Abstract
Type 2 diabetes (T2D) is associated with numerous comorbidities that significantly reduce quality of life, increase mortality and complicate treatment decisions. In a recent cardiovascular outcomes trial, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin was shown to reduce cardiovascular (CV) mortality and heart failure in high-risk patients with T2D with a previous CV event or with established CV disease (CVD). Recently published data from the Canagliflozin Cardiovascular Assessment Study (CANVAS-PROGRAM) study suggested that the cardiovascular benefits of empagliflozin are also seen with the SGLT2-inhibitor canagliflozin, indicating a class effect of SGLT2 inhibitors. Evidence for a class effect has also been shown by meta-analyses and real-world studies, including the Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL) and The Health Improvement Network (THIN) databases. These findings also suggest the results of EMPA-REG OUTCOME can be applied to patients with T2D with a broader CV risk profile, including people at low risk of CVD.
Keywords: Cardiovascular outcome trials, dapagliflozin, empagliflozin, canagliflozin, real-world data, sodium-glucose cotransporter 2, type 2 diabetes
People with type 2 diabetes mellitus (T2D) have a two- to four-fold increased risk for coronary heart disease compared to those without diabetes,1–3 as well as other vascular disorders (consisting of heart failure [HF], cardiac dysrhythmia, sudden death, hypertensive disease, pulmonary embolism, and aortic aneurysm). Heart failure is a particularly common complication of T2D and is associated with poor outcomes.4,5 The risk associated with diabetes is higher at younger ages and lower at higher ages. For instance, at the age of 60 years, a patient with T2D and cardiovascular disease (CVD) has a reduced life expectancy of 12 years compared with the general population, according to a study by the Emerging Risk Factors Collaboration (689,300 participants; 91 European cohorts),6 and of 2 years at age 67 years in Sweden.3 There is, therefore, a need for novel treatments for T2D that not only improve glycaemic control but also reduce the risk of CVD.
Historically, the aim of glucose-lowering therapy in diabetes was to reduce microvascular complications and interventional studies focused on intensive glucose reduction in T2D have only had a minor or no effect in reducing cardiovascular (CV) risk.7–9 In 1998, the UK Prospective Diabetes Study (UKPDS) found that a subgroup of obese patients randomised to metformin had a reduction in myocardial infarction (MI).10 Metformin has since become the standard first-line drug treatment for T2D.11 However, most people taking metformin ultimately require intensified treatment due to disease progression and insufficient glycaemic control. In the last two decades, numerous therapeutic options have emerged for T2D, including dipeptidyl peptidase 4 inhibitors (DPP-4i), glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
In 2008, following the withdrawal of rosiglitazone from the market because of its association with increased risk of HF and MI,12 the US Food and Drug Administration (FDA) mandated CV outcome trials (CVOTs) on glucose-lowering drugs (GLDs).13 In 2012, the European Medicines Agency (EMA) also published a guideline requiring CVOTs for new GLDs for which specific CV claims are made or that are suspected of having detrimental CV effects.14 As a result, two drugs – empagliflozin (an SGLT2 inhibitor) and liraglutide (a GLP-1 receptor agonist) – have a level A recommendation in the American Diabetes Association (ADA) 2018 Standards of Medical Care in Diabetes.15 This article discusses the findings of the two completed CVOTs on SGLT2 inhibitors to date, their applicability to real-world clinical practice, and the findings of real-world studies that included patients with T2D with a broader CV risk profile.
Cardiovascular outcome trials on sodium-glucose cotransporter 2 inhibitors
Early CVOTs of saxagliptin,16 alogliptin,17 sitagliptin18 and lixisenatide19 demonstrated the safety of GLDs but did not show superiority in CV outcomes compared with placebo. In 2015 the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME),20 showed a 38% relative risk reduction in CV death (hazard ratio [HR], 0.62; 95% confidence interval [CI], 0.49–0.77; p<0.001) and a 32% relative risk reduction in all–cause mortality (HR, 0.68; 95% CI, 0.57–0.82, p<0.001), as well as a 55% reduction in HF hospitalisations (HR, 0.65; 95% CI, 0.50–0.85; p=0.002). The effects were similar for both doses of empagliflozin, 10 mg or 25 mg once daily. These were surprising and unprecedented findings and attracted considerable attention. The EMPA-REG OUTCOME study involved 7,020 patients with T2D and established CVD who were on standard-of-care medications for both hypertension and dyslipidaemia. The size of the effect and the rapid onset of action of empagliflozin were almost certainly not due to glucose lowering, and prompted questions about its underlying cardioprotective actions, and whether the reported CVD benefits were intrinsic to empagliflozin or represented a class effect common to all SGLT2 inhibitors. In addition, EMPA-REG OUTCOME involved predominantly older patients with long-standing diabetes and 99% with a prior CVD event (MI, stroke, amputation, multivessel coronary artery disease, or coronary artery bypass graft), including HF in 10% of subjects. This led to speculation about the potential CV benefits of empagliflozin in lower risk patients.
