solution composed of (mM): NaCl 122; KCl 5; N-[2-hydroxyethyl] piperazine-N′-[2-ethane-sulfonic acid] (HEPES) 10; KH2PO4 0.5; NaH2PO4 0.5; MgCl2 1; CaCl2 1.8; and glucose 11, adjusted to pH 7.4 with 1 M NaOH, and were aerated continuously with air, at 37°C. The segments were longitudinally tensioned to 1 g and allowed to equilibrate for 60 minutes.22,25 During the equilibration period, the bathing solutions were replaced by Krebs’ solution every 15 minutes. The wires were connected to a force transducer to measure isometric tension via a MacLab A/D converter (Chart V5; A.D. Instruments, Castle Hill, Australia), stored, and displayed on a personal computer.
After equilibration, maximal contraction was elicited by switching to 80 mM high K+ solution containing (mM): NaCl 47.4; KCl 79.5; HEPES 10; KH2PO4 0.5; NaH2PO4 0.5; MgCl2 1; CaCl2 1.8; and glucose 11, adjusted to pH 7.4 with 1 M NaOH, for ∼1 minute and then returning to normal Krebs’ solution. When the spontaneous contraction reached equilibrium, ACh, M3 receptor agonist, at concentrations of 0.01 to 30 μM were cumulatively added (∼30 seconds for each addition) in order to induce contraction. The ACh was washed out 2 to 3 times and replaced by fresh Krebs’ solution for 30 minutes. Then, either vehicle alone (dimethyl sulfoxide [DMSO]), ginger extract (10, 100, or 300 μg/mL), or [6]-gingerol (1, 30, or 100 μM) were added for 10 minutes followed by the accumulating ACh concentrations. Contractile responses were measured from the baseline for each ACh concentration and expressed as a proportion of the maximal contraction produced by high K+ solution.
Experimental Protocol for In Vivo Study
For this study, ginger extract or [6]-gingerol was administered by a single oral gavage daily for 7 days. The rats (200-250 g weight) were randomly divided into 5 groups (n = 6) treated as follows: (1) the control group gavaged with vehicle (propylene glycol [PG]), (2) 10 mg/kg ginger extract group, (3) 20 mg/kg ginger extract group, (4) 100 mg/kg ginger extract group, (5) 2 mg/kg [6]-gingerol group. The ginger extract and [6]-gingerol were dissolved by PG and administered orally to the rats via a syringe and stainless steel gastric tube once a day. At day 8, the rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally) and the subdivided small intestine sections removed as described above, and then the following protocols were carried out.
Small Intestinal Contractile Responses to ACh
Contractile responses to ACh were recorded as described above but without any treatments with ginger extract or [6]-gingerol.
Histological Study
Isolated duodenum, jejunum, and ileum (2 cm long) were quickly rinsed in normal saline, harvested, fixed in 10% buffered formalin, dehydrated, and embedded in paraffin. Dewaxed sections of 3 μm were stained with hematoxylin-eosin (H&E) for light microscopy. To evaluate histological changes, all sections were photographed under the light microscope. Villi lengths, crypt depths, goblet cell counts, and circular and longitudinal muscle layer thicknesses were measured using ImageJ (version 1.51j8, National Institutes of Health, Baltimore, MA).
Drugs and Solutions
ACh and [6]-gingerol were obtained from Sigma (St Louis, MO). ACh was dissolved in distilled water. Propylene glycol was purchased from Ajax Finechem Pty Ltd (Unilab, New South Wales, Australia). DMSO was purchased from VWR International Ltd (Prolabo Chemicals, UK). Pentobarbital sodium solution (Nembutal) was obtained from Ceva Animal Health, Bangkok, Thailand.
Statistical Analysis
All data were expressed as means ± standard error of mean (SEM) of n animals. Contractions were expressed as percentage to high K+ (80 mM) induced maximum response. The EC50 values (defined as the concentration of ACh that induced 50% of the maximal contraction) and E max values (values of maximal contraction) were determined by fitting the original concentration-response curves using GraphPad Prism software (version 5.0). The multiple comparisons were analyzed using one-way ANOVA followed by Tukey’s test. P < .05 was considered statistically significant.
