Abstract
Background:
Arthroscopic surgery of the shoulder joint has become increasingly more common given its advantages over open surgery; however, one rare but potentially life-threatening complication is fluid extravasation into the surrounding tissues, causing edema, respiratory compromise, abnormal results on laboratory blood tests, and possibly death. Currently, no systematic review exists that summarizes the existing clinical research on this topic.
Purpose:
To perform a systematic review on fluid extravasation as a complication of shoulder arthroscopic surgery, specifically assessing clinical presentation, risk factors, management, and outcomes.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
Two reviewers independently searched 3 databases (PubMed, Ovid [MEDLINE], and Embase) from database inception until July 1, 2017. The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist guided the reporting and data abstraction. The methodological quality of these studies was assessed using the Methodological Index for Non-Randomized Studies (MINORS) checklist. The results are presented in a narrative summary fashion using descriptive statistics including ranges and agreement statistics.
Results:
A total of 26 studies (20 case reports, 4 case series, and 2 prospective comparative studies) encompassing 205 patients (mean age, 50.8 years [range, 15-83 years]) were included. The most common signs of fluid extravasation included chest wall swelling (n = 86) and neck swelling (n = 116). In 32 patients, observation alone was sufficient. Other patients required airway intubation (n = 16), diuretics (n = 7), steroids (n = 1), and percutaneous drainage of fluid (n = 1). Clinical edema resolved after 2 to 48 hours, and patients were discharged 1 to 20 days postoperatively. Serious complications included transfer to the intensive care unit (n = 14), anterior interosseous nerve palsy (n = 4), rhabdomyolysis (n = 1), and death (n = 1).
Conclusion:
Fluid extravasation has the potential to be a life-threatening complication of shoulder arthroscopic surgery; however, it is most commonly managed nonoperatively, and symptoms typically resolve with no evidence of long-term complications. Intraoperative surgical decisions, such as minimizing the surgical time and volume of irrigation fluid used, may limit fluid extravasation, while careful intraoperative monitoring may facilitate prompt diagnosis and management to optimize patient outcomes.
Keywords: shoulder, anesthesia/pain management, cardiovascular physiology, injury prevention, epidemiology, muscle injuries
Arthroscopic surgery of the shoulder joint has become increasingly more common worldwide since the turn of the millennium, with reports from the United States demonstrating a 250% increase in the number of patients undergoing arthroscopic shoulder surgery from 1996 to 2006 as well as data from the UK Hospital Episode Statistics suggesting a 7.5-fold increase in the frequency of shoulder arthroscopic procedures, such as acromioplasty, from 2000 to 2010.6
Despite the increasing trend in the popularity of shoulder arthroscopic surgery, there are complications associated with the procedure.2 One rare but potentially life-threatening complication is fluid extravasation into the surrounding tissues.11 Fluid extravasation during shoulder arthroscopic surgery was first described in 1990, with subsequent reports of edema of the chest, neck, face, and upper airway and respiratory compromise.9,14,15,31 Moreover, systemic absorption of large amounts of fluid can produce laboratory abnormalities of electrolyte and complete blood count panels and lead to dysfunction of remote organs, including the central nervous system,18 and possibly even death. Currently, there has been no systematic review of the literature assessing the complication of fluid extravasation in the context of shoulder arthroscopic surgery.
The purpose of this systematic review was to investigate fluid extravasation as a complication of shoulder arthroscopic surgery; specifically, this review study assessed the characterization of the clinical presentation, identification of risk factors, delineation of the management approach, and analysis of patient outcomes after this complication.
Methods
Search Strategy
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement was used for the reporting of study selection, as used in prior reviews.21,28 The online databases PubMed, Embase, and Ovid (MEDLINE) were utilized to search for literature addressing the complication of fluid extravasation in the setting of shoulder arthroscopic surgery from the time of database inception until July 1, 2017. The search terms “shoulder,” “arthroscopy,” “fluid,” and “extravasation” were used (Appendix Table A1).
Study Screening
Two reviewers (M.M. and J.K.) independently screened the titles, abstracts, and full-text articles. Any disagreements were discussed between the reviewers and the senior author (O.R.A.) to determine study inclusion when necessary. The references of the eligible included studies were then screened to include any additional articles that may not have been captured by the initial search. The search strategy is outlined in Appendix Table A1.
Assessment of Study Eligibility
The research questions and inclusion and exclusion criteria were determined a priori. The inclusion criteria were English-language studies, human studies, and studies investigating fluid extravasation as a complication of shoulder arthroscopic surgery. Studies of all levels of evidence that reported on clinical presentation, management, and/or outcomes involving fluid extravasation as a complication of shoulder arthroscopic surgery were included. The exclusion criteria included cadaveric studies, conference papers, book chapters, review articles, and technical reports with no outcome data.
Data Abstraction
The 2 reviewers collected data in duplicate and recorded them in an Excel spreadsheet (version 2007; Microsoft). Data regarding year of publication, author(s), study design, sample size, percentage of female patients, age, follow-up, arthroscopic procedure, indications for surgery, operative side, patient positioning, type of anesthesia, operative time, irrigation fluid type and volume, irrigation device, clinical presentation, management, and outcomes were abstracted from all included studies.
Quality Assessment
The 2 reviewers independently assessed the level of evidence (levels 1-4) of the included studies using the American Academy of Orthopaedic Surgeons classification system.41 The Methodological Index for Non-Randomized Studies (MINORS) tool was used as well to assess the methodological quality of the included studies.37 The MINORS tool grades comparative studies with a maximum score of 24 and noncomparative studies with a maximum score of 16. The senior author was consulted for any discrepancy between the reviewers. All eligible studies were included in this review regardless of level of evidence and study quality.
Assessment of Agreement
Interreviewer agreement for the titles, abstracts, and full-text articles was calculated using the kappa statistic. Intraclass correlation coefficients (ICCs) were calculated for the quality assessment using the MINORS criteria. Agreement was categorized a priori as follows: κ or ICC of ≥0.61 was considered substantial agreement, κ or ICC of 0.21 to 0.60 was moderate agreement, and κ or ICC of ≤0.20 was slight agreement.23
Statistical Analysis
Descriptive statistics calculated from the data included means, proportions, SDs, and ranges. Because of the limited reporting, these data were not combined in a meta-analysis and are summarized descriptively. All statistics were calculated using Minitab statistical software (version 17; Minitab).