As a class, the SGLT2 inhibitors – empagliflozin, dapagliflozin and canagliflozin are currently approved by the FDA and EMA although others are in clinical development – share the same mechanism of action. They all decrease renal reabsorption of glucose and induce an increase in glycosuria (Table 1).21–23 Approximately 90% of filtered glucose is absorbed by SGLT2 with the remaining 10% absorbed by SGLT1.23 By inhibiting this process, SGLT2 inhibitors reduce renal glucose reabsorption, increase urinary glucose excretion and reduce serum glucose concentration. They also affect several CV risk factors, including lowering glucose and blood pressure (BP), altering fasting lipid parameters, decreasing arterial stiffness, weight and visceral adiposity, and decreasing albuminuria and serum uric acid levels (Table 1).21,24–30 No significant differences have been observed in glucose lowering, body weight loss, and BP reduction among the individual SGLT2 inhibitors.21 It has also been suggested that treatment with SGLT2 inhibitors ameliorates oxidative stress31 and encourages a shift in fuel metabolism from fatty acids to beta-hydroxybutyrate in the heart, as well as other organs.32 Other hypotheses focus on the potential haemodynamic benefits including reduced pre- and afterload, enhanced myocardial oxygen supply and potential improvements of SGLT2 inhibitors in cardiac systolic and diastolic function.33 An additional interesting hypothesis is that empagliflozin reduces sodium and calcium overload in the myocardial cell and increases concentration of calcium in the myocardial mitochondria and thereby reducing HF development.34 If the observed CV benefits are related to the glucuretic/natriuretic effect and subsequent volume depletion together with reduction of systolic BP or via metabolic effects, they are likely to be common to all SGLT2 inhibitors. However, different drugs may have different specificity for SGLT2 and SGLT1,35 as well as different benefit/risk profiles, therefore it is impossible to draw conclusions based on the EMPA-REG OUTCOME study alone.
Table 1: Cardiovascular effects of sodium-glucose cotransporter 2 inhibitors based on clinical and mechanistic studies.
| CV effect | Reference |
|---|---|
| Blood pressure ↓ | 26,27 |
| Arterial stiffness ↓ | 30 |
| Glucose and insulin ↓ | 26,28 |
| Albuminuria ↓ | 27 |
| Uric acid ↓ | 21,26 |
| Weight ↓ | 29 |
| Visceral adiposity ↓ | 29 |
| Oxidative stress ↓ | 31 |
| Triglycerides ↓ | 25,26 |
| LDL-C ↑ | 24,25 |
| HDL-C ↑ | 25,26,28 |
CV = cardiovascular; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; SNS = sympathetic nervous system
The Canagliflozin Cardiovascular Assessment Study (CANVAS-PROGRAM) was published in June 2017 (Table 2).36 The rate of the primary endpoint, a composite of death from cardiovascular causes, nonfatal MI, or nonfatal stroke, was significantly lower with canagliflozin than with placebo for both doses pooled (26.9 versus 31.5 participants per 1000 patient-years; HR 0.86; 95% CI, 0.75–0.97; p<0.001 for noninferiority; p=0.02 for superiority). While these data suggest that a class effect of SGLT2 inhibitors is likely, unlike in EMPA-REG OUTCOME the reductions in the individual end points of all-cause and cardiovascular death were not significant in CANVAS-PROGRAM, although the point-estimates were in the same direction as in the EMPA-REG OUTCOME. One possible explanation for this is that, while almost all of the EMPA-REG OUTCOME subjects had established CVD, approximately 20% of the CANVAS-PROGRAM were at increased risk for cardiovascular events and around two-thirds had no prior history of CVD and were accordingly at lower CV-risk and event rates, reducing the statistical power. However, there were some notable differences between the safety findings of the two trials, with an increased risk of amputation with canagliflozin (6.3 versus 3.4 participants per 1000 patient-years; HR, 1.97; 95% CI, 1.41–2.75); amputations were primarily at the level of the toe or metatarsal. This was an unexpected finding, raising the question of whether it was a random finding or compound-specific, suggesting that important differences may exist between the SGLT2 inhibitors, and requires further investigation.
Table 2: Summary of key studies investigating the cardiovascular effects of sodium-glucose cotransporter 2 inhibitors.