Result
Quantitative Analysis of the Main Pungent Principles in Ginger Extract
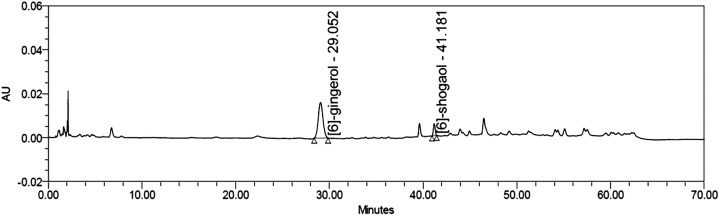
The HPLC analysis of the ginger extract showed the active compounds of 11.91% (w/w) of [6]-gingerol and 0.92% (w/w) of [6]-shogaol (Figure 1).
Figure 1.
HPLC chromatogram of active compounds in the ginger extract. Column: Luna C18 4.6 × 150 mm; Mobile phase: water/acetonitrile (gradient).
Ginger Extract and [6]-Gingerol Inhibited Spontaneous Contractility of Isolated Intestine
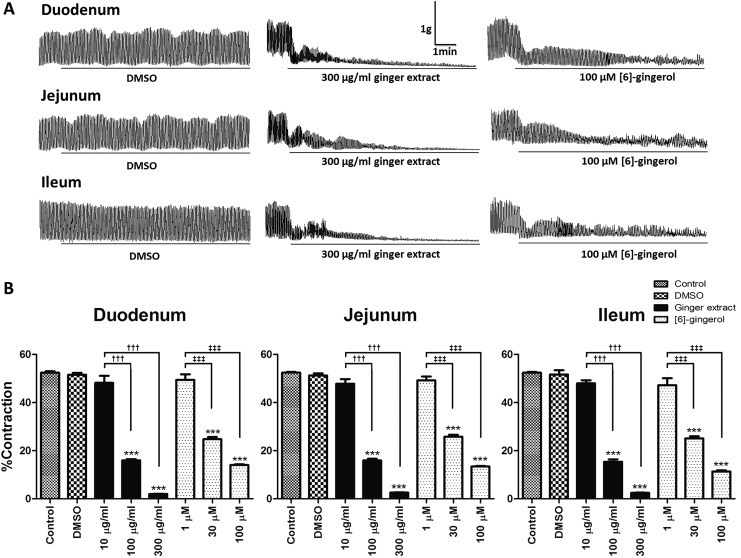
Spontaneous contraction of the duodenum, jejunum, and ileum became established after 60 minutes of incubation in Krebs’ solution. The amplitude of movements was 52.41 ± 0.64%, 52.86 ± 0.36%, and 52.44 ± 0.31% to high K+ (80 mM) induced maximum contraction in the duodenum, jejunum, and ileum, respectively. These spontaneous contractions were inhibited by ginger extract (100 and 300 μg/mL) or [6]-gingerol (30 and 100 μM) into the bath for 10 minutes (Figure 2A) and attenuated at lower concentrations (Figure 2B) irrespective of the intestinal segment tested. DMSO was without effect.
Figure 2.
Inhibitory effect of ginger extract or [6]-gingerol on spontaneous contraction of rat isolated duodenum, jejunum, and ileum. (A) Representative tracing of the duodenum, jejunum, and ileum spontaneous contraction before and after 10 minutes preincubation with DMSO, 300 μg/mL ginger extract, or 100 μM [6]-gingerol. (B) Contraction expressed as percentage to high K+ (80 mM) solution-induced maximum contraction of isolated duodenum, jejunum, and ileum before (control) and after incubation with DMSO, 10 to 300 μg/mL ginger extract, or 1 to 100 μM [6]-gingerol. All data were expressed as means ± SEM (n = 5). ***P < .001 versus the control or DMSO; ††† P < .001 versus the 10 μg/mL ginger extract; ‡‡‡ P < .001 versus the 1 μM [6]-gingerol.
