Abstract
The increasing prevalence of obesity has made nonalcoholic fatty liver disease (NAFLD) the most common chronic liver disease. As a consequence, NAFLD and especially its inflammatory form nonalcoholic steatohepatitis (NASH) are the fastest increasing etiology of end-stage liver disease and hepatocellular carcinoma. Physical inactivity is related to the severity of fatty liver disease irrespective of body weight, supporting the hypothesis that increasing physical activity through exercise can improve fatty liver disease. This review summarizes the evidence for the effects of physical exercise on NAFLD and NASH. Several clinical trials have shown that both aerobic and resistance exercise reduce the hepatic fat content. From clinical and basic scientific studies, it is evident that exercise affects fatty liver disease through various pathways. Improved peripheral insulin resistance reduces the excess delivery of free fatty acids and glucose for free fatty acid synthesis to the liver. In the liver, exercise increases fatty acid oxidation, decreases fatty acid synthesis, and prevents mitochondrial and hepatocellular damage through a reduction of the release of damage-associated molecular patterns. In conclusion, physical exercise is a proven therapeutic strategy to improve fatty liver disease.
Key words: Nonalcoholic fatty liver disease (NAFLD), Nonalcoholic steatohepatitis (NASH), Aerobic exercise, Resistance exercise, Lipid metabolism
INTRODUCTION
For millions of years, humans needed to invest significant physical effort in gathering food to meet their nutritional needs, which has hard-wired our metabolism to efficiently store nutrients at rare moments of caloric abundance. However, in the current day and age, no physical activity is required to obtain a daily caloric load for most people in developed as well as developing countries. The resulting obesity epidemic has caused nonalcoholic fatty liver disease (NAFLD) to rapidly become the most common etiology of chronic liver disease1–3. NAFLD can progress to nonalcoholic steatohepatitis (NASH), which places patients at risk for developing end-stage liver disease (i.e., cirrhosis) in which hepatocellular carcinoma (HCC) may develop4. Of great concern is the observation that HCC can also develop in noncirrhotic NASH5. Of all common cancers in the US, HCC is the only tumor with an increasing mortality6.
Diet and lifestyle modification leading to weight loss of 10% or more has been proven to be an effective strategy to achieve resolution of NASH in >90% of patients7. Unfortunately, greater than 50% of patients included in clinical trials have not been able to meet this weight loss threshold8. Likewise, treatment with medications, such as pioglitazone, vitamin E, or the bile acid derivative obeticholic acid, has been effective only in up to 45% of patients9,10.
Physical inactivity and its related reduced cardiorespiratory fitness have been associated with increased NASH severity11. Among obese people, sedentary individuals have increased risks of having a fatty liver in comparison with weight-matched physically active individuals12. These data provide support for the hypothesis that increasing physical activity through exercise, defined as a planned, structured, and repetitive physical activity with a specific intensity, frequency, and duration, has beneficial effects on NAFLD. Theoretically, it is also a cheap intervention with both therapeutic and preventive value. Simultaneously, exercise can reduce risk factors for cardiovascular disease in NASH patients, such as diabetes and hypertension13. The American Gastroenterological Association, the American Association for the Study of Liver Diseases, and American College of Gastroenterology all recommend physical exercise as a treatment for NAFLD14. The current recommendations do not specify what exercise regimen is most beneficial, and the mechanisms by which exercise affects the liver remain, at least in part, unknown. Here we aim to review the existing evidence for the effects of physical exercise on NAFLD, as well as the mechanistic principles that have been elucidated through human trials and basic scientific studies.
EFFECTS OF EXERCISE ON BIOPSY-PROVEN NASH
The true outcomes of fatty liver disease are end-stage liver disease (ESLD) and HCC. No studies with longitudinal follow-up have been performed to evaluate the effect of exercise on these outcomes, and such studies would probably not be feasible either. However, the liver’s remarkable regeneration capacity can result in the reversibility of steatohepatitis. It is most probably correct to assume that with recovery of NASH, the risks of developing ESLD and HCC are reduced as well. A small number of studies have used postintervention liver biopsy to evaluate the effect of exercise on the histologic reversibility of NASH. Eckard et al. performed a randomized controlled study on the effect of 6 months of various lifestyle modifications on NASH, including exercise and dietary modifications15. In this trial, the effect of a moderate exercise regimen (20- to 60-min routine, 4–7 days/week, including both resistance and aerobic training) with or without dietary intervention (unrestricted diet vs. low-fat diet vs. moderate fat/low-carbohydrate diet) was compared to a group undergoing no intervention. In each of the intervention groups there was a significant decrease in the NASH Activity Score (NAS), a histological grading of NASH15. Similarly, a randomized control trial (RCT) of 31 NASH patients reported that 48 weeks of intensive lifestyle intervention (moderate-intensity exercise with a goal of >200 min per week, reduced calorie diet, and behavioral guidance) led to a 2.4 point reduction in the NAS on postintervention liver biopsy, a significantly greater reduction than in the control arm16. Another study evaluated 120 candidates for living liver donation who were encouraged to do aerobic exercise and restrict their calorie intake to 25 cal/kg, after liver biopsy showed at least 30% steatosis. On repeat biopsy after a median of 10 weeks of intervention, steatosis was improved in >85% of patients17. We can infer from these results that exercise has a beneficial effect on the reversal of histologically proven fatty liver disease. However, because dietary interventions aiming at weight loss were included as well, these studies do not provide direct evidence that the effect on the liver was mediated by exercise or through the effect of weight loss.
EFFECTS OF EXERCISE ON NONINVASIVE MEASURES OF NASH
Although liver biopsy is the gold standard for diagnosis and grading of NAFLD, its risk of complications, potential to obtain nonrepresentative samples, and cost deter its widespread use18. Liver biopsy has therefore infrequently been used in the evaluation of the efficacy of exercise on NAFLD. Alternatively, several noninvasive techniques have been developed to assess liver fat content19, and their advantages and shortcomings are summarized in Table 1. In general, these techniques measure liver fat content or liver stiffness as an indication of fibrosis and do not necessarily distinguish NASH from simple steatosis. Liver stiffness measured by magnetic resonance (MR) elastography, however, appeared to closely correlate with a diagnosis of NASH on liver biopsy and had a sensitivity of 94% and a specificity of 73% to discern NASH from simple steatosis20.
Table 1.