| Study | Study type | Patient population | Inclusion criteria | Exclusion criteria | Key findings |
|---|---|---|---|---|---|
| CVOT | |||||
| EMPA-REG OUTCOME20 | Phase III clinical trial | n=7,020; >99% had established CVD | Diagnosis of T2D prior to informed consent Male or female patients on diet and exercise regimen who are drug-naive or pre-treated with any background therapy. Antidiabetic therapy has to be unchanged for 12 weeks prior to randomization. HbA1c of ≥7.0% and ≥10% for patients on background therapy or HbA1c ≥7.0% and ≤9.0% for drug naive patients Age ≥18 years BMI ≤45 at visit one Signed and dated informed consent High CV risk |
Uncontrolled hyperglycaemia with a glucose level >240 mg/dl (>13.3 mmol/L) after an overnight fast during placebo run-in and confirmed by a second measurement (not on the same day) Indication of liver disease, defined by serum levels of either ALT, aspartate aminotransferase or alkaline phosphatase above 3 x ULN as determined at screening and/or run in Planned cardiac surgery or angioplasty within 3 months Impaired renal function, defined as GFR <30 ml/min (severe renal impairment, Modification of Diet in Renal Disease formula) during screening or run in Bariatric surgery within the past two years and other gastrointestinal surgeries that induce chronic malabsorption Blood dyscrasias or any disorders causing haemolysis or unstable Red Blood Cell (e.g. malaria, babesiosis, haemolytic anaemia) Medical history of cancer (except for basal cell carcinoma) and/or treatment for cancer within the last 5 years Contraindications to background therapy according to the local label Treatment with anti-obesity drugs (e.g. sibutramine, orlistat) 3 months prior to informed consent or any other treatment at the time of screening (i.e. surgery, aggressive diet regimen, etc.) leading to unstable body weight Current treatment with systemic steroids at time of informed consent or change in dosage of thyroid hormones within 6 weeks prior to informed consent or any other uncontrolled endocrine disorder except T2D Pre-menopausal women (last menstruation <1 year prior to informed consent) who:
Participation in another trial with an investigational drug within 30 days prior to informed consent Any other clinical condition that would jeopardize patients' safety while participating in this clinical trial Acute coronary syndrome, stroke or transient ischaemic attack within 2 months prior to informed consent |
14% reduction composite of death from CV causes, nonfatal MI, or nonfatal stroke for canagliflozin versus placebo; 38% risk reduction in CV death, 35% reduction in heart failure hospitalisation and 32% risk reduction in all-cause mortality |
| CANVAS-PROGRAM36 | Phase III clinical trial | n=10,142; 65.6% had a history of CVD | Patients must have a diagnosis of T2D and ≥30 years old with history of CV event, or ≥50 years old with high risk of CV events Patients must have inadequate diabetes control (as defined by glycosylated haemoglobin greater than or equal to 7.0% to less than or equal to 10.5% at screening) and be either not currently on diabetes drug therapy or on therapy with any approved class of diabetes drugs |
A history of diabetic ketoacidosis, T1D, pancreas or beta-cell transplantation, or diabetes secondary to pancreatitis or pancreatectomy History of one or more severe hypoglycaemic (i.e. very low blood sugar) episode within 6 months before screening |
14% relative risk reduction in composite of death from CV causes, nonfatal MI, or nonfatal stroke for canagliflozin versus placebo |
| DECLARE TIMI-5858 | Phase III clinical trial | n=17,276; established CVD (40% have had a prior CV event) or multiple CV risk factors | Provision of informed consent prior to any study specific procedures Female or male aged ≥40 years Diagnosed with T2D High risk for CV events |
Diagnosis of T1D History of bladder cancer or history of radiation therapy to the lower abdomen or pelvis at any time Chronic cystitis and/or recurrent urinary tract infections Pregnant or breast-feeding patients |
Results not available yet |
| Meta-analyses of RCTs | |||||
| Meta-analysis of all SLGT2 inhibitors37 | Meta-analysis | n=70,910 | 16% relative risk reduction in MACE, 37% reduction in CV death | ||
| Meta-analysis of dapagliflozin38 | Meta-analysis | n=9,339 | 23% relative risk reduction in MACE | ||
| Real-world evidence | |||||
| CVD-REAL46 | Real-world observational study | n=309,056; 87% did not have a history of CVD | New user receiving or dispensed prescription of SGLT2 inhibitor medication or other glucose lowering drug, oral as well as injectable, including FDC products containing these medication groups T2D diagnosis on or prior to the index date ≥18 years old at index date >1 year of data history in the database prior to the index date |
Patients with a T1D diagnosis Patients with gestational diabetes within 1 year before index date |
39% reduced risk of HF hospitalisation, 51% lower risk of all-cause death in patients taking SGLT2 inhibitors |
| THIN49 | Real-world observational study | n=22,124; 20% had a previous CV event | Aged 18+ years at the index date, A diagnosis of T2D any time before the index date had been initiated treatment with dapagliflozin, remained at their practice at least 3 months after treatment initiation |
Patients with a T1D diagnosis | 50% lower risk of all-cause death |
| Swedish registry50 | Real-world observational study | n=37,603; CVD in 33% | A diagnosis of T2D Prescription for either DPP-4i-or SGLT2i, or insulin, 1 July 2013 to 31 December 2014 |
Patients with a diagnosis of gestational diabetes. Within one year of the index date Patients with T1D were excluded |
56% reduced risk of all-cause mortality and 49% reduced risk of CVD with dapagliflozin versus insulin |
ALT = alanine aminotransferase; BMI = body mass index; CANVAS-PROGRAM = Canagliflozin Cardiovascular Assessment Study; CV = cardiovascular; CVD = cardiovascular disease; CVD-REAL = Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors; CVOT = cardiovascular outcome trial; DECLARE = Dapagliflozin Effect on CardiovascuLAR Events; DPP-4i = dipeptidyl peptidase-4 inhibitors; EMPA-REG OUTCOME = Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; FDC = fixed-dose combinations; GFR = glomerular filtration rate; HbA1C = glycosylated haemoglobin; HF = heart failure; MACE = major adverse cardiac events; MI = myocardial infarction; RCT = randomised controlled trial; SGLT2 = sodium-glucose cotransporter 2; SGLT2i = sodium-glucose cotransporter 2 inhibitor; T1D = type 1 diabetes; T2D = type 2 diabetes; TIMI = Thrombolysis in Myocardial Infarction; THIN = The Health Improvement Network; ULN = upper limit of normal.