Results
Search Strategy
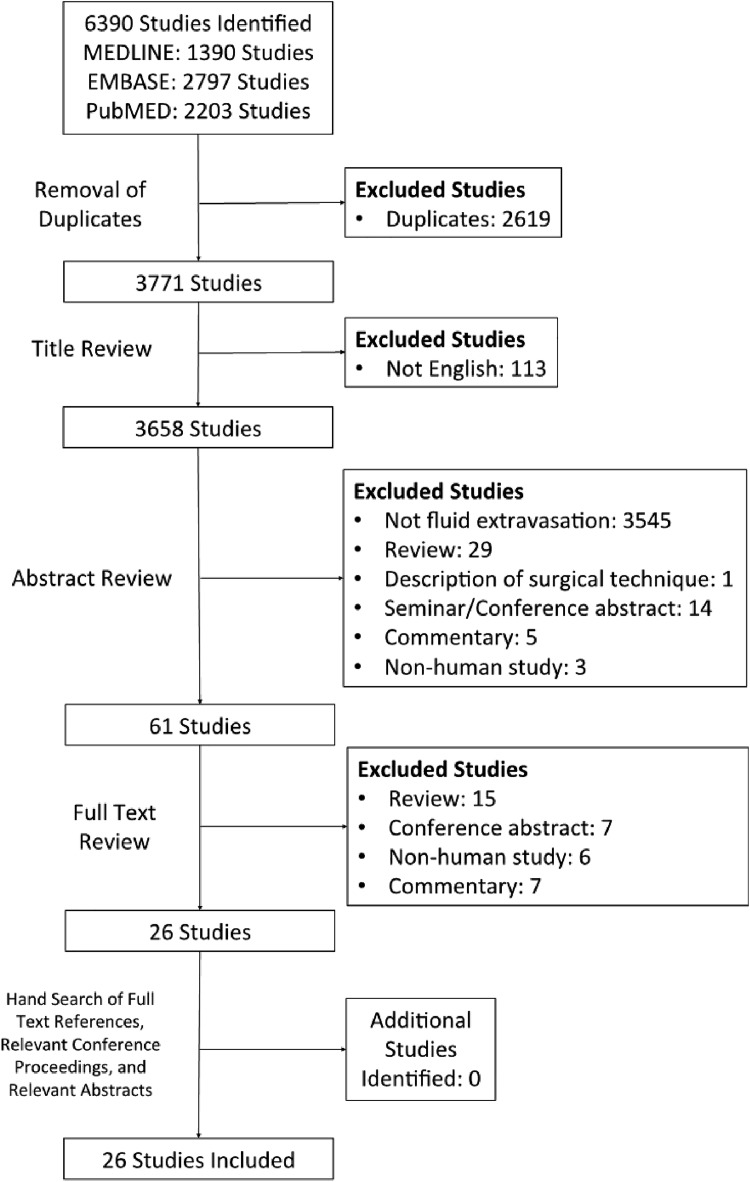
The initial search of the 3 databases resulted in 6390 total studies. A total of 2619 studies were immediately removed as duplicates, resulting in 3771 studies for title screening. A systematic screening approach removed articles failing to meet inclusion criteria and resulted in 26 available full-text articles for review (Figure 1). There was substantial agreement between reviewers at the title (κ = 0.852; 95% CI, 0.809-0.895), abstract (κ = 0.889; 95% CI, 0.864-0.914), and full-text (κ = 1.000) screening stages.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram demonstrating the systematic review of the literature on fluid extravasation in shoulder arthroscopic surgery.
Study Quality
In total, 20 retrospective case reports (n < 5 patients), 4 prospective case series (n > 5 patients), and 2 prospective comparative studies were identified, corresponding to levels of evidence of 4, 4, and 2, respectively. For noncomparative studies, the median MINORS score was 9 of 16 (range, 7-12), whereas the median MINORS score for comparative studies was 14.5 of 24 (range, 14-15). Overall, 100% of studies had a clearly stated aim, 92.3% had appropriate endpoints, 7.6% had an appropriate follow-up period, and 100% had loss of follow-up of less than 5%. However, only 23.1% of studies had a prospective collection of data, and no studies had an unbiased assessment of study endpoints. The overall interrater agreement for the MINORS score was high, with an ICC of 0.831 (95% CI, 0.811-0.851).
Patient Demographics
In total, 205 patients (205 shoulders) underwent shoulder arthroscopic surgery and experienced fluid extravasation as a complication related to their arthroscopic procedure. The mean patient age was 50.8 years (range, 15-83 years), and there were 84 (41%) female patients (Table 1). The site of arthroscopic surgery and indication for shoulder arthroscopic surgery are summarized in Table 2. Only 1 study,11 a case report, identified a particular anatomic abnormality, which was described as abnormal communication between the superior glenoid extending medially to the infraspinous fossa along the teres minor.
TABLE 1.
Study and Patient Characteristicsa
| Author (Year) | Country | Study Design (Level of Evidence) | Mean MINORS Score | No. of Patients/Shoulders | Age, Mean (Range), y | Sex, Male/Female, n | Mean Follow-up, mo |
|---|---|---|---|---|---|---|---|
| Antonucci et al1 (2006) | Italy | Retrospective case report (4) | 8 | 2/2 | 58 (53-63) | 2/0 | NR |
| Blumenthal et al3 (2003) | Switzerland | Retrospective case report (4) | 9 | 1/1 | 55 | 1/0 | NR |
| Borgeat et al4 (2000) | Switzerland | Retrospective case report (4) | 9 | 1/1 | 69 | 0/1 | NR |
| Carr and Murphy7 (1995) | USA | Prospective comparative study (2) | 15 | 24/24 | 38 (17-78) | 18/6 | NR |
| Chellam et al8 (2015) | India | Prospective case series (4) | 12 | 32/32 | 51.7 (18-70) | 12/20 | NR |
| Edwards et al11 (2014) | UK | Retrospective case report (4) | 9 | 1/1 | 53 | 0/1 | NR |
| Ercin et al12 (2016) | Turkey | Retrospective case report (4) | 9 | 1/1 | 24 | 1/0 | NR |
| Errando13 (2011) | Spain | Retrospective case report (4) | 8 | 1/1 | 58 | 0/1 | NR |
| Gogia et al14 (2012) | India | Retrospective case report (4) | 8 | 1/1 | 18 | 1/0 | NR |
| Gupta et al15 (2016) | India | Prospective case series (4) | 12 | 36/36 | 47.7 (15-60) | 34/12 | NR |
| Gwak et al16 (2013) | Republic of Korea | Prospective comparative study (2) | 14 | 30/30 | 56 | 13/17 | NR |
| Hynson et al17 (1993) | USA | Retrospective case report (4) | 8 | 1/1 | 46 | 0/1 | NR |
| Ichai et al18 (1996) | France | Retrospective case report (4) | 7 | 1/1 | 40 | 0/1 | NR |
| Jirativanont et al20 (2010) | Thailand | Retrospective case report (4) | 9 | 1/1 | 68 | 1/0 | NR |
| Khan et al22 (2013) | India | Retrospective case report (4) | 9 | 1/1 | 49 | 1/0 | NR |
| Lim et al25 (2006) | Singapore | Retrospective case report (4) | 9 | 1/1 | 18 | 1/0 | 4 |
| Manjuladevi et al26 (2013) | India | Retrospective case report (4) | 8 | 3/3 | 49.3 (43-53) | 2/1 | NR |
| Orebaugh30 (2003) | USA | Retrospective case report (4) | 8 | 1/1 | 49 | 1/0 | NR |
| Ozhan et al31 (2010) | Turkey | Retrospective case report (4) | 9 | 1/1 | 33 | 1/0 | NR |
| Pope and Wottowa33 (2016) | USA | Retrospective case report (4) | 9 | 4/4 | 55.8 (49-64) | 2/2 | 12 |
| Saeki and Kawamoto35 (2011) | Japan | Retrospective case report (4) | 9 | 1/1 | 73 | 1/0 | NR |
| Sharma and Achar36 (2013) | India | Retrospective case report (4) | 9 | 1/1 | 44 | 1/0 | NR |
| Smith and Shah38 (2008) | UK | Prospective case series (4) | 11 | 35/35 | 51.5 (19-83) | 18/17 | NR |
| Sperber and Wredmark39 (1999) | Sweden | Prospective case series (4) | 12 | 11/11 | 46 (31-58) | 10/1 | NR |
| 11/11 | 48 (31-65) | 9/2 | NR | ||||
| Venkat et al40 (2009) | Republic of Korea | Retrospective case report (4) | 8 | 1/1 | 60 | 1/0 | NR |
| Yoshimura et al42 (2005) | Japan | Retrospective case report (4) | 9 | 1/1 | 77 | 0/1 | NR |
aMINORS, Methodological Index for Non-Randomized Studies; NR, not reported.