Noninvasive Modalities for Assessment of Fatty Liver Disease
| Modality | Technique | Advantages | Shortcomings |
|---|---|---|---|
| US | Increased echogenicity makes steatotic livers appear brighter than spleen and kidney | Low cost; widely available; reasonable sensitivity/specificity | Lower performance with steatosis <30% and in morbidly obese patients; operator dependent |
| US-CAP | Measures the degree of ultrasound attenuation by hepatic fat using vibration control transient elastography | Can roughly distinguish steatosis categories | Overlap between stages; not validated in large patient cohorts |
| CT | Decreased attenuation of fatty liver (10 HU less than spleen, or liver attenuation <40 HU) | Widely available | Not sensitive for detecting mild steatosis (5%–30%); radiation exposure |
| MRS | Protons in triglycerides resonate with specific spectral peaks | High sensitivity; correlates strongly with the histological fat percentage | Not widely available; increased cost, cannot be used as a screening tool |
| MRE | Contrast MRI with a low frequency vibration source to assess stiffness | High sensitivity for fibrosis; differentiates between steatosis and NASH | Not widely available; long procedural time; low image resolution |
| Transient elastography | Velocity of electric shear indicates liver stiffness | Short procedure time; can be done at bedside; immediate results | Mainly looks at fibrosis; operator dependent; difficult to get accurate and valid results (requires at least 10 measurements) |
US, ultrasound; US-CAP, ultrasound with controlled attenuation parameter; CT, computed tomography; MRS, magnetic resonance spectroscopy; MRE, magnetic resonance elastography.
Over the past decades, several trials have been performed using these surrogate endpoints to estimate the effect of physical exercise on NASH. Randomized trials reported since 2005 are summarized in Table 2. In 2012, Keating et al. performed a meta-analysis of 12 trials (11 of them randomized) investigating the effect of exercise on liver fat content. In the pooled analysis, 439 subjects were included. A small reduction in liver fat was seen, however, only if studies that looked at diet and exercise were left out of the analysis21. Most studies included were small (n = 14–45 in 11 studies with 1 study of 130 subjects), and exercise regimens were often short (in 7 studies 10 weeks or shorter), which are potential reasons why the reported effect was limited.
Table 2.
Randomized Controlled Trials of Exercise and the Effect on Nonalcoholic Fatty Liver Disease (NAFLD)
| Reference | n | Exercise Intervention | Main Results |
|---|---|---|---|
| Bacchi et al., 201345 | 40 | AE vs. RE, 3×/week for 16 weeks | Equal effects on reducing intrahepatic fat |
| Balducci et al., 2015110 | 606 | AE + RE vs. control, 2×/week for 12 months | Reduced fatty liver index |
| Cassidy et al., 201339 | 28 | AE vs. controls, 3×/week for 12 weeks | Decreased hepatic lipid content, improvement in cardiac function |
| Cuthbertson et al., 201658 | 69 | AE vs. control, 3–5×/week for 16 weeks | Decreased hepatic lipid content, improved peripheral insulin sensitivity |
| Eckard et al., 201315 | 56 | AE vs. AE + low fat diet, 4–7×/week for 6 months | Decrease in NASH activity score on liver biopsy |
| Finucane et al., 201032 | 100 | AE vs. control, 1×/week for 12 weeks | Decreased hepatic lipid content, improved cardiorespiratory fitness |
| Goodpaster et al., 201033 | 130 | Diet + AE for 6 months vs. diet + AE for 12 months, 5×/week | 12-Month intervention resulted in greater decrease in hepatic fat content, with equal reduction in insulin resistance |
| Hallsworth et al., 201146 | 21 | RE vs. control, 3×/week for 8 weeks | Decreased hepatic lipid content, improved insulin resistance |
| Hallsworth et al., 201528 | 29 | High intensity AE vs. control, 3×/week for 12 weeks | Decreased hepatic lipid content, improved cardiorespiratory fitness |
| Houghton et al., 201740 | 24 | AE + RE vs. control, 3×/week for 12 weeks | Decreased hepatic lipid content and plasma triglycerides |
| Larson-Meyer et al., 200834 | 23 | Diet + AE vs. diet vs. control, 5×/week for 6 months | Decreased hepatic lipid content |
| Lee et al., 201241 | 45 | AE vs. RE vs. control, 3×/week for 12 weeks | Decreased hepatic lipid content. RE improved insulin sensitivity |
| Levinger et al., 2009111 | 55 | RE vs. control, 3×/week for 10 weeks | No reduction in ALT/AST or inflammatory markers |
| Monteiro et al., 201542 | 32 | AE vs. AE + RE vs. control, 3×/week for 20 weeks | Decreased hepatic fat content |
| de Piano et al., 2012105 | 58 | AE vs. AE + RE, 3×/week for 12 months | AE + RE results in reduced ALT and insulin resistance, and increased adipokine levels |
| Promrat et al., 201016 | 31 | AE + diet vs. control, 1×/week for 48 weeks | Decrease in NASH activity score on liver biopsy |
| Pugh et al., 2013103 | 20 | AE vs. control, 3×/week for 16 weeks | No difference in hepatic fat content. Improved ALT/AST levels |
| Pugh et al., 2014104 | 31 | AE vs. control, 3×/week for 16 weeks | No difference in hepatic fat content. Improved ALT/AST levels. Improved cardiovascular risk factors |
| Shah et al., 200935 | 18 | Diet + AE/RE vs. diet, 3×/week for 6 months | Comparable decrease in hepatic lipid content and insulin resistance |
| Shoojaee-Moradie et al., 200754 | 17 | AE vs. control, 3×/week for 6 weeks | No difference in intrahepatic fat content. Decreased circulating FFA, increased insulin sensitivity |
| Skrypnik et al., 2016106 | 44 | AE vs. AE + RE, 3×/week for 3 months | AE + RE results in greater reduction in ALT and AST |
| Slentz et al., 201143 | 196 | AE vs. RE vs. AE + RE, 3×/week for 8 months | Regimens including AE result in greater reduction in hepatic fat content, ALT and insulin resistance |
| Straznicky et al., 2012112 | 63 | Diet + AE vs. diet vs. control, 300 min/week for 12 weeks | Decreased insulin resistance, ALT, γ-GT; no significant differences between diet + AE and diet alone |
| Sullivan et al., 201236 | 18 | AE vs. control, 3×/week for 16 weeks | Decreased hepatic lipid content, decreased circulating FFA, improved insulin sensitivity |
| Tamura et al., 200537 | 14 | Diet + AE vs. diet, 5–6×/week for 2 weeks | Decreased hepatic lipid content in both groups. Improved insulin sensitivity in AE group |
| Thompson et al., 200962 | 41 | AE vs. control, 4×/week for 24 weeks | Decreased IL-6, ALT, and FFA |
| Wong et al., 201324 | 154 | AE vs. control, 3×/week for 12 months | Decreased hepatic lipid content |
| Yoshimura et al., 2014113 | 33 | Diet + AE vs. diet, 300 min/week for 12 weeks | Equal decrease in hepatic lipid content |
| Zelber-Sagi et al., 201438 | 82 | RE vs. control, 3×/week for 12 weeks | Improved steatosis and inflammation |
| Zhang et al., 201644 | 220 | AE vs. control, 150 min/week for 12 months | Decreased hepatic lipid content. Effect disappeared when adjusted for weight loss |
AE, aerobic exercise; RE, resistance exercise.