The ongoing large CV outcome trial Dapagliflozin Effect on Cardiovascular Events (DECLARE)-Thrombolysis in Myocardial Infarction (TIMI)-58 is investigating the effect of dapagliflozin on CV outcomes in a broad population of patients with either established CVD (40% have had a prior CV event) or multiple CV risk factors, with an estimated completion date of 2019 and will give further answers to the effect of SGLT2 inhibitors in a low risk T2D population and whether a class effect exists (NCT01032629).
Meta-analyses
A recent meta-analysis and systematic review included data from six regulatory submissions (n=37,525) and 57 clinical trials (n=33,385) and involved seven different SGLT2 inhibitors. The SGLT2 inhibitors were found to reduce the risk of major adverse CV events (MACE; relative risk 0.84 [95% CI 0.75–0.95]; p=0.006), CV death (relative risk 0.63 [0.51–0.77]; p<0.0001), HF (0.65 [0.50–0.85]; p=0.002), and death from any cause (relative risk 0.71 [0.61–0.83]; p<0.0001). While the efficacy results were largely driven by empagliflozin, results for other SGLT2 inhibitors were not clearly different, supporting the hypothesis that that the CV benefits of SGLT2 inhibitors are consistent across the drug class.37 In addition, a meta-analysis investigating the CV safety of dapagliflozin analysed data from 21 clinical trials (n=9,339), including five phase IIb studies of 12 to 24 weeks duration and 16 phase III studies of up to 208 weeks duration. At baseline, 3,214 patients (34.4%) had a history of CVD (coronary artery disease, cerebrovascular disease, peripheral vascular disease or congestive HF). The results showed that dapagliflozin was not associated with increased CV risk and may have a beneficial effect both in the overall population, although confidence limits were overlapping 1.00 (HR 0.77; 95% CI 0.54–1.10 for MACE) and in those with a history of CVD (HR 0.80; 95% CI 0.53–1.22).38 These results are consistent with meta-analyses of the CV effect of other SGLT2 inhibitors.39
Real-world evidence
As analytical tools continue to grow in power and sophistication, real-world evidence is an important component of pharmaceutical product development and is increasingly used to support regulatory decision-making.11,40–42 Real-world studies are considered statistically less rigorous than randomised controls and may have inadvertent biases. Two important forms of bias in this respect that may lead to overestimation of differences between two treatment strategies are the so-called immortal time bias and the time-lag bias.43 Furthermore, there can be differences between studies in clinical practice and the quality and detail of the data, making studies hard to interpret.44 Despite these limitations, however, real-world data have the potential to improve clinical outcomes by increasing the understanding of how best to incorporate new therapies into everyday clinical practice. These data help fill the knowledge gap between clinical trials and actual clinical practice.45 While clinical trials remain the gold standard for drug approval, real-world studies can provide valuable information on how drugs perform within specific subgroups often excluded in clinical trials (age, gender, differences in disease severity, comorbidities, differences in therapeutic adherence). Real-world studies are useful for assessing long-term response and safety, as well as for generating hypotheses to guide future clinical directions.