TABLE 2.
Operative Detailsa
| Author (Year) | Arthroscopic Procedure | Indication for Surgery | Operative Side, Left/Right, n | Patient Positioning | Type of Anesthesia | Operative Time, Mean ± SD (Range), min | Irrigation Fluid | Irrigation Fluid Volume, Mean ± SD (Range), L | Irrigation Pump Pressure, Mean or Range, mm Hg |
|---|---|---|---|---|---|---|---|---|---|
| Antonucci et al1 (2006) | Rotator cuff repair (n = 2) | Rotator cuff tear (n = 2) | 1/1 | Lateral decubitus | Regional nerve block + sedation | 195 | Ringer’s lactate | NR | 36 |
| Blumenthal et al3 (2003) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 1/0 | Lateral decubitus | Regional nerve block | 110 | Ringer’s lactate | NR | NR |
| Borgeat et al4 (2000) | Acromioplasty, rotator cuff repair, acromioclavicular joint resection | Osteoarthritis, rotator cuff tear, impingement syndrome | NR | NR | NR | 120 | Normal saline | NR | 115 |
| Carr and Murphy7 (1995) | Acromioplasty (n = 8), Bankart repair (n = 8), diagnostic ± labral debridement (n = 8) | Impingement syndrome (n = 8), Bankart lesion (n = 8), diagnostic ± labral tear (n = 8) | NR | Lateral decubitus | NR | Acromioplasty: 116.4 (82.2-148.2), Bankart repair: 109.2 (96-142.2), diagnostic ± labral debridement: 87 (16.8-165) | Normal saline with 1% epinephrine | Acromioplasty: 20.75 (7-33), Bankart repair: 23.63 (12-36), diagnostic ± labral debridement: 2.81 (1.5-6) | No |
| Chellam et al8 (2015) | Rotator cuff repair (n = 18), Bankart repair (n = 12), rotator cuff and Bankart repairs (n = 2) | Rotator cuff tear (n = 18), Bankart lesion (n = 12), rotator cuff tear and Bankart lesion (n = 2) | NR | Lateral decubitus | General anesthesia + regional nerve block | 146.9 ± 44.3 | NR | 39.7 ± 20.8 | 30-65 |
| Edwards et al11 (2014) | Arthroscopic capsular release (n = 1) | Adhesive capsulitis (n = 1) | 0/1 | Beach chair | General anesthesia | 2 | Normal saline | NR | 50 |
| Ercin et al12 (2016) | Bankart repair (n = 1) | Labral tear (n = 1) | NR | Beach chair | General anesthesia | 120 | Normal saline | 20 | 48 |
| Errando13 (2011) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 1/0 | Lateral decubitus | Regional nerve block + sedation | 130 | Normal saline | 35 | No |
| Gogia et al14 (2012) | NR | Recurrent dislocations (n = 1) | 1/0 | Lateral decubitus | General anesthesia | 180 | Normal saline with epinephrine 1:3,000,000 | 36 | 91 |
| Gupta et al15 (2016) | NR | NR | NR | Lateral decubitus | General anesthesia | 189.2 (60-390) | Normal saline | 24.6 (5-52) | 40-80 |
| Gwak et al16 (2013) | Rotator cuff repair (n = 30) | Rotator cuff tear (n = 30) | 9/21 | Lateral decubitus | General anesthesia + regional nerve block | 92 | Normal saline | 21 | 80-90 |
| Hynson et al17 (1993) | Arthroscopic surgery and debridement (n = 1) | Chronic pain (n = 1) | 0/1 | Lateral decubitus | Regional nerve block | 105 | Ringer’s lactate with epinephrine 1:1,000,000 | NR | Yes |
| Ichai et al18 (1996) | NR | Chronic pain (n = 1) | 0/1 | NR | General anesthesia + regional nerve block | 90 | 1.5% glycine | 18 | NR |
| Jirativanont et al20 (2010) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 1/0 | NR | General anesthesia + regional nerve block | 240 | NR | 76 | NR |
| Khan et al22 (2013) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 0/1 | Lateral decubitus | General anesthesia | 210 | Normal saline | NR | 91 |
| Lim et al25 (2006) | Labral repair (n = 1) | Labral tear (n = 1) | 0/1 | Lateral decubitus | General anesthesia + regional nerve block | 115 | Sterile water with 0.1 mg/L epinephrine | 22 | 40 |
| Manjuladevi et al26 (2013) | Rotator cuff repair (n = 3) | Rotator cuff tear (n = 3) | 0/3 | NR | General anesthesia | 270 | NR | 56 | NR |
| Orebaugh30 (2003) | Subacromial decompression and rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 0/1 | Beach chair | Regional nerve block + sedation | 180 | Ringer’s lactate with epinephrine | NR | 91 |
| Ozhan et al31 (2010) | Acromioplasty and rotator cuff repair (n = 1) | Impingement syndrome (n = 1) | 0/1 | Beach chair | General anesthesia + regional nerve block | 140 | Ringer’s lactate | NR | 48 |
| Pope and Wottowa33 (2016) | Rotator cuff repair (n = 2), acromioplasty (n = 2) | Rotator cuff tear (n = 2), impingement syndrome (n = 2) | 2/2 | Beach chair | General anesthesia ± regional nerve block | NR | NR | NR | NR |
| Saeki and Kawamoto35 (2011) | NR | NR | 1/0 | Lateral decubitus | General anesthesia + regional nerve block | NR | Normal saline | 24 | 60 |
| Sharma and Achar36 (2013) | Bankart repair (n = 1) | Rotator cuff injury (n = 1) | 1/0 | Lateral decubitus | General anesthesia | NR | Normal saline | 20 | 48 |
| Smith and Shah38 (2008) | Subacromial decompression (n = 25), capsular release (n = 7), diagnostic (n = 2), rotator cuff repair (n = 1) | Impingement syndrome (n = 25), adhesive capsulitis (n = 7), diagnostic (n = 2), rotator cuff tear (n = 1) | 16/19 | Beach chair | Regional nerve block ± general anesthesia | 27.4 (10-63) | Normal saline | 3.2 (1-12) | 50 |
| Sperber and Wredmark39 (1999) | Acromioplasty (n = 11) | Impingement syndrome (n = 11) | NR | Beach chair | General anesthesia | 22 (17-27) | Normal saline with 2% ethanol | NR | 100-150 |
| Acromioplasty (n = 11) | Impingement syndrome (n = 11) | NR | Beach chair | General anesthesia | 35 (27-44) | Normal saline with 2% ethanol | NR | 100-150 | |
| Venkat et al40 (2009) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 1/0 | Lateral decubitus | General anesthesia | 45 | Normal saline with epinephrine 1:300,000 | NR | 100 |
| Yoshimura et al42 (2005) | Rotator cuff repair (n = 1) | Rotator cuff tear (n = 1) | 0/1 | Lateral decubitus | General anesthesia | 95 | Normal saline | NR | 60 |
aNR, not reported.
Operative Details
Of the 205 included patients, 134 were draped in the lateral decubitus position and 63 in the beach-chair position; the positioning for the remaining 8 patients was not reported. Of the 26 included studies, 18 reported the use of an irrigation pump to maintain visualization during the procedure, while the remaining studies either utilized gravity or did not specify a mechanism. The pump pressure reported by these 18 studies ranged from 29 to 150 mm Hg. The irrigation fluid used during the arthroscopic procedures was normal saline in 13 studies, Ringer’s lactate in 5 studies, sterile water in 1 study, glycine in 1 study, and unspecified in 6 studies. Further, of the 26 included studies, 15 reported the total irrigation volume, which ranged from 1 to 76 L. Additionally, 25 studies reported the total operative time, which ranged from 2 to 390 minutes. Operative details are summarized in Table 2.
Clinical Presentation
According to reports of when the signs and symptoms of fluid extravasation first appeared, they were observed midsurgery in 98 patients; after the removal of drapes in 98 patients; after extubation in 5 patients, after which 4 of these patients were reintubated; and 1 day to 3 weeks postoperatively in the remaining 4 patients. The signs and symptoms of fluid extravasation included chest wall swelling in 86 patients, neck swelling in 116 patients, facial swelling in 13 patients, and respiratory distress in 16 patients. Additionally, in 6 patients, the symptoms manifested as chest discomfort; in 4 patients, the symptoms included sore throat; in 4 patients, the symptoms included anterior interosseous nerve palsy; and in 16 patients, the symptoms presented as respiratory distress. The oxygen saturation reported for patients who experienced respiratory distress ranged from 50% to 95%, whereas the oxygen saturation reported for patients who did not experience respiratory distress ranged from 92% to 95%. A pleural effusion was diagnosed in 1 patient, and another patient displayed tracheal deviation as a result of fluid extravasation. Additionally, bradycardia was reported in 2 patients, with both having a heart rate of 38 bpm.
Two studies, including 68 patients, found a significant increase in patients’ neck circumference postoperatively by 1.17 to 2.35 cm.8,15 Two studies, including 71 patients, also found an increase in patients’ weight postoperatively, ranging from 0.9 to 1.17 kg, and they found that both the duration of surgery and the volume of irrigation fluid used significantly correlated with the amount of postoperative weight gain.15,38 Furthermore, these same 2 studies found significant decreases in patients’ hemoglobin and hematocrit levels postoperatively by 0.6 g/dL and 1.5%, respectively; however, there was no significant change in serum sodium levels.15,38 Finally, 2 studies identified significant increases in intramuscular pressure of the shoulder musculature (eg, deltoid, supraspinatus), ranging from increases of 8.63 mm Hg to over 15 mm Hg after arthroscopic surgery.7,39 Clinical presentation details for the studies reviewed are summarized in Table 3.
TABLE 3.