Since Keating and colleagues’ meta-analysis, additional and larger RCTs have been done that clearly demonstrate the beneficial effect of physical exercise on NASH. Golabi et al. conducted a systematic review of these studies published between 2011 and 2016. In this work, only trials of at least 8-week intervention were included. On reviewing eight randomized trials, the effect of physical exercise on the reduction of hepatic fat content was assessed. With the use of MR spectroscopy or liver biopsy, a pooled analysis of a total of 433 adult participants revealed a 30.2% reduction in hepatic fat as a result of the exercise intervention, and a 49.8% reduction in liver fat resulting from exercise combined with dietary intervention22.
Whitsett et al. conducted a systematic review of 18 studies23. Besides randomized trials, prospective and well-conducted retrospective cohort studies were also included. The included studies together evaluated more than 6,000 patients with NAFLD, with two studies in particular having a study population greater than 1,000. The intervention duration varied greatly from 1 to 52 weeks, and the most commonly employed imaging modality to determine change in hepatic steatosis was hydrogen-MR spectroscopy (H-MRS). The authors concluded that exercise significantly improves hepatic fat content.
A recent randomized trial not included in the above systematic reviews, but worth mentioning because of its relatively large size, was conducted by Wong et al. In this study of 145 NASH patients, lifestyle intervention (aerobic exercise, resistance exercise, and dietary restriction) demonstrated a 64% remission rate (i.e., achievement of <5% intrahepatic triglyceride content) in the intervention group, compared to a 20% remission rate in the control arm, which underwent no intervention24.
EXERCISE REGIMEN AND INTENSITY
Several studies addressed which modality, intensity, and duration of exercise are most efficacious in ameliorating NASH. A retrospective trial of 813 biopsy-proven NAFLD patients asking them to self-report on their physical activity status found that only those patients who met the vigorous exercise criteria, corresponding to 7 or more metabolic equivalents25, had a decreased odds ratio of developing NASH26. Those patients who doubled the time of recommended vigorous exercise further decreased the adjusted odds for advanced fibrosis26. Another self-reported retrospective trial from Japan, on the physical activity of 1,149 patients with fatty liver disease, corroborated this finding. Vigorous physical activity showed a significant preventative effect in the progression of fatty liver to NASH27. Modified high-intensity interval training (HIIT) of five cycles of high-intensity cycling followed by 3-min recovery periods, three times/week for 12 weeks demonstrated reduction in liver fat and improvement in early diastolic filling in 23 NAFLD patients compared to standard controls28. This improvement in early diastolic filling is beneficial to NAFLD patients, as it has been well documented that cardiorespiratory fitness, independent of visceral fat, is a predictor of liver fat11,29,30. These studies establish that vigorous exercise results in significant reduction in hepatic steatosis.
A number of studies tried to establish scientific evidence for this matter in an experimental setting. A recent study of 48 overweight and obese patients compared aerobic exercise regimens of various doses and intensities31. Patients were randomly assigned to low-intensity/high-volume, high-intensity/low-volume, low-intensity/low-volume, or no exercise. Each exercise group experienced significant reduction in liver fat, but there was no significant difference between the different regimens. This led to the conclusion that aerobic exercise, even if done at low intensity and low volume, would have a beneficial effect on the reduction of liver fat31. Several randomized trials provide evidence that aerobic exercise indeed reduces hepatic fat content at different intensities and frequencies32–44.
Bacchi et al. conducted an RCT comparing the effects of resistance training versus aerobic training in 31 NASH patients over a 4-month period. In both arms of the trial, there was a significant reduction in liver fat on MRS. However, there was no difference between the two exercise regimens45. Lee et al. also found a similar beneficial effect of resistance exercise compared with aerobic exercise41. Hallsworth et al. reported on 19 patients with NAFLD who were either subjected to 8 weeks of resistance training or no exercise. A significant reduction in liver fat was achieved46. Zelber-Sagi et al. also found a significant effect of resistance exercise on hepatic fat content38. On the contrary, in 29 obese/overweight adults who were randomized to either 8 weeks of resistance exercises or sham control exercise regimen no significant difference in liver fat by MR spectroscopy was achieved47. A randomized trial including 196 subjects revealed that regimens including aerobic exercise resulted in greater reduction in hepatic fat content than a resistance exercise program43.
In conclusion, although various exercise regimens have been shown to affect liver fat content, there is no definitive evidence to recommend one regimen over another. Aerobic exercise has shown an effect on hepatic fat content in many studies, but resistance exercise may provide an option to patients unable to perform aerobic exercises, for example, due to a limited cardiorespiratory reserve. It is therefore emphasized in recent practice guidelines that the choice of training for patients with NAFLD should be tailored based on patients’ preferences and on the highest likelihood of continuation by the individual patient in the long term19,48.
THE EFFECT OF EXERCISE ON THE LIVER IS INDEPENDENT OF WEIGHT LOSS
Importantly, the decrease in the hepatic fat content was achieved even when overall weight loss was not observed in a multitude of studies15–18,22,31,45–47, which is consistent with the idea that exercise has a direct effect on the liver. However, the mechanisms by which exercise reduces liver fat are still greatly unknown. The next sections of this review summarize the available evidence on possible metabolic and molecular pathways involved in the reduction of hepatic fat by exercise.
EFFECTS ON INSULIN RESISTANCE AND FREE FATTY ACIDS
In order to begin to understand the molecular pathways that are involved in the effect of physical exercise on fatty liver disease, it is worth looking at other outcome measures than hepatic fat content. For example, insulin resistance is thought to be a driving force in NASH and its related metabolic syndrome49. Improving insulin resistance is among the mechanisms by which physical exercise has been proposed to improve NASH. In support of this hypothesis, several studies in humans have reported on the beneficial effect of exercise on insulin resistance35–37,45,46,50–54. For example, in a comparative trial of 16 weeks of aerobic versus resistance training, Bacchi et al. used a euglycemic clamp technique to demonstrate that both programs substantially increased insulin sensitivity, along with an improvement in other markers of the metabolic syndrome, like HbA1C and visceral fat45.