Four recent real-world studies have added to the body of evidence in support of the hypothesis that SGLT2 inhibitors exert their CV benefits via a class effect (Table 2). The Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL) study is the first large real-world study of patients with T2D.46 It collected real-world data from six countries and more than 300,000 patients, 87% of whom did not have a history of CVD. Inclusion criteria were new users receiving a SGLT2 inhibitor or other GLDs with established T2D on or prior to the index date, age ≥18 years and availability of >1 year (6 months in Germany) historical data prior to the index date. The study period and inclusion of patients was 2012-2015, thus in large part before the publication of the EMPA-REG OUTCOME study and not influenced by the results or inclusion criteria in that study. Patients in the SGLT2 inhibitor and GLD groups were matched by propensity score analysis. In the primary analysis, patients prescribed SGLT2 inhibitors had a 39% lower associated risk for HF hospitalisation compared with other GLDs (HR 0.61, p<0.001). For all analyses (multivariable adjusted, intention to treat, stepwise removal of thiazolidinedione, insulin and sulfonylurea from the control group), SGLT2 inhibitors showed lower incidences of HF hospitalisations than other GLDs (p<0.001). In addition, patients in the SGLT2 inhibitor group had lower associated risk of all-cause death compared with other GLDs (HR 0.49, p<0.001).46
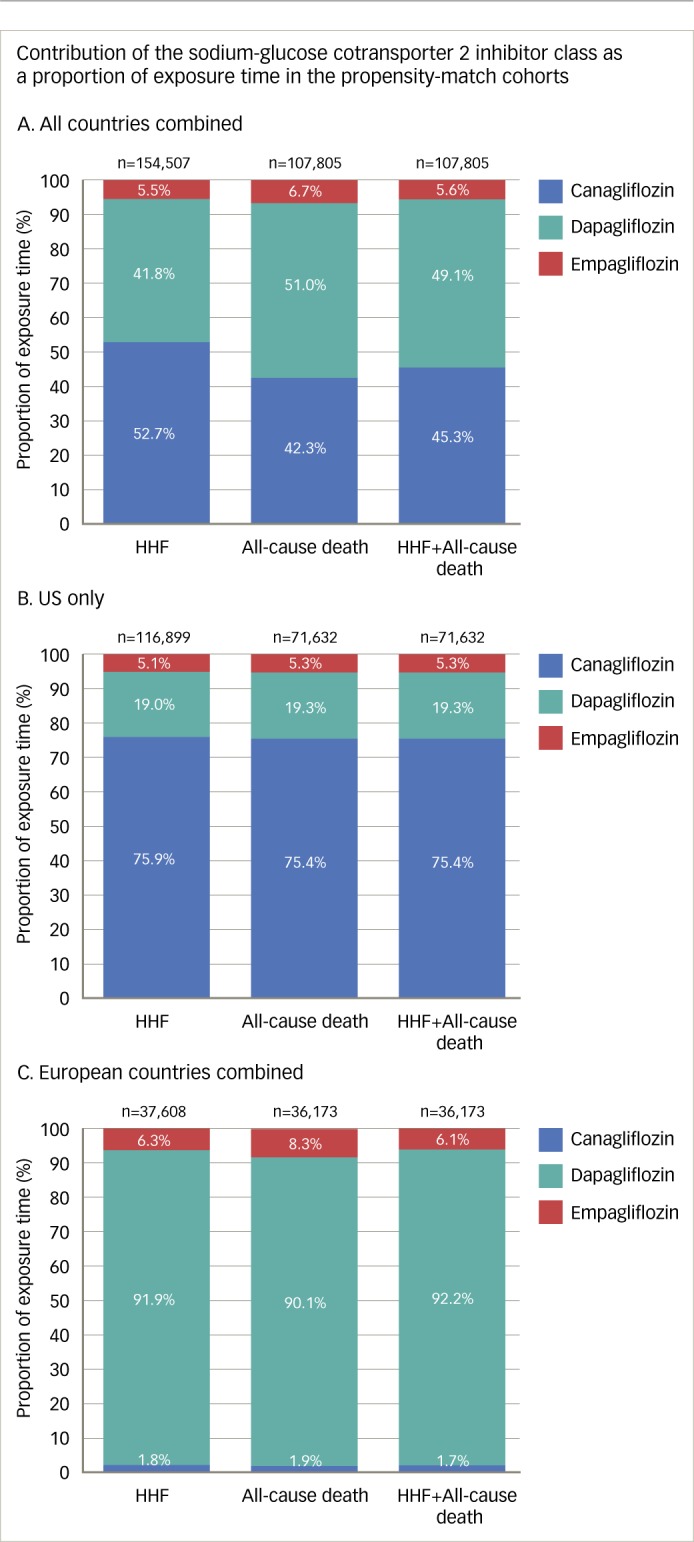
One limitation of the CVD-REAL study was that other CV events such as MI and stroke were not examined, however HF is the most common CV comorbidity in T2D.4 Importantly, there was no significant heterogeneity in findings across countries, despite geographic variations in the use of SGLT2 inhibitors (76% canagliflozin in the US and ~92% dapagliflozin in Europe, with empagliflozin accounting for <7% of total exposure time; Figure 1). Furthermore, there is no information of the dose distribution of the patients treated with canagliflozin and empagliflozin.46 These findings suggest that the observed CV benefits are likely to be class related. The benefits in terms of HF hospitalisation and all-cause mortality were strikingly similar to those reported in EMPA-REG OUTCOME (where both doses of empagliflozin had very similar effects), suggesting that the benefits translate to real-world clinical practice. In addition, the study involved a broad population of patients with T2D, the majority (87%) of whom did not have known CVD, suggesting that the benefits of SGLT2 inhibitors might extend to those with a lower risk of CVD. The CVD-REAL study thus suggests that the beneficial effects of SGLT2 inhibitors may be extended to broader and lower risk group of patients with T2D than previously evaluated in clinical trials.
Figure 1: The CVD-REAL study.
The CVD-REAL Nordic study identified all patients with T2D who were prescribed GLDs between 2012 and 2015 from the Prescribed Drug Registries and linked them with the National Patient Registry and Cause of Death Registry in Denmark, Norway and Sweden (n=91,320). This represented a broad T2D population and included 25% with prior registered established CVD. New use of SGLT2 inhibitors was associated with lower risk of MACE compared to new use of another GLD, and in a similar magnitude as in the EMPA-REG OUTCOME and CANVAS-PROGRAM.47
Recently the CVD-REAL Nordic study group presented data showing that, compared with new use of DPP-4i, new use of dapagliflozin was associated with lower risk for HF hospitalisation (HR 0.62; 95% CI, 0.50–0.77 p<0.001); MACE (HR 0.79; 95% CI, 0.67-0.94; p=0.006); and all-cause mortality (HR 0.59; 95% CI, 0.49-0.72 p=0.004).48 Again, these findings were similar to those in EMPA-REG OUTCOME but in contrast to a placebo as a comparator, the comparator was single group of compounds with CV-safe data — the DPP-4i.