Ginger Extract or [6]-Gingerol Attenuated ACh-Induced Contractions
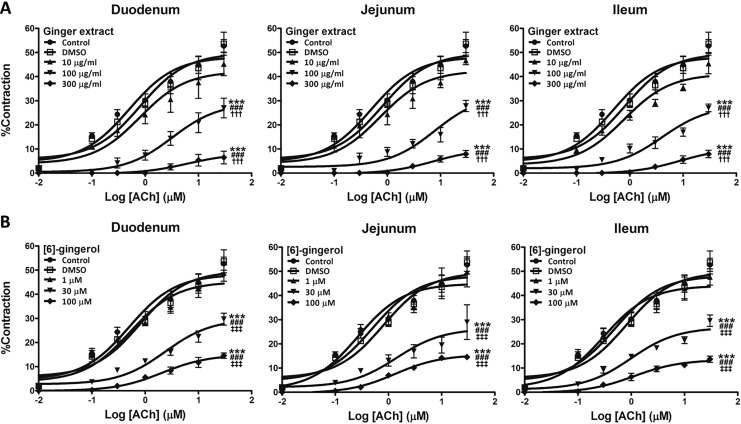
In all 3 anatomical sections of the small intestine, the patterns of responses were similar. Thus, with 100 and 300 μg/mL, extract produced clear attenuations in the ACh-induced contractions (Figure 3A), and effects replicated by [6]-gingerol (30-100 μM, Figure 3B). These effects were reflected in reduced maximal contractions (E max) and increased EC50 (Table 1). In contrast, neither the lowest concentrations (10 μg/mL ginger extract and 1 μM [6]-gingerol) nor the vehicles had any detectable effects.
Figure 3.
Inhibitory effect of ginger extract (A) and [6]-gingerol (B) on cumulative ACh-induced contractions of rat isolated duodenum, jejunum, and ileum. Small intestinal segments were preincubated with either DMSO, 10 to 300 μg/mL ginger extract, or 1 to 100 μM [6]-gingerol for 10 minutes, and then concentration-contraction responses to ACh were obtained. Contractions were expressed as percentage of 80 mM K+ solution-induced maximum response. All data were expressed as means ± SEM (n = 5-6). ***P < .001 versus the control; ### P < .001 versus the DMSO; ††† P < .001 versus the 10 μg/mL ginger extract; ‡‡‡ P < .001 versus the 1 μM [6]-gingerol.
Table 1.
EC50 and E max of ACh-Induced Contractions on Rat Duodenum, Jejunum, and Ileum in the Absence (Control) or Presence of DMSO, 10 to 300 μg/mL Ginger Extract, or 1 to 100 μM [6]-Gingerola.
| Group | Duodenum | Jejunum | Ileum | n | |||
|---|---|---|---|---|---|---|---|
| EC50 (μM) | E max (%) | EC50 (μM) | E max (%) | EC50 (μM) | E max (%) | ||
| Control | 0.59 ± 0.09 | 52.29 ± 3.81 | 0.58 ± 0.27 | 52.94 ± 2.91 | 0.60 ± 0.26 | 52.26 ± 2.76 | 6 |
| DMSO | 0.57 ± 0.13 | 50.30 ± 5.74 | 0.57 ± 0.26 | 53.88 ± 2.06 | 0.61 ± 0.17 | 52.56 ± 2.67 | 6 |
| Ginger extract | |||||||
| 10 μg/mL | 0.84 ± 0.42 | 46.71 ± 0.572 | 0.85 ± 0.15 | 46.03 ± 3.38 | 0.86 ± 0.13 | 47.39 ± 4.21 | 5 |
| 100 μg/mL | 4.16 ± 0.76*** | 26.87 ± 3.73*** | 4.12 ± 0.45*** | 28.36 ± 2.01*** | 4.14 ± 0.50*** | 26.73 ± 1.43*** | 6 |
| 300 μg/mL | 6.01 ± 0.34*** | 6.62 ± 2.56*** | 6.18 ± 0.64*** | 7.89 ± 1.46*** | 6.15 ± 0.68*** | 7.92 ± 1.54*** | 5 |
| [6]-Gingerol | |||||||
| 1 μM | 0.72 ± 0.22 | 48.74 ± 3.36 | 0.70 ± 0.44 | 49.27 ± 4.31 | 0.71 ± 0.62 | 47.57 ± 3.36 | 5 |
| 30 μM | 2.05 ± 0.39*** | 29.92 ± 3.08*** | 2.13 ± 0.93*** | 29.16 ± 3.48*** | 2.08 ± 0.57*** | 29.56 ± 2.35*** | 5 |
| 100 μM | 4.87 ± 0.57*** | 14.52 ± 1.12*** | 4.89 ± 0.62*** | 16.69 ± 1.99*** | 4.80 ± 0.54*** | 13.57 ± 1.95*** | 5 |
Abbreviation: DMSO, dimethyl sulfoxide.
a Values are means ± SEM of the number (n) of individual segments. EC50 is the concentration of ACh eliciting a half-maximal contraction induced by 80 mM high K+ solution. E max is the maximum response of each segment expressed as a contraction percentage of the contraction induced by 80 mM high K+ solution.