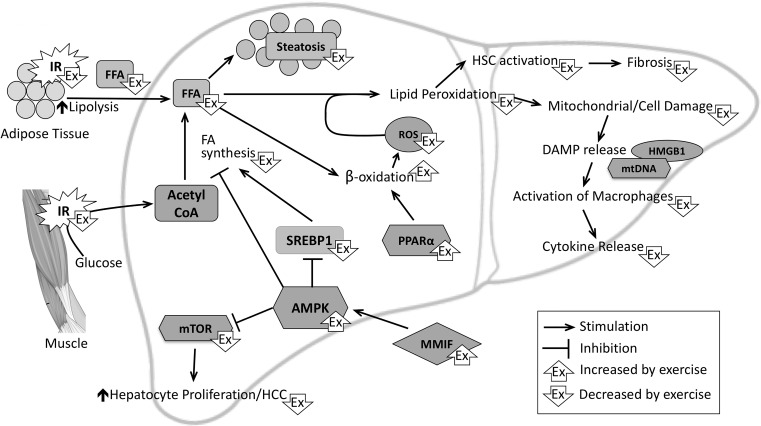
Mechanistically, insulin resistance in adipose tissue results in an incomplete suppression of lipase, leading to enhanced lipolysis and release of free fatty acids (FFAs), which are elevated in serum of NAFLD patients55,56 and are taken up by the liver57. An improvement in insulin resistance is thought to reduce this flux of FFA to the liver (Fig. 1). In a randomized trial of 69 NAFLD patients, it was shown that 4 months of physical exercise predominantly affects peripheral rather than hepatic insulin resistance58. Studies on the acute metabolic effects of exercise in 15 prediabetic adults confirmed that the improvement in insulin sensitivity mainly occurs in adipose and muscle tissues59. Although some studies did not detect a significant reduction in fasting serum FFA46,58, several others have demonstrated a resulting decrease in FFA in human patients36,52,54,59–62, as well as in experimental rodent models of NASH63,64. In addition, it has been shown that FFA in return increases insulin resistance in skeletal muscle and that physical exercise can direct FFA into triglyceride formation with a resulting increase in insulin sensitivity65.
Figure 1.
Schematic overview of metabolic and molecular pathways involved in the pathobiology of nonalcoholic steatohepatitis (NASH) and the effects of physical exercise thereon. Peripheral insulin resistance causes an increase in delivery of glucose and FFA to the liver. FA synthesis further increases FFA levels. When the mechanisms for FA storage as triglycerides (steatosis) and metabolism (β-oxidation) become overwhelmed, ROS production increases, resulting in mitochondrial and hepatocyte damage, DAMP release, and amplification of inflammation. Exercise affects these pathways at multiple levels, as indicated. Of note, multiple other pathways are involved in the pathogenesis of NASH. As the effects of exercise have not been investigated on these pathways, they are not included in this diagram. AMPK, AMP-activated protein kinase; DAMP, damage-associated molecular pattern; FA, fatty acid; FFA, free fatty acids; HCC, hepatocellular carcinoma; HMGB1, high-mobility group box-1; HSC, hepatic stellate cell; IR, insulin resistance; MMIF, macrophage migration inhibitory factor; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; PPARα, peroxisome proliferator-activated receptor-α; ROS, reactive oxygen species; SREBP-1, sterol regulatory element-binding protein 1.
EXERCISE AND FATTY ACID SYNTHESIS
Insulin resistance in skeletal muscle is the cause of diversion of glucose to the liver for FFA synthesis (de novo lipogenesis)66. In addition to storage into triglycerides leading to steatosis, FFAs are considered the metabolically and immunologically active form of fat contributing to cell damage and inflammation that are characteristic of NASH (Fig. 1)67,68. Animal models have shown that the main transcription factor controlling lipogenesis, sterol regulatory element-binding protein 1 (SREBP-1), is elevated in the pathobiology of NASH69–71. The best available evidence that physical exercise can modify de novo synthesis of FFA in human patients with fatty liver disease is reported by Oh et al., who exposed middle-aged, sedentary, obese men to exercise regimens in various intensity and frequency. They found that 12 weeks of either resistance or high-intensity aerobic exercise led to a decrease in the expression of SREBP-1c in circulating peripheral blood mononuclear cells (PBMCs)52,72. As PBMCs have the same embryonic origin as liver cells, it is felt that they accurately represent the changes in hepatocytes.
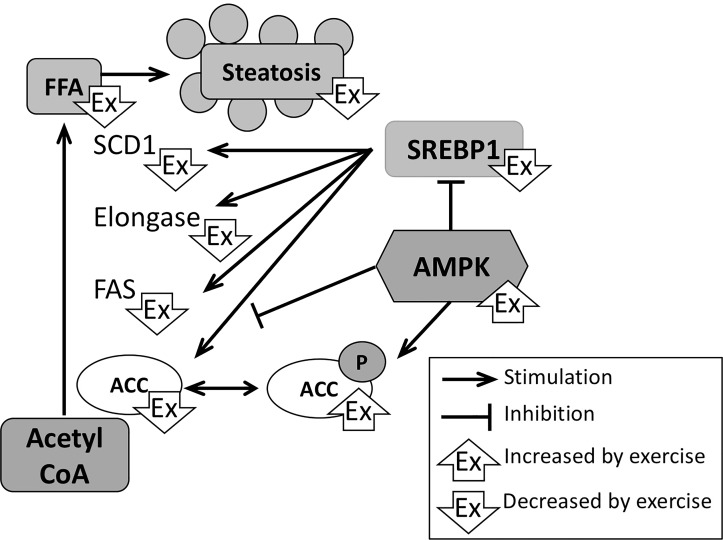
In lipogenesis, acetyl-coA derived from the Krebs cycle gets converted into long-chain fatty acids facilitated by a number of enzymes, such as acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), elongases, and stearoyl-CoA desaturase 1 (SCD1) (Fig. 2). Although evidence in humans is limited (changes in levels of these enzymes in the studies by Oh et al. were nonsignificant), several rodent studies have shown that exercise reduces expression levels of FAS, elongases, and SCD1 in fatty livers, resulting in decreased levels of FFA and reversal of steatosis69,73–77. Exercise also increased phosphorylation of ACC, resulting in its inactivity76,77. Epigenetic mechanisms (e.g., reduction of DNA hypermethylation) have been proposed to be responsible for the beneficial effect of physical exercise on the metabolic pathways including de novo lipogenesis78.