Reproduced under an open access license from Kosiborod et al., 2017.46
HHF = hospitalisation for heart failure; SGLT2 = sodium-glucose cotransporter-2.
Another study investigated all-cause mortality in patients with T2D taking dapagliflozin from The Health Improvement Network (THIN) database. This was a UK population-based, retrospective open cohort study (n=22,124) that compared outcomes in patients with T2D taking dapagliflozin with matched controls with T2D who were taking other GLDs.49 The study included any patient administered dapagliflozin at any time point between 1 January 2013 and 1 September 2015. Inclusion criteria were: age ≥18 years at the index date, a diagnosis of T2D, having being registered at their practice at a year prior to treatment initiation and to remain at the practice for at least 3 months after treatment initiation. The mean age of participants was 58.4 years and the mean duration of diabetes was approximately 9 years. Approximately 20% of the study population (n=4,350) had a previous CV event (ischaemic heart disease, stroke and/or HF).
Data from THIN over a median observation period of 1 year showed that all-cause mortality was significantly lower in patients taking dapagliflozin than those taking other GLDs (adjusted incidence rate ratio [IRR] 0.50, 95% CI: 0.33–0.75, p=0.001), similar to the mortality reductions reported in EMPA-REG OUTCOME.49 The risk of all-cause mortality was also lower in patients taking dapagliflozin in the low CV risk population, (IRR 0.44, 95% CI: 0.25–0.78, p=0.002). The low risk population was defined as the absence of all CVD outcomes (MI and ischaemic heart disease, stroke and transient ischaemic attack [TIA] and HF) at baseline. Although showing a similar numerical trend, no significant difference in the risk of CV events was detected between low risk patients who received dapagliflozin and matched controls receiving GLDs. This observational study therefore suggested that the CV benefits of empagliflozin in the EMPA-REG OUTCOME study are seen with dapagliflozin in both high as well as low risk patients.
A report of a Swedish registry study that investigated the association of oral GLDs, compared with that of insulin, with the risk of all-cause mortality, CVD and severe hypoglycaemia has also been published recently.50 The study included all patients with T2D who were new users of either DPP-4i or SGLT2 inhibitors (only dapagliflozin was available in Sweden during the study period) during 2013-2014. Of 37,603 participants, 21,758 were matched 1:1 to DPP-4i/dapagliflozin versus insulin groups, with median follow-up times of 1.51 years and 1.53 years respectively. The DPP-4i/dapagliflozin group was associated with a reduced risk of all-cause mortality, CVD and hypoglycaemia compared to the insulin group (HR [95% CI] 0.56 [0.49–0.64]), 0.85 [0.73–0.99] and 0.26 [0.12–0.57] respectively. In separate analyses for the two drug classes, dapagliflozin was associated with lower risks of both all-cause mortality and CVD (HR 0.44 [0.28–0.70]) and 0.51 [0.30–0.86]) respectively, while DPP-4i use was associated with lower risk of all-cause mortality (HR 0.59 [0.51–0.67]), but not with CVD (HR 0.87 [0.75–1.01]).50
Finally, another recently published retrospective cohort study in the US (118,018 new users of SGLT2 inhibitors, including 73,024 taking canagliflozin) found no evidence of increased risk of below-knee lower extremity amputation for new users of canagliflozin compared with non-SGLT2 inhibitor antihyperglycaemic agents in a broad population of patients with T2D.51
Clinical implications of the totality of evidence
The findings of EMPA-REG OUTCOME are potentially paradigm changing, for the first time showing a reduction in CV events and mortality in patients with T2D with established CVD and have already influenced diabetes and cardiovascular guidelines.52–54 The recent CANVAS-PROGRAM data suggest that the cardiovascular benefits observed in EMPA-REG OUTCOME is likely to be a class effect, and its broader inclusion criteria suggests that the CV benefits of SGLT2 inhibitors may extend to lower risk patients. Real-world studies have further extended the inclusion criteria of the EMPA-REG OUTCOME and CANVAS-PROGRAM. These data suggest that the CV benefits of SGLT2 inhibitors may also be applicable to the general diabetic population, not only those with established CVD, a finding that should be confirmed in a clinical trial setting. The CVD-REAL data were consistent across countries although there was considerable heterogeneity of use of specific SGLT2 inhibitors, adding to the body of evidence in favour of a class effect. Of note, the results of CVD-REAL were unchanged after the sequential removal of several GLD classes from the comparator group, suggesting that the findings reflect the beneficial effects of SGLT2 inhibitors rather than adverse effects of comparator GLDs.55 A major limitation so far with real life data is the limited information on safety data and future real life analyses should also include such information. However, data from the DECLARE study is needed before any firm conclusions about a class effect can be made.