***P < .001 versus EC50 or E max of the control and DMSO.
In Vivo Actions of ACh on Preparations Obtained from Extract and Gingerol Treated Rats
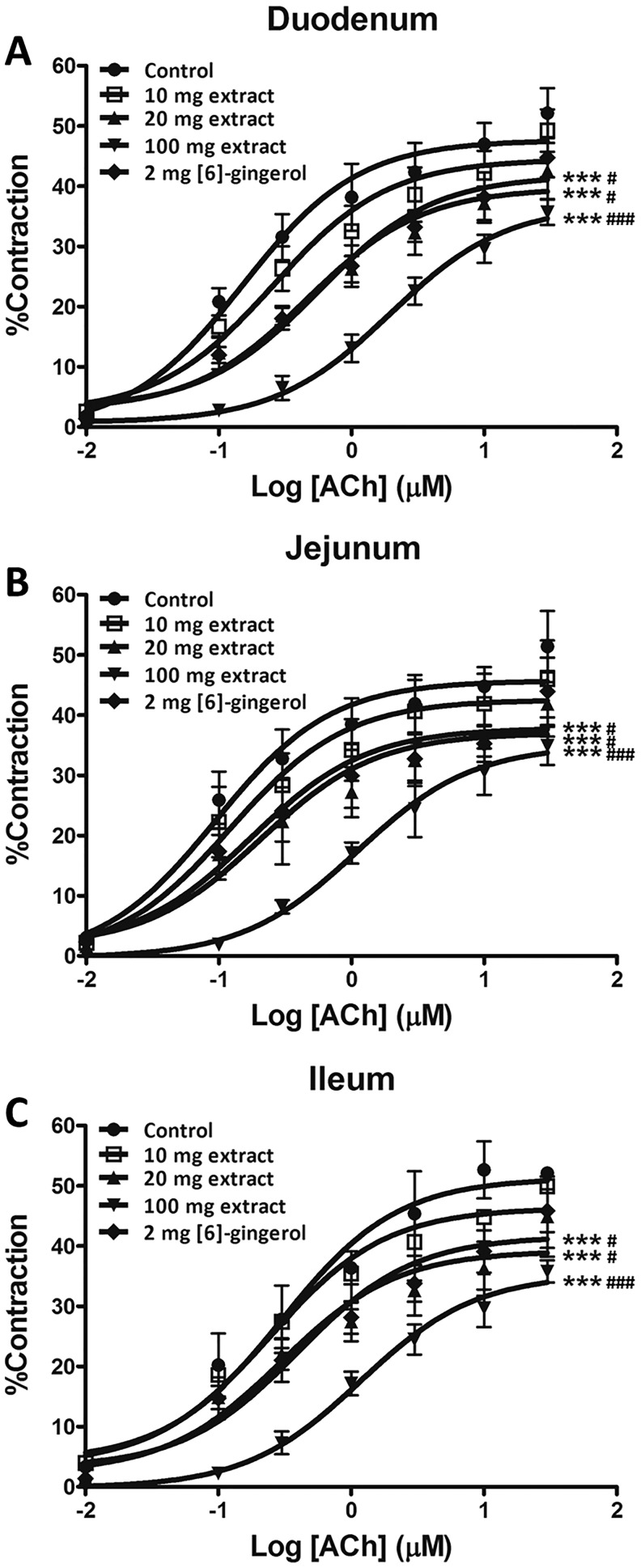
All intestinal sections from animals pretreated with higher doses of extract (20 and 100 mg/kg/d) or [6]-gingerol (2mg/kg/d) were consistently less responsive to all test concentrations of ACh (Figure 4) as reflected in reduced E max and right-shifted EC50 (Table 2). Nevertheless, pretreatment with the lower dose of ginger extract (10 mg/kg/d) or vehicle have no measureable effect on either E max or EC50 (Table 2).
Figure 4.
Concentration response curves of ACh-induced contraction in duodenum (A), jejunum (B), and ileum (C) isolated from rats orally administered with 10 to 100 mg/kg/d ginger extract or 2 mg/kg/d [6]-gingerol for 7 days. Values are means ± SEM (n = 6). ***P < .001 versus the control; # P < .05, ### P < .001 versus the 10 mg/kg ginger extract group.