Figure 2.
Effects of physical exercise on hepatic fatty acid synthesis. In NASH, the increase in glucose delivery to the liver results in increased FFA synthesis. Exercise reduces the expression of various enzymes that mediate the conversion of acetyl-coA to FFA. An increase in AMPK by exercise stimulates the phosphorylation and therefore inactivation of these enzymes (here depicted for ACC) and of SREBP-1, which is a main transcription factor for expression of ACC, FAS, elongase, and SCD1. ACC, acetyl coenzyme A carboxylase; AMPK; FAS, fatty acid synthase; FFA; SCD1, stearoyl coenzyme A desaturase 1; SREBP-1. P denotes phosphorylation.
EFFECTS ON FATTY ACID OXIDATION AND MITOCHONDRIA
The liver can neutralize metabolically and immunologically active FFA in three major pathways: esterification of FFA into triglycerides and sequestration into lipid droplets (steatosis), excretion in very-low-density lipoprotein, and fatty acid oxidation in hepatocyte mitochondria (β-oxidation). β-Oxidation was found to be increased in human NASH, as measured by fasting serum β-hydroxybuturate levels79. However, structural defects to liver mitochondria, such as the loss of cristae, were observed simultaneously79, indicating that a compensatory increase in β-oxidation may lead to mitochondrial damage and dysfunction in the long term. Indeed, in other studies, a positive correlation between NASH severity and reduced liver mitochondrial performance was observed80,81. Haus et al. measured fatty acid oxidation in PBMCs before and after a 7-day aerobic exercise course in 17 NAFLD patients. The participants were found to have a significant increase in fatty acid oxidation, as measured with indirect calorimetry50.
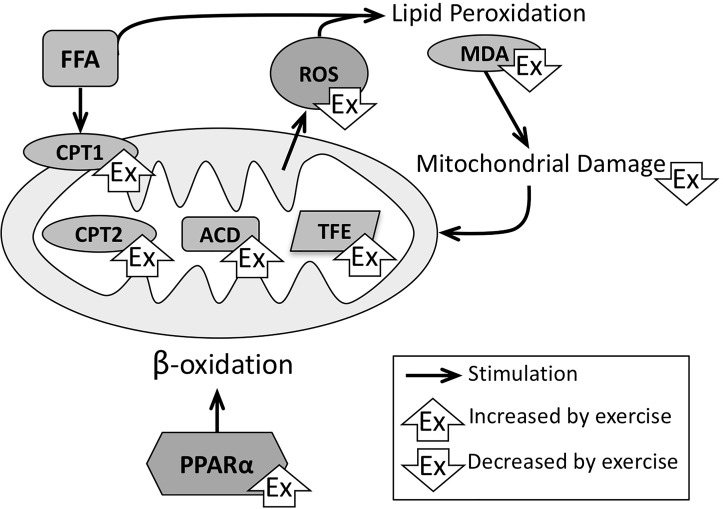
In rodent studies, it was confirmed that running improves liver mitochondrial function and increased palmitate oxidation in freshly excised livers, concomitantly with an increase in hepatic carnitine palmitoyl-CoA transferase 1 (CPT-1)76,77,82,83, an enzyme necessary for transport of FA from the cytosol across the mitochondrial membrane (Fig. 3). Other investigators confirmed these findings and demonstrated that exercise increased CPT-2, acyl-coenzyme A dehydrogenase (ACD), and trifunctional enzyme, which are rate-limiting enzymes in fatty acid oxidation in the liver64. In mice, Gonçalves et al. demonstrated with electron microscopy that exercise caused an improvement in abnormal liver mitochondria84. Exercise studies in rodents have also shown an improvement in mitochondrial respiration84 and an increase in cytochrome C76, indicating that, in addition to β-oxidation, downstream oxidative phosphorylation is enhanced by aerobic exercise. Peroxisome proliferator-activated receptor-α (PPARα) regulates the expression of enzymes responsible for mitochondrial fatty acid oxidation and thereby stimulates β-oxidation in the liver (Fig. 3). Several studies have shown that exercise increases PPARα73,77, which may indicate that exercise, in part, acts along a mechanism similar to the action of thiazolidinediones9.
Figure 3.
Effects of NASH and exercise on mitochondrial function. When β-oxidation fails to appropriately neutralize the access in FFA, ROS formation leads to lipid peroxidation products, which in return cause more mitochondrial damage. Physical exercise stimulates PPARα, which has beneficial effects on multiple aspects of β-oxidation and therefore improves mitochondrial quality and function. ACD, acyl coenzyme A dehydrogenase; CPT1/2, carnitine palmitoyl coenzyme A transferase 1/2; FFA; MDA, malondialdehyde; PPARα; TFE, trifunctional enzyme.
However, when β-oxidation becomes overwhelmed by the abundance of FFA and mitochondria sustain damage, reactive oxygen species (ROS) are formed and create double bonds in polyunsaturated FA (lipid peroxidation), resulting in toxic metabolites capable of causing further mitochondrial damage (Fig. 3). In the pathology of human NASH, multiple markers of this oxidative stress have been shown to be elevated85–89. Multiple clinical trials have shown that exercise reduces ROS formation by various methodologies. The studies by Oh et al. revealed that 12-week exercise programs significantly reduced serum levels of the thiobarbituric acid-reactive substances that reflect the levels of ROS production and lipid peroxidation60,90,91. This was corroborated by a trial that established 7 days of aerobic exercise was sufficient to significantly reduce the ROS levels in PBMCs during an oral glucose challenge in 17 NASH patients, measured using chemiluminescence50. In rats, Hu et al. measured improved serum levels of the lipid peroxidation marker malondialdehyde (MDA) after 8 weeks of treadmill exercise. With mass spectrometry, they found carbonylation (indicative of oxidative damage) to eight mitochondrial proteins, which was improved by exercise92.