Summary and concluding remarks
Reduction of cardiovascular complications has become an important treatment goal in T2D and for the first time there are licensed GLDs that also reduce CV event and mortality.20,36,56,57 While it must be emphasized that only empagliflozin is currently recommended by the ADA for CV risk reduction, the totality of evidence from EMPA-REG OUTCOME, CANVAS-PROGRAM and real-world studies suggest that the CV benefits observed with empagliflozin in the EMPA-REG OUTCOME study may be a class effect. There are some signals on increased risk of amputations with canagliflozin in the CANVAS-PROGRAM study that should be considered in high risk patients. However, in the absence of data from the ongoing prospective DECLARE study, it is not possible to conclude that a class effect exists. Individualisation remains the cornerstone for selection of therapy for hyperglycaemia in T2D.
Results from the CVD-REAL and THIN databases have demonstrated that data derived from rigorous, large international epidemiologic studies are a valuable addition to those generated by clinical trials, as they suggest effectiveness of treatments outside the limitation of the inclusion and exclusion criteria in randomised controlled trials and in a broader patient population, that is more representative of those seen in primary care. It is important to note that retrospective studies have inherent limitations, possibility of selection bias and residual confounding and relatively short follow-up. Moreover, SGLT2 inhibitors have been used in real-world practice for a relatively limited time and long-term follow up will be needed to determine whether the observed CV benefits are sustained. There is also a need for further studies examining the mechanisms underlying the CV benefits of SGLT2 inhibitors although CV-mortality-preventive and CV-safe treatment should not be withheld awaiting such explanatory findings.
Acknowledgments
Acknowledgements: Medical writing assistance was provided by Katrina Mountfort, Touch Medical Media and supported by AstraZeneca. Baptist Gallwitz would like to thank Anna Norhammer for her help and input to this article.
Funding Statement
Support: The publication of this article was supported by AstraZeneca. The views and opinions expressed are those of the author and do not necessarily reflect those of AstraZeneca.
References
- 1.Emerging Risk Factors Collaboration. Sarwar N, Gao P. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah AD, Langenberg C, Rapsomaniki E. et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–13. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norhammar A, Bodegård J, Nyström T. et al. Incidence, prevalence and mortality of type 2 diabetes requiring glucoselowering treatment, and associated risks of cardiovascular complications: a nationwide study in Sweden, 2006-2013. Diabetologia. 2016;59:1692–701. doi: 10.1007/s00125-016-3971-y. [DOI] [PubMed] [Google Scholar]
- 4.Cavender MA, Steg PG, Smith SC Jr. et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death: outcomes at 4 years from the Reduction of Atherothrombosis for Continued Health (REACH) registry. Circulation. 2015;132:923–31. doi: 10.1161/CIRCULATIONAHA.114.014796. [DOI] [PubMed] [Google Scholar]
- 5.Johansson I, Edner M, Dahlström U. et al. Is the prognosis in patients with diabetes and heart failure a matter of unsatisfactory management? An observational study from the Swedish Heart Failure Registry. Eur J Heart Fail. 2014;16:409–18. doi: 10.1002/ejhf.44. [DOI] [PubMed] [Google Scholar]
- 6.Emerging Risk Factors Collaboration. Di Angelantonio E, Kaptoge S. et al. Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME. et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ADVANCE Collaborative Group. Patel A, MacMahon S. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study (UKPDS) Group. Intensive bloodglucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 10.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 11.Garrison LP Jr, Neumann PJ, Erickson P. et al. Using real-world data for coverage and payment decisions: the ISPOR Real- World Data Task Force report. Value Health. 2007;10:326–35. doi: 10.1111/j.1524-4733.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–95. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 13. FDA. Guidance for Industry - Diabetes Mellitus — Evaluating Cardiovascular Risk in New Antidiabetic Therapies to Treat Type 2 Diabetes. 2008. Available at: www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf (accessed 14 February 2017).
- 14. European Medicines Agency. Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_ guideline/2012/06/WC500129256.pdf (accessed 14 February 2017).