Table 2.
EC50 and E max of ACh-Induced Contraction on Duodenum, Jejunum, and Ileum Isolated From Rats Pretreated with PG (Control), 10 to 100 mg/kg/d Ginger Extract, or 2 mg/kg/d [6]-Gingerol for 7 Daysa.
| Group | Duodenum | Jejunum | Ileum | n | |||
|---|---|---|---|---|---|---|---|
| EC50 (μM) | E max (%) | EC50 (μM) | E max (%) | EC50 (μM) | E max (%) | ||
| Control | 0.57 ± 0.023 | 50.25 ± 3.43 | 0.58 ± 0.50 | 51.12 ± 6.10 | 0.59 ± 0.20 | 56.29 ± 4.05 | 6 |
| Ginger extract | |||||||
| 10 mg/kg | 0.60 ± 0.25 | 45.93 ± 3.29 | 0.61 ± 0.30 | 46.01 ± 6.39 | 0.61 ± 0.27 | 51.19 ± 6.48 | 6 |
| 20 mg/kg | 0.83 ± 0.17**## | 40.21 ± 4.11** | 0.84 ± 0.23**## | 41.29 ± 5.56** | 0.82 ± 0.22**## | 40.10 ± 5.29** | 6 |
| 100 mg/kg | 1.86 ± 0.36***### | 38.87 ± 3.42***### | 1.74 ± 0.62***### | 36.22 ± 3.89***### | 1.62 ± 0.51***### | 35.93 ± 1.91***### | 6 |
| [6]-Gingerol | |||||||
| 2 mg/kg | 0.79 ± 0.23*# | 42.38 ± 3.91** | 0.78 ± 0.47* | 43.92 ± 5.04** | 0.78 ± 0.22* | 41.52 ± 3.46** | 6 |
Abbreviation: PG, propylene glycol.
a Values are means ± SEM of the number (n) of individual segments. EC50 is the concentration of ACh eliciting a half-maximal contraction induced by 80 mM high K+ solution. E max is the maximum response of each segment expressed as a contraction percentage of the contraction induced by 80 mM high K+ solution.
*P < .05, **P < .01, ***P < .001 versus EC50 or E max of the control; # P < .05, ## P < .01, ###P < .001 versus EC50 or E max of the 10 mg/kg group.
Histology of Small Intestine of Pretreated Rats
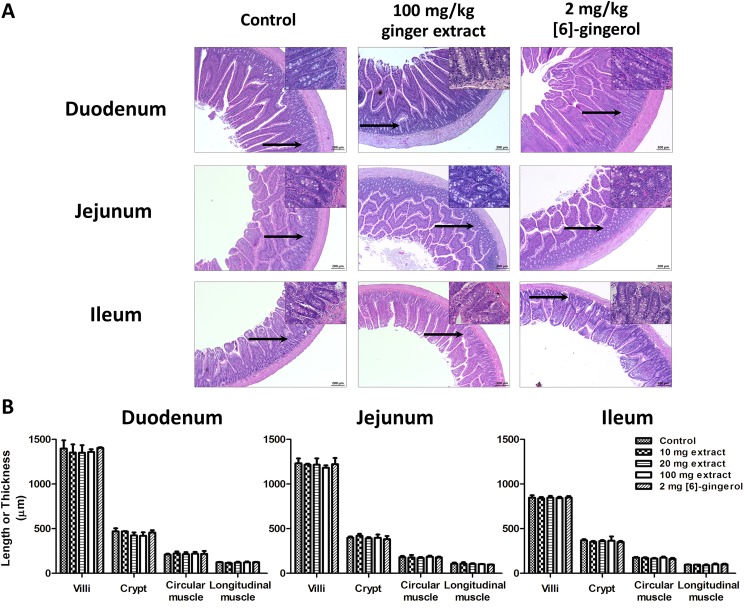
There were no changes in key morphological features (lengths of villi, and crypts, nor circular, or longitudinal muscle layers of duodenum, jejunum, and ileum) after treatment with ginger extract or [6]-gingerol (Figure 5). Goblet cell density and their structures were unaffected nor were there any changes in the enterocyte, Paneth cells, or smooth muscle cells (insets). No lymphocyte invasion, vacuolation, or edema formation could be seen, demonstrating the absence of any gross inflammatory reaction.
Figure 5.