THE ROLE OF AMPK IN EXERCISE-MEDIATED IMPROVEMENT OF LIVER LIPID METABOLISM
When ATP consumption is increased during physical exercise, the formation of ADP and AMP is sensed by AMP-activated protein kinase (AMPK). AMPK shifts liver lipid metabolism away from FFA synthesis through phosphorylation (and thereby suppression) of ACC and FAS, and of SREBP-1 to reduce expression of these lipogenic enzymes (Fig. 2). In addition, AMPK increases fatty acid oxidation through activation of CPT-193. Several basic scientific studies have documented that exercise activates AMPK-regulated pathways of lipid metabolism in the liver. Treadmill exercise resulted in increased levels of phosphorylated AMPK and ACC in livers of mice77,94,95. Moon et al. showed that this effect can be mediated by macrophage migration inhibitory factor, a cytokine of which metabolic effects are increasingly becoming known94 (Fig. 1).
EFFECTS ON HEPATOCYTE DAMAGE AND ACTIVATION OF INFLAMMATION
The activation of inflammation and the innate immune system, and the role of macrophages as predominant effector immune cells in NASH are well established96. Innate immune activation is thought to be a consequence of hepatocyte damage induced by the above-described toxic consequences of FFA. Indeed, elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as markers of hepatocyte damage positively correlate with NASH severity97. With cellular damage, the release of damage-associated molecular patterns (DAMPs) such as high-mobility group box-1 protein (HMGB1) and mitochondrial DNA can activate pattern recognition receptors on macrophages98,99, which are a major source of inflammatory cytokines leading to amplification of the inflammation.
In several human trials, various exercise regimens reduced the serum levels of ALT and AST43,100–107. In some studies, a direct correlation between reduced ROS and improvement in transaminase levels was found60. In a randomized trial comparing aerobic versus resistance training, 12 weeks of aerobic exercise resulted in a greater reduction in ALT and AST than resistance training100. In a small study in 15 obese women who failed a lifestyle intervention program, it was found that 24 weeks of a combination of voluntary and electrically stimulated movement of the quadriceps and hamstrings was also able to significantly lower ALT levels60. Contrarily, the meta-analysis of randomized trials by Keating et al. was unable to detect an effect of exercise on ALT, possibly because patients in most included trials had baseline ALT levels that already fell within the normal range21.
In several studies, levels of serum cytokines and inflammatory markers (IL-6, IL-8, TNF-α, ferritin, CRP) were significantly reduced, concurrently with a decrease in liver transaminases51. In addition, Oh et al. demonstrated that a high-intensity aerobic exercise program caused a reduction in TLR4, TLR5, CD11b, and CD14 expression on PBMCs, indicating an improvement in innate immune activation. Among the high-intensity aerobic group, they reported an increase in nuclear respiratory factor 2 (nrf2), a transcription factor that inhibits the macrophage inflammatory response52.
We can infer from these trials that physical exercise, or even electrically stimulated exercise, has a beneficial effect on hepatocellular damage and the consequent inflammatory activation in NAFLD patients.
EFFECTS ON PROGRESSION OF NASH TO FIBROSIS AND HCC
Besides the effects on the development of NASH, some studies have investigated the effects of exercise on downstream outcomes, such as fibrosis and progression to liver cancer. In rats with NASH, a treadmill running regimen reduced markers of fibrosis (collagen 1α1 mRNA, α-smooth muscle actin, and fibrosis scores) through decreased activation of hepatic stellate cells, which activation by lipid peroxidation products is thought to be the cause of fibrosis in the pathobiology of NASH (Fig. 1)108.
In a mouse model of NASH that progresses to HCC, Piguet et al. demonstrated that exercise-induced AMPK decreased mammalian target of rapamycin (mTOR) signaling, which reduced hepatocyte proliferation and tumor formation (Fig. 1)95. Although not in the setting of NASH, mice with diethylnitrosamine-induced primary liver cancer had a reduction in tumor burden when doing voluntary treadmill training, also indicating the beneficial effect of exercise on liver cancer progression109.
CONCLUSIONS
From the available literature, it is evident that physical exercise has a beneficial effect on NAFLD. Various regimens of aerobic and resistance training have been shown to reduce hepatic fat content through improvements in insulin resistance, liver fatty acid metabolism, liver mitochondrial function, and activation of inflammatory cascades. These data provide justification for the current guidelines that recommend an exercise regimen that fits with the patient’s individual abilities and preferences, in order to facilitate long-term compliance with a more active lifestyle14.
REFERENCES
- 1. Armstrong MJ, Houlihan DD, Bentham L, Shaw JC, Cramb R, Olliff S, Gill PS, Neuberger JM, Lilford RJ, Newsome PN. Presence and severity of non-alcoholic fatty liver disease in a large prospective primary care cohort. J Hepatol. 2012;56(1):234–40. [DOI] [PubMed] [Google Scholar]
- 2. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011;140(1):124–31. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–30.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 4. Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10(11):656–65. [DOI] [PubMed] [Google Scholar]
- 5. Mittal S, El-Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, May SB, Kramer JR, Richardson PA, Davila JA. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–31 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, Henley SJ, Holtzman D, Lake A, Noone AM, and others. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016;122(9):1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;149(2):367–78.e5; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 8. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia 2012;55(4):885–904. [DOI] [PubMed] [Google Scholar]
- 9. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, and others. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, and others. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015;385(9972):956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kantartzis K, Thamer C, Peter A, Machann J, Schick F, Schraml C, Konigsrainer A, Konigsrainer I, Krober S, Niess A, and others. High cardiorespiratory fitness is an independent predictor of the reduction in liver fat during a lifestyle intervention in non-alcoholic fatty liver disease. Gut 2009;58(9):1281–8. [DOI] [PubMed] [Google Scholar]
- 12. Palve KS, Pahkala K, Suomela E, Aatola H, Hulkkonen J, Juonala M, Lehtimaki T, Ronnemaa T, Viikari JSA, Kahonen M, and others. Cardiorespiratory fitness and risk of fatty liver: The Young Finns Study. Med Sci Sports Exerc. 2017;49(9):1834–41. [DOI] [PubMed] [Google Scholar]