Clinical Presentation of Patientsa
| Author (Year) | Oxygen Saturation, % | Heart Rate, bpm | Blood Pressure, mm Hg | Chest Swelling | Neck Swelling | Face Swelling | Respiratory Distress | Other |
|---|---|---|---|---|---|---|---|---|
| Antonucci et al1 (2006) | 65-75 | 38 | NR | Yes | Yes | Yes | Yes | NR |
| Blumenthal et al3 (2003) | 50 | 38 | NR | Yes | Yes | Yes | Yes | NR |
| Borgeat et al4 (2000) | NR | NR | NR | Yes | Yes | Yes | Yes | NR |
| Carr and Murphy7 (1995) | NR | NR | NR | NR | NR | NR | NR | Mean increase in intramuscular pressure: deltoid, 8.63 mm Hg (P = .001); supraspinatus: 10.46 mm Hg (P < .001) |
| Chellam et al8 (2015) | NR | NR | Hypertension | NR | Yes | NR | NR | Mean increase in neck circumference: 1.17 ± 1.16 cm |
| Edwards et al11 (2014) | NR | NR | NR | Yes | Yes | Yes | NR | Elevation of airway pressure |
| Ercin et al12 (2016) | NR | NR | NR | Yes | NR | NR | NR | NR |
| Errando13 (2011) | 95 | NR | NR | Yes | Yes | NR | NR | Compression of carotid and internal jugular on ultrasound |
| Gogia et al14 (2012) | 70 | NR | NR | NR | Yes | NR | Yes | Pulmonary edema on chest radiography |
| Gupta et al15 (2016) | NR | NR | NR | Yes | Yes | NR | NR | Significant increase in neck, chest, midarm, and midthigh circumferences and weight; significant decrease in hemoglobin levels; no significant change in sodium levels; significant decrease in body temperature and increase in airway pressure |
| Gwak et al16 (2013) | 90-95 | NR | NR | Yes | Yes | NR | Yes (n = 3) | Chest discomfort (n = 6) and sore throat (n = 4); pulmonary function test: restrictive pattern secondary to reduction in static compliance of respiratory system, caused by soft tissue edema around the chest wall, and obstructive pattern, caused by upper airway swelling and edema |
| Hynson et al17 (1993) | NR | NR | 240/100 | Yes | Yes | NR | Yes | NR |
| Ichai et al18 (1996) | NR | NR | SBP 70 | Yes | NR | NR | NR | Cerebral edema and brain death |
| Jirativanont et al20 (2010) | NR | NR | NR | Yes | Yes | Yes | Yes | NR |
| Khan et al22 (2013) | NR | NR | NR | Yes | Yes | Yes | NR | NR |
| Lim et al25 (2006) | NR | NR | NR | Yes | NR | NR | NR | Rhabdomyolysis |
| Manjuladevi et al26 (2013) | NR | NR | NR | Yes | Yes | Yes | Yes | NR |
| Orebaugh30 (2003) | 67 | NR | NR | Yes | Yes | Yes | Yes | Pleural effusion |
| Ozhan et al31 (2010) | 93 | NR | NR | Yes | Yes | Yes | NR | Tracheal deviation |
| Pope and Wottowa33 (2016) | NR | NR | NR | NR | NR | NR | NR | Ipsilateral anterior interosseous nerve palsy |
| Saeki and Kawamoto35 (2011) | NR | NR | NR | Yes | Yes | NR | NR | Increased airway pressure |
| Sharma and Achar36 (2013) | NR | NR | NR | Yes | Yes | NR | NR | NR |
| Smith and Shah38 (2008) | NR | NR | NR | NR | NR | NR | NR | 1.2% weight gain; hemoglobin and hematocrit decreased by 0.6 g/dL (P < .0001) and 1.5% (P < .0001), respectively |
| Sperber and Wredmark39 (1999) | NR | NR | SBP 90 | NR | NR | NR | NR | Shoulder swelling; in 50% of cases, considerable (>15 mm Hg) increase in intramuscular pressure; fluid absorption of 37-100 mL |
| Venkat et al40 (2009) | NR | NR | NR | NR | NR | NR | Yes | NR |
| Yoshimura et al42 (2005) | 92 | NR | SBP 75 | Yes | Yes | Yes | Yes | NR |
aNR, not reported; SBP, systolic blood pressure.
Management and Outcomes
Of the 205 included patients, symptom management was reported for 58 patients. Of these 58 patients, 5 who initially received regional anesthesia were converted to general anesthesia and intubated at the onset of their symptoms. A further 6 patients were reintubated after developing symptoms after extubation. Moreover, 10 patients who were already intubated before the start of the procedure remained intubated postoperatively. The use of nasal prongs was reported for 1 patient, and the data were not routinely measured or reported throughout the other included studies. In total, diuretics were administered to 7 patients to reduce fluid overload, with intravenous furosemide 20 to 40 mg being specified in 6 patients. One patient was given prednisone to reduce airway edema. One patient experienced stridor secondary to fluid accumulation within the soft tissue of the neck, with evidence of internal jugular vein and carotid artery compression, requiring puncture of the soft tissue at the thoracic level with a 14-gauge intravenous catheter. Overall, 32 patients were managed nonoperatively and did not require intubation or medication administration. The time that elapsed until the resolution of edema was reported in 15 patients, which ranged from 2 to 48 hours.
One patient developed rhabdomyolysis, requiring 4 sessions of hemodialysis. Transfer to the intensive care unit was warranted for 14 patients. Additionally, 1 study reported a case of fluid extravasation, resulting in hypervolemic hyponatremia with a sodium level of 116 mmol/L and hematocrit level of 31%.18 Hypertonic saline was administered to the patient, and computed tomography demonstrated severe cerebral edema. The patient received a diagnosis of brain death, as confirmed by cerebral angiography. No long-term complications were reported in the remaining patients. Discharge times were reported for 25 patients, ranging from 1 to 20 days postoperatively. Management and outcome details are summarized in Table 4.
TABLE 4.
Fluid Extravasation Timing, Management, and Outcomesa
| Author (Year) | Timing of Symptom Manifestation | Management | Outcome |
|---|---|---|---|
| Antonucci et al1 (2006) | Midsurgery (procedure suspended) | 1. Induction of general anesthesia; intubation 2. Induction of general anesthesia; intubation | 1. Resolution of edema and extubation after 12 h 2. Resolution of edema and extubation after 24 h |
| Blumenthal et al3 (2003) | Midsurgery | Prednisone 200 mg | Resolution of edema and extubation after 24 h |