- 15.American Diabetes Association. 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S8604. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 16.Scirica BM, Bhatt DL, Braunwald E. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 17.White WB, Cannon CP, Heller SR. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 18.Green JB, Bethel MA, Armstrong PW. et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 19.Pfeffer MA, Claggett B, Diaz R. et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstock J, Aggarwal N, Polidori D. et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–8. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis. 2009;53:875–83. doi: 10.1053/j.ajkd.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4:195–220. doi: 10.1007/s13300-013-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inzucchi SE, Zinman B, Wanner C. et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardy E, Ptanszynska A, de Bruin T.W.A. et al. Changes in lipid profiles of patients with type 2 diabetes mellitus on dapagliflozin therapy. Diabetologia. 2013;56(Suppl.1):S1–566. #947. [Google Scholar]
- 26.Hach T, Gerich J, Salsali A. et al. Empagliflozin improves glycaemic parameters and cardiovascular risk factors in patients with type 2 diabetes: pooled data from four pivotal phase III trials. Diabetologie und Stoffwechsel. 2014;;9:142. [Google Scholar]
- 27.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–71. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinclair A, Bode B, Harris S. et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocr Disord. 2014;;14:37. doi: 10.1186/1472-6823-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cefalu WT, Leiter LA, Yoon KH. et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 noninferiority trial. Lancet. 2013;382:941–50. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 30.Cherney DZ, Perkins BA, Soleymanlou N. et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;;13:28. doi: 10.1186/1475-2840-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terami N, Ogawa D, Tachibana H. et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;;9:e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrannini E, Mark M, Mayoux E. CV Protection in the EMPAREG OUTCOME Trial: A “Thrifty Substrate” Hypothesis. Diabetes Care. 2016;39:1108–14. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 33.Sattar N, McLaren J, Kristensen SL. et al. SGLT2 Inhibition and cardiovascular events: why did EMPA-REG Outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–9. doi: 10.1007/s00125-016-3956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baartscheer A, Schumacher CA, Wust RC. et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60:568–73. doi: 10.1007/s00125-016-4134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Washburn WN, Poucher SM. Differentiating sodium-glucose co-transporter-2 inhibitors in development for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs. 2013;22:463–86. doi: 10.1517/13543784.2013.774372. [DOI] [PubMed] [Google Scholar]
- 36.Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 37.Wu JH, Foote C, Blomster J. et al. Effects of sodium-glucose cotransporter-2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2016;4:411–9. doi: 10.1016/S2213-8587(16)00052-8. [DOI] [PubMed] [Google Scholar]
- 38.Sonesson C, Johansson PA, Johnsson E, Gause-Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta-analysis. Cardiovasc Diabetol. 2016;15:37. doi: 10.1186/s12933-016-0356-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monami M, Dicembrini I, Mannucci E. Effects of SGLT- 2 inhibitors on mortality and cardiovascular events: a comprehensive meta-analysis of randomized controlled trials. Acta Diabetol. 2017;54:19–36. doi: 10.1007/s00592-016-0892-7. [DOI] [PubMed] [Google Scholar]
- 40. FDA. Use of real-world evidence to support regulatory decision-making for medical devices. Guidance for industry and food and drug administration staff. 2017. Available at: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm513027.pdf (accessed 26 July 2017).
- 41. European Commission. STAMP Commission Expert Group. Real world evidence. 2016. Available at: https://ec.europa.eu/health//sites/health/files/files/committee/stamp/2016-03_stamp4/4_real_world_evidence_background_paper.pdf (accessed 26 July 2017).
- 42.Califf RM, Robb MA, Bindman AB. et al. Transforming evidence generation to support health and health care decisions. N Engl J Med. 2016;375:2395–400. doi: 10.1056/NEJMsb1610128. [DOI] [PubMed] [Google Scholar]
- 43.Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care. 2018;41:6–10. doi: 10.2337/dc17-1223. [DOI] [PubMed] [Google Scholar]
- 44.Cowley A. What is real-world data? 2016 Available at: http://crcaustralia.com/media-releases/real-world-data/ (accessed 21 January 2017). [Google Scholar]
- 45.Cziraky M, Pollock M. Real-world evidence studies. 2015. Available at: http://www.appliedclinicaltrialsonline.com/realworld-evidence-studies (accessed 4th April 2017). [Google Scholar]
- 46.Kosiborod M, Cavender MA, Fu AZ. et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Birkeland KI, Jorgensen ME, Carstensen B. et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol. 2017;5:709–17. doi: 10.1016/S2213-8587(17)30258-9. [DOI] [PubMed] [Google Scholar]
- 48.Persson F, Nyström T, Jørgensen ME. et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes Metab. 2018;20:344–51. doi: 10.1111/dom.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toulis KA, Willis BH, Marshall T. et al. All-cause mortality in patients with diabetes under treatment with dapagliflozin: a population-based, open-cohort study in The Health Improvement Network database. J Clin Endocrinol Metab. 2017;102:1719–25. doi: 10.1210/jc.2016-3446. [DOI] [PubMed] [Google Scholar]
- 50.Nyström T, Bodegard J, Nathanson D. et al. Novel oral glucoselowering drugs are associated with lower risk of all-cause mortality, cardiovascular events and severe hypoglycaemia compared with insulin in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:831–41. doi: 10.1111/dom.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan Z, DeFalco FJ, Ryan PB. et al. Risk of lower extremity amputations in people with type 2 diabetes mellitus treated with sodium-glucose co-transporter-2 inhibitors in the USA: A retrospective cohort study. Diabetes Obes Metab. 2018;20:582–9. doi: 10.1111/dom.13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piepoli MF, Hoes AW, Agewall S. et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–81. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ponikowski P, Voors AA, Anker SD. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 54.ADA. American Diabetes Accociation Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40:S1–S2. [Google Scholar]
- 55.Kosiborod M, Cavender MA, Fu AZ. et al. Lower risk of heart failure and death in patients initiated on SGLT-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study. Circulation. 2017;136:249–59. doi: 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marso SP, Daniels GH, Brown-Frandsen K. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marso SP, Bain SC, Consoli A. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 58. ClinicalTrials.gov. Multicenter trial to evaluate the effect of dapagliflozin on the incidence of cardiovascular events (DECLARE-TIMI58). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT01730534 (accessed 5 April 2017).