- 13. Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary prevention of cardiovascular disease in diabetes mellitus. J Am Coll Cardiol. 2017;70(7):883–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55(6):2005–23. [DOI] [PubMed] [Google Scholar]
- 15. Eckard C, Cole R, Lockwood J, Torres DM, Williams CD, Shaw JC, Harrison SA. Prospective histopathologic evaluation of lifestyle modification in nonalcoholic fatty liver disease: A randomized trial. Therap Adv Gastroenterol. 2013;6(4):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava J, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis (NASH). Hepatology 2010;51(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, Jung DH, Kim KH, Yu E, Shim JH, and others. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: Analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27(8):1341–7. [DOI] [PubMed] [Google Scholar]
- 18. Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10(11):666–75. [DOI] [PubMed] [Google Scholar]
- 20. Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44(10):1236–43. [DOI] [PubMed] [Google Scholar]
- 21. Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 2012;57(1):157–66. [DOI] [PubMed] [Google Scholar]
- 22. Golabi P, Locklear CT, Austin P, Afdhal S, Byrns M, Gerber L, Younossi ZM. Effectiveness of exercise in hepatic fat mobilization in non-alcoholic fatty liver disease: Systematic review. World J Gastroenterol. 2016;22(27):6318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitsett M, VanWagner LB. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J Hepatol. 2015;7(16):2041–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wong VW, Chan RS, Wong GL, Cheung BH, Chu WC, Yeung DK, Chim AM, Lai JW, Li LS, Sea MM, and others. Community-based lifestyle modification programme for non–alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2013;59(3):536–42. [DOI] [PubMed] [Google Scholar]
- 25. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of physical activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–81. [DOI] [PubMed] [Google Scholar]
- 26. Kistler KD, Brunt EM, Clark JM, Diehl AM, Sallis JF, Schwimmer JB, Group NCR. Physical activity recommendations, exercise intensity, and histological severity of nonalcoholic fatty liver disease. Am J Gastroenterol. 2011;106(3):460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsunoda K, Kai Y, Kitano N, Uchida K, Kuchiki T, Nagamatsu T. Impact of physical activity on nonalcoholic steatohepatitis in people with nonalcoholic simple fatty liver: A prospective cohort study. Prev Med. 2016;88:237–40. [DOI] [PubMed] [Google Scholar]
- 28. Hallsworth K, Thoma C, Hollingsworth KG, Cassidy S, Anstee QM, Day CP, Trenell MI. Modified high-intensity interval training reduces liver fat and improves cardiac function in non-alcoholic fatty liver disease: A randomized controlled trial. Clin Sci. (Lond) 2015;129(12):1097–105. [DOI] [PubMed] [Google Scholar]
- 29. Nguyen-Duy T-B, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284(6):E1065–71. [DOI] [PubMed] [Google Scholar]
- 30. Perseghin G, Ntali G, De Cobelli F, Lattuada G, Esposito A, Belloni E, Canu T, Costantino F, Ragogna F, Scifo P, and others. Abnormal left ventricular energy metabolism in obese men with preserved systolic and diastolic functions is associated with insulin resistance. Diabetes Care 2007;30(6):1520–6. [DOI] [PubMed] [Google Scholar]
- 31. Keating SE, Hackett DA, Parker HM, O’Connor HT, Gerofi JA, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, and others. Effect of aerobic exercise training dose on liver fat and visceral adiposity. J Hepatol. 2015;63(1):174–82. [DOI] [PubMed] [Google Scholar]
- 32. Finucane FM, Sharp SJ, Purslow LR, Horton K, Horton J, Savage DB, Brage S, Besson H, De Lucia Rolfe E, Sleigh A, and others. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: A randomised controlled trial. Diabetologia 2010;53(4):624–31. [DOI] [PubMed] [Google Scholar]
- 33. Goodpaster BH, Delany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, Thomas SB, Brown J, McTigue K, Hames KC, and others. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. JAMA 2010;304(16):1795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larson-Meyer DE, Newcomer BR, Heilbronn LK, Volaufova J, Smith SR, Alfonso AJ, Lefevre M, Rood JC, Williamson DA, Ravussin E, and others. Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity (Silver Spring) 2008;16(6):1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17(12):2162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sullivan S, Kirk EP, Mittendorfer B, Patterson BW, Klein S. Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 2012;55(6):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, and others. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2005;90(6):3191–6. [DOI] [PubMed] [Google Scholar]
- 38. Zelber-Sagi S, Buch A, Yeshua H, Vaisman N, Webb M, Harari G, Kis O, Fliss-Isakov N, Izkhakov E, Halpern Z, and others. Effect of resistance training on non-alcoholic fatty-liver disease a randomized-clinical trial. World J Gastroenterol. 2014;20(15):4382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, Jakovljevic DG, Trenell MI. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 2016;59(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Houghton D, Thoma C, Hallsworth K, Cassidy S, Hardy T, Burt AD, Tiniakos D, Hollingsworth KG, Taylor R, Day CP, and others. Exercise reduces liver lipids and visceral adiposity in patients with nonalcoholic steatohepatitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2017;15(1):96–102 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee S, Bacha F, Hannon T, Kuk JL, Boesch C, Arslanian S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 2012;61(11):2787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Monteiro PA, Chen KY, Lira FS, Saraiva BT, Antunes BM, Campos EZ, Freitas IF Jr. Concurrent and aerobic exercise training promote similar benefits in body composition and metabolic profiles in obese adolescents. Lipids Health Dis. 2015;14:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slentz CA, Bateman LA, Willis LH, Shields AT, Tanner CJ, Piner LW, Hawk VH, Muehlbauer MJ, Samsa GP, Nelson RC, and others. Effects of aerobic vs. resistance training on visceral and liver fat stores, liver enzymes, and insulin resistance by HOMA in overweight adults from STRRIDE AT/RT. Am J Physiol Endocrinol Metab. 2011;301(5):E1033–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, Han HW, Chen S, Sun Q, and others. Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A randomized clinical trial. JAMA Intern Med. 2016;176(8):1074–82. [DOI] [PubMed] [Google Scholar]
- 45. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013;58(4):1287–95. [DOI] [PubMed] [Google Scholar]
- 46. Hallsworth K, Fattakhova G, Hollingsworth KG, Thoma C, Moore S, Taylor R, Day CP, Trenell MI. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60(9):1278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keating SE, Hackett DA, Parker HM, Way KL, O’Connor HT, Sainsbury A, Baker MK, Chuter VH, Caterson ID, George J, and others. Effect of resistance training on liver fat and visceral adiposity in adults with obesity: A randomized controlled trial. Hepatol Res. 2017;47(7):622–31. [DOI] [PubMed] [Google Scholar]