| Borgeat et al4 (2000) | Midsurgery (procedure terminated) | Oxygen via nasal prongs | Surgery completed in 2 d via open approach |
| Carr and Murphy7 (1995) | After surgery | NR | No complications (muscular weakness, neurological injury); no relationship between elevated intramuscular pressure and type of surgical procedure, duration of surgery, or fluid volume |
| Chellam et al8 (2015) | After surgery | Intubation (n = 2) | Resolution of edema and extubation on POD 2; hypertension was most direct predictor for the increase in neck circumference (P = .002, r = 0.49) |
| Edwards et al11 (2014) | Midsurgery (procedure terminated) | Intubation | Resolution of edema and extubation after 5 h |
| Ercin et al12 (2016) | Midsurgery (procedure terminated) | Chest radiography | Resolution of edema after 6 h |
| Errando13 (2011) | Removal of drapes | Soft tissue puncture with 14-gauge IV catheter; manual fluid dressing | Resolution of edema in hours |
| Gogia et al14 (2012) | Midsurgery (expedited closure) | Intubation; IV furosemide 40 mg | Resolution of edema and extubation after 24 h; discharged home on POD 2 |
| Gupta et al15 (2016) | Midsurgery | NR | Significant correlation between change in neck circumference and weight gain with amount of irrigation fluid used and duration of surgery |
| Gwak et al16 (2013) | Midsurgery | NR | Patients experienced restrictive and obstructive pulmonary abnormalities |
| Hynson et al17 (1993) | After surgery, after extubation | Intubation | Resolution of edema and extubation after overnight stay |
| Ichai et al18 (1996) | Midsurgery (procedure terminated) | CT of head and angiography; hypertonic saline infusion | Brain death on cerebral angiography; autopsy revealed severe cerebral edema and no other abnormalities |
| Jirativanont et al20 (2010) | After surgery, after extubation | Intubation | Resolution of edema and extubation after 24 h |
| Khan et al22 (2013) | Removal of drapes | Remained intubated; dexamethasone 8 mg; furosemide 20 mg | Resolution of edema and extubation after 16 h |
| Lim et al25 (2006) | Removal of drapes | IV furosemide; 4 sessions of hemodialysis | Resolution of shoulder muscle wasting on MRI after 3 mo |
| Manjuladevi et al26 (2013) | 1. After surgery, after extubation 2. Removal of drapes 3. Removal of drapes | 1. Intubation 2. Remained intubated 3. Remained intubated | 1. Resolution of edema and extubation after 48 h 2. Extubated after 2 h 3. Extubated on POD 1 |
| Orebaugh30 (2003) | Midsurgery (procedure terminated) | Induction of general anesthesia; intubation; diuresis | Resolution of edema after 24 h; extubation on POD 2; discharged home on POD 5 |
| Ozhan et al31 (2010) | Midsurgery | Remained intubated; IV dexamethasone 8 mg; IV furosemide 20 mg | Resolution of edema and extubation after 10 h; discharged home on POD 2 |
| Pope and Wottowa33 (2016) | 1. POD 5 2. Several days postoperatively 3. 2-3 wk postoperatively 4. POD 1 | 1. EMG 2. EMG; right AIN release 3. EMG 4. EMG; right AIN release | 1. FPL strength 4/5 after 15 mo 2. FPL strength 4/5 after 7 mo 3. Complete FPL recovery after 16 mo 4. FPL strength 4/5 after 9 mo |
| Saeki and Kawamoto35 (2011) | Removal of drapes | Remained intubated | Resolution of edema after 12 h |
| Sharma and Achar36 (2013) | Removal of drapes | Remained intubated | Resolution of edema and extubation after 8 h |
| Smith and Shah38 (2008) | After surgery | NR | Strong correlation between amount of fluid used and weight gain (R = 0.89, P < .0001) as well as duration of surgery and weight gain (R = 0.70, P < .0001) |
| Sperber and Wredmark39 (1999) | Midsurgery | NR | Resolution of shoulder swelling on POD 2 |
| Midsurgery | NR | No correlation between operative time and volume of absorption | |
| Venkat et al40 (2009) | After surgery, after extubation | Intubation | Resolution of edema and extubation after 12 h |
| Yoshimura et al42 (2005) | Midsurgery | Intubation; IV suxamethonium 40 mg; IV furosemide 30 mg | Resolution of edema and extubation after 2 h |
aAIN, anterior interosseous nerve; CT, computed tomography; EMG, electromyography; FPL, flexor pollicis longus; IV, intravenous; MRI, magnetic resonance imaging; NR, not reported; POD, postoperative day.
Discussion
The most significant finding of this systematic review was that although fluid extravasation has the potential to be a life-threatening complication of shoulder arthroscopic surgery, it was most commonly managed nonoperatively, and patients’ symptoms ultimately resolved with no evidence of long-term complications. Additionally, patients most commonly experienced neck swelling, chest wall swelling, and, less frequently, respiratory distress. When not managed conservatively, patients were intubated, given diuretics or steroids, or underwent percutaneous drainage of gross neck edema. Clinical edema typically resolved within 24 hours, and patients were often discharged within 1 week. Serious complications, including rhabdomyolysis and death, were rare.
Several reports have previously described common factors that may aid in prognosticating a patient’s risk for experiencing fluid extravasation during an arthroscopic procedure of the shoulder. These include a high pump pressure; a large volume of irrigation fluids; a lengthy operative procedure due to the larger amount of irrigation fluids used; the lateral decubitus position due to gravity-assisted movement of fluid from the shoulder to the neck, although its superiority over the beach-chair position has yet to be proven17,34; obesity; older age and looser subcutaneous soft tissue facilitating fluid movement into the extra-articular space; subacromial arthroscopic surgery due to the lack of encapsulation of the subacromial space, allowing fluid dissection into surrounding tissue27,42; surgery involving resection of the glenohumeral capsule, allowing fluid movement into the extracapsular space17; limited surgeon experience leading to the overuse of irrigation fluid pumps to improve the visual field; and anatomic abnormalities leading to pathological tears in the parascapular musculature or iatrogenic lesions of the deltoid muscle at the acromion.4,11,25