(A) Typical histological cross-section images of rat duodenum, jejunum, and ileum isolated from rats orally administered with PG (control), 10 to 100 mg/kg/d ginger extract, or 2 mg/kg/d [6]-gingerol for 7 days (H&E; ×100 original magnification, insets ×400 original magnification of the area indicated by black arrows). (B) The lengths of the villi and crypts, and thicknesses of the circular and longitudinal muscle layers of duodenum, jejunum, or ileum. Values are means ± SEM (n = 5).
Discussion
The present study demonstrated direct inhibitory action and effect of oral administration of ginger extract and [6]-gingerol on the ACh-induced contraction in rat duodenum, jejunum, and ileum, in agreement with previous findings.22,25 In addition, the amplitude and frequency of spontaneous contractions were stably generated for at least 60 minutes driven by rhythmic slow waves generated by interstitial cells of Cajal, the pacemaker cells between and within the muscle layers of the small intestine.27–30 Ginger extract or [6]-gingerol concentration-dependently reduced the amplitudes of spontaneous contractions of all portions of the isolated rat small intestine. These results agree with previous studies showing [6]-gingerol inhibited spontaneous contractile movements of rat-isolated colonic segments by blocking calcium influx through L-type calcium channels.31 Nevertheless, the effect of ginger extract or [6]-gingerol may be mediated through additional actions such as inhibition of M3 receptors or at any point along their downstream signaling pathways.
Rats fed with [6]-gingerol yielded small intestinal segments showing responses to exogenous ACh that were dose-dependently reduced. Likewise, 20 mg of ginger extract containing ∼2 mg of [6]-gingerol had roughly the same attenuated effect (Figure 4), implying that [6]-gingerol was the main active ingredient. The question arising is how the contractile action of ACh is attenuated by oral dosing. There are several explanations for these reduced actions. (1) There is residual [6]-gingerol or extract components remaining in the intestinal that occludes or desensitizes ginger action. Pharmacokinetic studies in rats with oral dosing of [6]-gingerol32,33 suggest that its blood levels would reach around 0.5 to 1 μM (after scaling for our doses) but rapidly decline after 15 to 60 minutes and barely detectable after 240 minutes. In contrast, the early intraluminal concentrations after dosing with [6]-gingerol are roughly estimated to be ∼6 mM based on the amount of [6]-gingerol ingested and the volume of vehicle. Thus, judging from the kinetics in the vascular compartment, gut luminal [6]-gingerol would clear in <60 minutes. Tissue contents of [6]-gingerol were also measured by Jiang et al,33 and the stomach and gut walls have particularly high concentrations (300 μM) for tissue water at 4 hours after dosing. While Jiang et al dosed with 120 mg/kg of [6]-gingerol compared to our 2 mg/kg, and allowing a further 50% decline during another 20 hours when the animal was terminated, we estimate tissue water to contain 10 μM of [6]-gingerol. This is enough to explain the reduced effect of ACh at this time point. Furthermore, gut wall tissue content of [6]-gingerol would be raised by carryover from successive [6]-gingerol doses. (2) The histological landscape was remarkably normal and consistent in appearance compared to controls and showed no suggestion of any villus retraction, irritation, inflammation, or cell necrosis. This apparent robustness of the gut wall compares with the susceptibility of cultured breast cancer cells that were vulnerable to lower concentrations of 100 μM.34 (3) The repetitive [6]-gingerol dosing has downregulated or desensitized the ACh triggered molecular signaling pathways. Explanation (1) provides the most plausible explanation for the reduced contractile effect of ACh. However, the extensive extrapolation from the Jiang et al study does not make our conclusion definitive. We also surmise that because the brain contents of [6]-gingerol would be very low,33 [6]-gingerol-containing material acts directly on the gut rather than centrally. The plasma [6]-gingerol concentrations barely reached 1 μM (extrapolated to our conditions). This concentration was ineffective in the bathing solution of our isolated preparations; thus, it is likely that [6]-gingerol, when given orally, acts from the luminal side of the gut wall rather than the serosal side. As an orally active spasmolytic agent, our data suggest that its action would persist for longer than the pharmacokinetics would suggest. Nevertheless, as an anti-emetic, confirmation would require its role in quelling activation of gut wall afferent nerves that activate the vomiting reflex or even give the sensation of nausea.