Specifically, pump pressures should be maintained below 150 mm Hg. Additionally, normal saline is the irrigation solution of choice, as acid-base and electrolyte disturbances may occur when excess amounts of solution other than normal saline (eg, Ringer’s lactate) enter the systemic circulation. While there is no established upper limit regarding the amount of irrigation fluid to be used during shoulder arthroscopic surgery, studies of symptomatic patients reported a range of volumes from 20 to 36 L, and as such, volumes lower than 20 L may be considered safe until more precise estimates are determined.12–14 Surgeons may limit the use of irrigation fluid and improve intraoperative visualization by considering the use of electrocautery devices, epinephrine-infused irrigation fluid, and hypotensive anesthesia at the discretion of the anesthesiologist.19,29
Additionally, the authors of one of the included studies suggested that the maximum operative time should be limited to between 90 and 120 minutes based on data trends demonstrating a correlation between length of surgery and fluid extravasation.22 Further, the use of irrigation pump devices may be eliminated entirely and replaced by gravity-driven irrigation systems to limit irrigation fluid pressures.22 Operative techniques that may reduce the inadvertent spread of irrigation fluid to the extra-articular space include the use of longer cannulas to prevent dislodging, avoiding the use of cannulas entirely and using the arthroscope itself for inflow,25,26 minimizing capsular rents, and allowing low continuous outflow so that fluid can exit the subacromial space.14,26 Finally, while it is believed that the lateral decubitus position increases the risk for fluid extravasation, the beach-chair position carries its own risks, of particular concern for anesthesiologists, including the risk of air embolism, hypotension, bradycardia, and central nervous system ischemia.24,32
Anesthesiologists may play a role in preventing fluid extravasation based on their choice of using regional versus general anesthesia. In the setting of fluid extravasation, the benefits of using regional anesthesia alone include not only fewer side effects, a reduced hospital stay, reduced postoperative analgesia requirements, and excellent muscle relaxation but also the ability of the patient to communicate symptoms that he or she may be experiencing, such as throat discomfort, dysphagia, or respiratory difficulties.3,5,10 However, in the event of fluid extravasation, the airway is not initially secured, and subsequent intubation may be difficult because of fluid accumulation in the neck. On the other hand, the initial use of general anesthesia and endotracheal intubation ensures a secure airway, and the use of hypotensive anesthetic agents aid in minimizing the use of irrigation fluid intraoperatively.14 However, it still remains vitally important to carefully examine the patient before extubation for signs of fluid extravasation, and if present, one must consider continued intubation and monitoring in a controlled setting postoperatively until the resolution of edema.26
Intraoperative monitoring of the signs and symptoms of fluid extravasation is central to prompt diagnosis and management, with several recommendations highlighted within the studies included in this review. Specifically, one common issue reported in the literature involved the difficulty of observing impending complications from fluid extravasation while the patient was covered by surgical drapes. This requires continuous monitoring for the swelling of structures near the shoulder, including the neck, face, and chest,1 as well as appropriate placement of surgical drapes to expose the shoulder, structures at the base of the neck,11 and a portion of the nearby chest wall.25 Studies have suggested monitoring for fluid extravasation by checking airway pressures and compliances, looking for airway edema on direct laryngoscopy, intraoperative measurements of patients’ neck circumferences, and observation of fluid extravasation via ultrasound imaging.14,26 Ultimately, patient outcomes may be optimized with efficient communication between anesthesiologists and surgeons as well as preparedness for emergency procedures such as intubation.31
Future Research
While the current literature improves the orthopaedic community’s understanding of fluid extravasation as a complication of shoulder arthroscopic surgery, future research should aim to quantify the degree to which the identified risk factors correlate with this complication. Presently, the literature is mixed, with one study suggesting that some previously identified risk factors, such as fluid volume and procedure duration, do not correlate with rises in intramuscular pressures of the deltoid and supraspinatus muscles.22 As such, large-scale studies and registries are needed to identify the patients most at risk for this complication to implement preventative measures. Additionally, considering that fluid extravasation from shoulder arthroscopic surgery directly poses a risk to airway structures, further research is required to determine the optimal management of the patient from an anesthesiological perspective, including the decision to use general anesthesia or regional anesthesia alone. Future studies should also evaluate anatomic variants that predispose a patient to fluid extravasation as well as long-term sequelae of this complication.