Conclusion
We conclude that the ginger extract and [6]-gingerol could reduce or inhibit contraction via M3 receptor activation, irrespective of the position along the length of the small intestine. Orally dosing of ginger extract or [6]-gingerol appeared not to have any detectable effect on gut wall structure or integrity. However, in isolated intestinal segments from these pretreated animals showed a dose-related attenuation of ACh-induced contractions. These outcomes support ginger for the treatment of nausea and vomiting but the molecular targets need to be defined.
Acknowledgments
The authors are grateful to Dr. C. Norman Scholfield, Naresuan University, for provision of meaningful discussion and editing the manuscript.
Footnotes
Author Contributions: Ginger ethanolic extract preparation was done by Tanwarat Kajsongkram. Krongkarn Chootip, Sakara Tunsophon, and Rachanee Chanasong designed the experimental study. Experimental work and analysis was done by Usana Chatturong. The article was written and complied by Usana Chatturong and Krongkarn Chootip.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Research Council of Thailand and Thailand Institute of Scientific and Technological Research, Pathum Thani, Thailand. We also thank the Center of Excellence for Innovation in Chemistry (PERCH-CIC) and the Commission on Higher Education for research facility support.
ORCID iD: Krongkarn Chootip  http://orcid.org/0000-0001-6973-158X
http://orcid.org/0000-0001-6973-158X
Ethical Approval: The handling and use of animals for this study was approved by Naresuan University Animal Care (NUACUC, Naresuan University, Phitsanulok, Thailand; Ethic Number: NU-AE590715) and was in accordance with the National Institute of Health guide for the use and handling of experimental animals.
References
- 1. Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–420. [DOI] [PubMed] [Google Scholar]
- 2. Tohma H, Gülçin İ, Bursal E, Gören AC, Alwasel SH, Köksal E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc) determined by HPLC-MS/MS. J Food Meas Charact. 2017;11:556–566. [Google Scholar]
- 3. Kim HS, Park HD. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS One. 2013;8:e76106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ryan JL, Heckler CE, Roscoe JA, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen JC, Huang LJ, Wu SL, Kuo SC, Ho TY, Hsiang CY. Ginger and its bioactive component inhibit enterotoxigenic Escherichia coli heat-labile enterotoxin-induced diarrhea in mice. J Agric Food Chem. 2007;55:8390–8397. [DOI] [PubMed] [Google Scholar]
- 6. Hu ML, Rayner CK, Wu KL, et al. Effect of ginger on gastric motility and symptoms of functional dyspepsia. World J Gastroenterol. 2011;17:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaiyakunapruk N, Kitikannakorn N, Nathisuwan S, Leeprakobboon K, Leelasettagool C. The efficacy of ginger for the prevention of postoperative nausea and vomiting: a meta-analysis. Am J Obstet Gynecol. 2006;194:95–99. [DOI] [PubMed] [Google Scholar]
- 8. Smith C, Crowther C, Willson K, Hotham N, McMillian V. A randomized controlled trial of ginger to treat nausea and vomiting in pregnancy. Obstet Gynecol. 2004;103:639–645. [DOI] [PubMed] [Google Scholar]
- 9. Willetts KE, Ekangaki A, Eden JA. Effect of a ginger extract on pregnancy-induced nausea: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2003;43:139–144. [DOI] [PubMed] [Google Scholar]
- 10. Lien HC, Sun WM, Chen YH, Kim H, Hasler W, Owyang C. Effects of ginger on motion sickness and gastric slow-wave dysrhythmias induced by circular vection. Am J Physiol Gastrointest Liver Physiol. 2003;284:G481–G489. [DOI] [PubMed] [Google Scholar]
- 11. Pillai AK, Sharma KK, Gupta YK, Bakhshi S. Anti-emetic effect of ginger powder versus placebo as an add-on therapy in children and young adults receiving high emetogenic chemotherapy. Pediatr Blood Cancer. 2011;56:234–238. [DOI] [PubMed] [Google Scholar]
- 12. Wu KL, Rayner CK, Chuah SK, et al. Effects of ginger on gastric emptying and motility in healthy humans. Eur J Gastroenterol Hepatol. 2008;20:436–440. [DOI] [PubMed] [Google Scholar]