Limitations
The primary limitation of this review on fluid extravasation in shoulder arthroscopy pertains to the low overall level of the current evidence that was available in the literature. Most commonly, the included studies were retrospective case reports, yielding a small overall sample size. Additionally, the reviewed literature was limited to the English language. Furthermore, there was a lack of consistency in the reporting of relevant data, including patient characteristics, operative details, clinical presentation, management strategies, and patient outcomes. Given the heterogeneity of the data, it was not possible to perform a meta-analysis and calculate event rates because of inherent data limits. Moreover, this review may be prone to publication bias, in that the most interesting case reports were available for review, while unpublished, milder cases with uneventful postcomplication courses were not able to be captured.
Conclusion
Fluid extravasation has the potential to be a life-threatening complication of shoulder arthroscopic surgery; however, it is most commonly managed conservatively, and patients’ symptoms ultimately resolve with no evidence of long-term complications in most cases. Intraoperative surgical decisions, such as minimizing the duration of surgery and volume of irrigation fluid used, may limit the occurrence of fluid extravasation, while careful intraoperative monitoring may facilitate prompt diagnosis and management to optimize patient outcomes.
Appendix
TABLE A1.
Detailed Search Strategy
| MEDLINE: 1390 Studies | Embase: 2797 Studies | PubMed: 2203 Studies | |||
|---|---|---|---|---|---|
| Strategy | No. of Studies | Strategy | No. of Studies | Strategy | No. of Studies |
| (1) shoulder.mp. OR Shoulder joint/ OR Shoulder/ | 66,524 | (1) shoulder.mp. OR shoulder/ | 82,687 | (1) shoulder | 66,641 |
| (2) Arthroscopy/ OR arthroscop*.mp. | 30,459 | (2) arthroscopic surgery/ OR arthroscopy/ OR arthroscop*.mp. | 38,728 | (2) arthroscop* | 31,596 |
| (3) fluid.mp. OR extravas*.mp. OR edema.mp. OR Edema/ OR swell*.mp. OR complication*.mp. OR Postoperative Complications/ OR adverse.mp. | 2,227,734 | (3) shoulder arthroscopy/ | 1519 | (3) fluid OR extravas* OR edema* OR swelling OR complication* OR adverse | 4,555,967 |
| (4) 1 AND 2 AND 3 | 1390 | (4) fluid.mp. OR extravasation/ OR extravas*.mp. OR edema/ or edema.mp. OR swell*.mp. OR complication*.mp. OR postoperative complication/ OR adverse.mp. | 4,727,848 | (4) 1 AND 2 AND 3 | 2203 |
| (5) 1 AND 2 | 8258 | ||||
| (6) 3 OR 5 | 8258 | ||||
| (7) 4 AND 6 | 2797 | ||||
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: O.R.A. is a consultant for ConMed, Smith & Nephew, and DJO.
References
- 1. Antonucci S, Orlandi P, Mattei PA, Amato F. Airway obstruction during arthroscopic shoulder surgery: anesthesia for the patient or for the surgeon? Minerva Anestesiol. 2006;72(12):995–1000. [PubMed] [Google Scholar]
- 2. Berjano P, Gonzalez BG, Olmedo JF, Perez-Espana LA, Munilla MG. Complications in arthroscopic shoulder surgery. Arthroscopy. 1998;14(8):785–788. [DOI] [PubMed] [Google Scholar]
- 3. Blumenthal S, Nadig M, Gerber C, Borgeat A. Severe airway obstruction during arthroscopic shoulder surgery. Anesthesiology. 2003;99(6):1455–1456. [DOI] [PubMed] [Google Scholar]
- 4. Borgeat A, Bird P, Ekatodramis G, Dumont C. Tracheal compression caused by periarticular fluid accumulation: a rare complication of shoulder surgery. J Shoulder Elbow Surg. 2000;9(5):443–445. [DOI] [PubMed] [Google Scholar]
- 5. Brown AR, Weiss R, Greenberg C, Flatow EL, Bigliani LU. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9(3):295–300. [DOI] [PubMed] [Google Scholar]
- 6. Carr AJ, Price AJ, Glyn-Jones S, Rees JL. Advances in arthroscopy: indications and therapeutic applications. Nat Rev Rheumatol. 2015;11(2):77–85. [DOI] [PubMed] [Google Scholar]
- 7. Carr CF, Murphy JM. Deltoid and supraspinatus muscle pressures following various arthroscopic shoulder procedures. Arthroscopy. 1995;11(4):401–403. [DOI] [PubMed] [Google Scholar]
- 8. Chellam S, Chiplonkar S, Pathak K. Change in neck circumference after shoulder arthroscopy: an observational study. Indian J Anaesth. 2015;59(6):365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiplonkar S, Pathak K, Chellam S. Change in neck circumference after shoulder arthroscopy: an observational study. Indian J Anaesth. 2015;59(6):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conn RA, Cofield RH, Byer DE, Linstromberg JW. Interscalene block anesthesia for shoulder surgery. Clin Orthop Relat Res. 1987;(216):94–98. [PubMed] [Google Scholar]
- 11. Edwards DS, Davis I, Jones NA, Simon DW. Rapid tracheal deviation and airway compromise due to fluid extravasation during shoulder arthroscopy. J Shoulder Elbow Surg. 2014;23(7):e163–e165. [DOI] [PubMed] [Google Scholar]
- 12. Ercin E, Bilgili MG, Ones HN, Kural C. Postoperative pectoral swelling after shoulder arthroscopy. Joints. 2016;3(3):158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Errando CL. Ultrasound observation of tissue fluid infiltration causing stridor in a woman undergoing shoulder arthroscopy. Rev Esp Anestesiol Reanim. 2011;58(9):582–584. [DOI] [PubMed] [Google Scholar]