- 13. Sharma SS, Kochupillai V, Gupta SK, Seth SD, Gupta YK. Antiemetic efficacy of ginger (Zingiber officinale) against cisplatin-induced emesis in dogs. J Ethnopharmacol. 1997;57:93–96. [DOI] [PubMed] [Google Scholar]
- 14. Sharma SS, Gupta YK. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale). J Ethnopharmacol. 1998;62:49–55. [DOI] [PubMed] [Google Scholar]
- 15. Navari RM. Management of chemotherapy-induced nausea and vomiting in pediatric patients. Pediatr Drugs. 2017;19:213–222. [DOI] [PubMed] [Google Scholar]
- 16. Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81. [DOI] [PubMed] [Google Scholar]
- 17. Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 18. Schwörer H, Racke K, Kilbinger H. Cisplatin increases the release of 5-hydroxytryptamine (5-HT) from the isolated vascularly perfused small intestine of the guinea-pig: involvement of 5-HT 3 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1991;344:143–149. [DOI] [PubMed] [Google Scholar]
- 19. Fox AJ, Morton IK. An examination of the 5-HT3 receptor mediating contraction and evoked [3H]-acetylcholine release in the guinea-pig ileum. Br J Pharmacol. 1990;101:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Montgomery LE, Tansey EA, Johnson CD, Roe SM, Quinn JG. Autonomic modification of intestinal smooth muscle contractility. Adv Physiol Educ. 2016;40:104–109. [DOI] [PubMed] [Google Scholar]
- 21. Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res. 2004;40:237–247. [DOI] [PubMed] [Google Scholar]
- 22. Borrelli F, Capasso R, Pinto A, Izzo AA. Inhibitory effect of ginger (Zingiber officinale) on rat ileal motility in vitro. Life Sci. 2004;74:2889–2896. [DOI] [PubMed] [Google Scholar]
- 23. Jin Z, Lee G, Kim S, Park CS, Park YS, Jin YH. Ginger and its pungent constituents non-competitively inhibit serotonin currents on visceral afferent neurons. Korean J Physiol Pharmacol. 2014;18:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdel-Aziz H, Windeck T, Ploch M, Verspohl EJ. Mode of action of gingerols and shogaols on 5-HT 3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur J Pharmacol. 2006;530:136–143. [DOI] [PubMed] [Google Scholar]
- 25. Pertz HH, Lehmann J, Roth-Ehrang R, Elz S. Effects of ginger constituents on the gastrointestinal tract: role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med. 2011;77:973–978. [DOI] [PubMed] [Google Scholar]
- 26. Yamahara J, Rong HQ, Iwamoto M, Kobayashi G, Matsuda H, Fujimura H. Active components of ginger exhibiting anti-serotonergic action. Phytother Res. 1989;3:70–71. [Google Scholar]
- 27. Brijs J, Hennig GW, Kellermann AM, Axelsson M, Olsson C. The presence and role of interstitial cells of Cajal in the proximal intestine of shorthorn sculpin (Myoxocephalus scorpius). J Exp Biol. 2017;220(pt 3):347–357. [DOI] [PubMed] [Google Scholar]
- 28. Koh SD, Kim TW, Jun JY, Glasgow NJ, Ward SM, Sanders KM. Regulation of pacemaker currents in interstitial cells of Cajal from murine small intestine by cyclic nucleotides. J Physiol. 2000;527(pt 1):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McHale N, Hollywood M, Sergeant G, Thornbury K. Origin of spontaneous rhythmicity in smooth muscle. J Physiol. 2006;570(pt 1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. [DOI] [PubMed] [Google Scholar]
- 31. Cai ZX, Tang XD, Wang FY, et al. Effect of gingerol on colonic motility via inhibition of calcium channel currents in rats. World J Gastroenterol. 2015;21:13466–13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gundala SR, Mukkavilli R, Yang C, et al. Enterohepatic recirculation of bioactive ginger phytochemicals is associated with enhanced tumor growth-inhibitory activity of ginger extract. Carcinogenesis. 2014;35:1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jiang SZ, Wang NS, Mi SQ. Plasma pharmacokinetics and tissue distribution of [6]-gingerol in rats. Biopharm Drug Dispos. 2008;29:529–537. [DOI] [PubMed] [Google Scholar]
- 34. Bernard MM, McConnery JR, Hoskin DW. [10]-Gingerol, a major phenolic constituent of ginger root, induces cell cycle arrest and apoptosis in triple-negative breast cancer cells. Exp Mol Pathol. 2017;102:370–376. [DOI] [PubMed] [Google Scholar]