- 14. Gogia A, Bajaj J, Sahni A, Saigal D. Negative-pressure pulmonary oedema in a patient undergoing shoulder arthroscopy. Indian J Anaesth. 2012;56(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gupta S, Manjuladevi M, Vasudeva Upadhyaya KS, Kutappa AM, Amaravathi R, Arpana J. Effects of irrigation fluid in shoulder arthroscopy. Indian J Anaesth. 2016;60(3):194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gwak MS, Kim WH, Choi SJ, et al. Arthroscopic shoulder surgery under general anesthesia with brachial plexus block. Anaesthesist. 2013;62(2):113–120. [DOI] [PubMed] [Google Scholar]
- 17. Hynson JM, Tung A, Guevara JE, Katz JA, Glick JM, Shapiro WA. Complete airway obstruction during arthroscopic shoulder surgery. Anesth Analg. 1993;76(4):875–878. [DOI] [PubMed] [Google Scholar]
- 18. Ichai C, Ciais JF, Roussel LJ, et al. Intravascular absorption of glycine irrigating solution during shoulder arthroscopy: a case report and follow-up study. Anesthesiology. 1996;85(6):1481–1485. [DOI] [PubMed] [Google Scholar]
- 19. Jensen KH, Werther K, Stryger V, Schultz K, Falkenberg B. Arthroscopic shoulder surgery with epinephrine saline irrigation. Arthroscopy. 2001;17(6):578–581. [DOI] [PubMed] [Google Scholar]
- 20. Jirativanont T, Tritrakarn TD. Upper airway obstruction following arthroscopic rotator cuff repair due to excess irrigation fluid. Anaesth Intensive Care. 2010;38(5):957–958. [PubMed] [Google Scholar]
- 21. Kay J, De Sa D, Memon M, Simunovic N, Paul J, Ayeni OR. Examining the role of perioperative nerve blocks in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(4):704–715.e1. [DOI] [PubMed] [Google Scholar]
- 22. Khan F, Padmanabha S, Shantaram M, Aravind M. Airway compromise due to irrigation fluid extravasation following shoulder arthroscopy. J Anaesthesiol Clin Pharmacol. 2013;29(4):578–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 24. Liguori GA, Kahn RL, Gordon J, Gordon MA, Urban MK. The use of metoprolol and glycopyrrolate to prevent hypotensive/bradycardic events during shoulder arthroscopy in the sitting position under interscalene block. Anesth Analg. 1998;87(6):1320–1325. [DOI] [PubMed] [Google Scholar]
- 25. Lim J-K, Ang K-C, Wang S-C, Kumar VP. Rhabdomyolysis following shoulder arthroscopy. Arthroscopy. 2006;22(12):1366.e1–5. [DOI] [PubMed] [Google Scholar]
- 26. Manjuladevi M, Gupta S, Upadhyaya KV, Kutappa AM. Postoperative airway compromise in shoulder arthroscopy: a case series. Indian J Anaesth. 2013;57(1):52–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matthews LS, Fadale PD. Subacromial anatomy for the arthroscopist. Arthroscopy. 1989;5(1):36–40. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint--Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. [PubMed] [Google Scholar]
- 29. Morrison DS, Schaefer RK, Friedman RL. The relationship between subacromial space pressure, blood pressure, and visual clarity during arthroscopic subacromial decompression. Arthroscopy. 1995;11(5):557–560. [DOI] [PubMed] [Google Scholar]
- 30. Orebaugh SL. Life-threatening airway edema resulting from prolonged shoulder arthroscopy. Anesthesiology. 2003;99(6):1456–1458. [DOI] [PubMed] [Google Scholar]
- 31. Ozhan MO, Suzer MA, Cekmen N, Caparlar CO, Eskin MB. Tracheal compression during shoulder arthroscopy in the beach-chair position. Curr Ther Res Clin Exp. 2010;71(6):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463–469. [DOI] [PubMed] [Google Scholar]
- 33. Pope D, Wottowa C. Mixed neuropathy presenting clinically as an anterior interosseous nerve palsy following shoulder arthroscopy: a report of four cases. J Shoulder Elbow Surg. 2016;25(10):1699–1703. [DOI] [PubMed] [Google Scholar]
- 34. Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2011;27(4):532–541. [DOI] [PubMed] [Google Scholar]
- 35. Saeki N, Kawamoto M. Tracheal obstruction caused by fluid extravasation during shoulder arthroscopy. Anaesth Intensive Care. 2011;39(2):317–318. [PubMed] [Google Scholar]
- 36. Sharma M, Achar SK. Airway oedema during shoulder arthroscopy: how we played it safe! Indian J Anaesth. 2013;57(3):319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological Index for Non-Randomized Studies (MINORS): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 38. Smith CD, Shah MM. Fluid gain during routine shoulder arthroscopy. J Shoulder Elbow Surg. 2008;17(3):415–417. [DOI] [PubMed] [Google Scholar]
- 39. Sperber A, Wredmark T. Intramuscular pressure and fluid absorption during arthroscopic acromioplasty. J Shoulder Elbow Surg. 1999;8(5):414–418. [DOI] [PubMed] [Google Scholar]
- 40. Venkat G, Moon YL, Na WC, So KY. Upper airway compromise by extravasated fluid: a rare complication after arthroscopic repair of atrophic cuff tear. Orthopedics. 2009;32(10):776–778. [DOI] [PubMed] [Google Scholar]
- 41. Wright JG. Levels of evidence and grades of recommendations. Available at: http://www2.aaos.org/bulletin/apr05/fline9.asp. Accessed December 20, 2015.
- 42. Yoshimura E, Yano T, Ichinose K, Ushijima K. Airway obstruction involving a laryngeal mask airway during arthroscopic shoulder surgery. J Anesth. 2005;19(4):325–327. [DOI] [PubMed] [Google Scholar]