- 48. EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts 2016;9(2):65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 50. Haus JM, Solomon TP, Kelly KR, Fealy CE, Kullman EL, Scelsi AR, Lu L, Pagadala MR, McCullough AJ, Flask CA, and others. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98(7):E1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kawaguchi T, Shiba N, Maeda T, Matsugaki T, Takano Y, Itou M, Sakata M, Taniguchi E, Nagata K, Sata M. Hybrid training of voluntary and electrical muscle contractions reduces steatosis, insulin resistance, and IL-6 levels in patients with NAFLD: A pilot study. J Gastroenterol. 2011;46(6):746–57. [DOI] [PubMed] [Google Scholar]
- 52. Oh S, Shida T, Yamagishi K, Tanaka K, So R, Tsujimoto T, Shoda J. Moderate to vigorous physical activity volume is an important factor for managing nonalcoholic fatty liver disease: A retrospective study. Hepatology 2015;61(4):1205–15. [DOI] [PubMed] [Google Scholar]
- 53. Sun WH, Song MQ, Jiang CQ, Xin YN, Ma JL, Liu YX, Ma L, Lin ZH, Li CY, Liu L, and others. Lifestyle intervention in non-alcoholic fatty liver disease in Chengyang District, Qingdao, China. World J Hepatol. 2012;4(7):224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shojaee-Moradie F, Baynes KC, Pentecost C, Bell JD, Thomas EL, Jackson NC, Stolinski M, Whyte M, Lovell D, Bowes SB, Gibney J, Jones RH, Umpleby AM. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia 2007;50(2):404–13. [DOI] [PubMed] [Google Scholar]
- 55. Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: A cross-sectional study. Sci Rep. 2014;4:5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gambino R, Bugianesi E, Rosso C, Mezzabotta L, Pinach S, Alemanno N, Saba F, Cassader M. Different serum free fatty acid profiles in NAFLD subjects and healthy controls after oral fat load. Int J Mol Sci. 2016;17(4):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lavoie JM, Gauthier MS. Regulation of fat metabolism in the liver: Link to non-alcoholic hepatic steatosis and impact of physical exercise. Cell Mol Life Sci. 2006;63(12):1393–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cuthbertson DJ, Shojaee-Moradie F, Sprung VS, Jones H, Pugh CJ, Richardson P, Kemp GJ, Barrett M, Jackson NC, Thomas EL, and others. Dissociation between exercise-induced reduction in liver fat and changes in hepatic and peripheral glucose homoeostasis in obese patients with non-alcoholic fatty liver disease. Clin Sci. (Lond) 2016;130(2):93–104. [DOI] [PubMed] [Google Scholar]
- 59. Malin SK, Rynders CA, Weltman JY, Barrett EJ, Weltman A. Exercise intensity modulates glucose-stimulated insulin secretion when adjusted for adipose, liver and skeletal muscle insulin resistance. PLoS One 2016;11(4):e0154063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oh S, Maruyama T, Eguchi K, Shida T, Arai E, Isobe T, Okamoto Y, Shoda J. Therapeutic effect of hybrid training of voluntary and electrical muscle contractions in middle-aged obese women with nonalcoholic fatty liver disease: A pilot trial. Ther Clin Risk Manag. 2015;11:371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50(4):1105–12. [DOI] [PubMed] [Google Scholar]
- 62. Thompson D, Markovitch D, Betts JA, Mazzatti D, Turner J, Tyrrell RM. Time course of changes in inflammatory markers during a 6-mo exercise intervention in sedentary middle-aged men: A randomized-controlled trial. J Appl Physiol. (1985) 2010;108(4):769–79. [DOI] [PubMed] [Google Scholar]
- 63. Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. (1985) 2003;94(6):2127–34. [DOI] [PubMed] [Google Scholar]
- 64. Aoi W, Naito Y, Hang LP, Uchiyama K, Akagiri S, Mizushima K, Yoshikawa T. Regular exercise prevents high-sucrose diet-induced fatty liver via improvement of hepatic lipid metabolism. Biochem Biophys Res Commun. 2011;413(2):330–5. [DOI] [PubMed] [Google Scholar]
- 65. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117(6):1690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rabol R, Petersen KF, Dufour S, Flannery C, Shulman GI. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc Natl Acad Sci USA 2011;108(33):13705–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu J, Han L, Zhu L, Yu Y. Free fatty acids, not triglycerides, are associated with non-alcoholic liver injury progression in high fat diet induced obese rats. Lipids Health Dis. 2016;15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3(4):445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cintra DE, Ropelle ER, Vitto MF, Luciano TF, Souza DR, Engelmann J, Marques SO, Lira FS, de Pinho RA, Pauli JR, and others. Reversion of hepatic steatosis by exercise training in obese mice: The role of sterol regulatory element-binding protein-1c. Life Sci. 2012;91(11–12):395–401. [DOI] [PubMed] [Google Scholar]
- 70. Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes 2005;54(5):1314–23. [DOI] [PubMed] [Google Scholar]
- 71. Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98(7):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Oh S, So R, Shida T, Matsuo T, Kim B, Akiyama K, Isobe T, Okamoto Y, Tanaka K, Shoda J. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep. 2017;7:43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wu H, Jin M, Han D, Zhou M, Mei X, Guan Y, Liu C. Protective effects of aerobic swimming training on high-fat diet induced nonalcoholic fatty liver disease: Regulation of lipid metabolism via PANDER-AKT pathway. Biochem Biophys Res Commun. 2015;458(4):862–8. [DOI] [PubMed] [Google Scholar]
- 74. Suk M, Shin Y. Effect of high-intensity exercise and high-fat diet on lipid metabolism in the liver of rats. J Exerc Nutrition Biochem. 2015;19(4):289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tsuzuki T, Shinozaki S, Nakamoto H, Kaneki M, Goto S, Shimokado K, Kobayashi H, Naito H. Voluntary exercise can ameliorate insulin resistance by reducing iNOS-mediated S-nitrosylation of Akt in the liver in obese rats. PLoS One 2015;10(7):e0132029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol. 2008;294(3):G619–26. [DOI] [PubMed] [Google Scholar]
- 77. Cho J, Lee I, Kim D, Koh Y, Kong J, Lee S, Kang H. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J Exerc Nutrition Biochem. 2014;18(4):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi JH, Miller JD, Roberts LR, LeBrasseur NK, Robertson KD. High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics 2017;12(1):55–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001;120(5):1183–92. [DOI] [PubMed] [Google Scholar]
- 80. Croci I, Byrne NM, Choquette S, Hills AP, Chachay VS, Clouston AD, O’Moore-Sullivan TM, Macdonald GA, Prins JB, Hickman IJ. Whole-body substrate metabolism is associated with disease severity in patients with non-alcoholic fatty liver disease. Gut 2013;62(11):1625–33. [DOI] [PubMed] [Google Scholar]
- 81. Portincasa P, Grattagliano I, Lauterburg BH, Palmieri VO, Palasciano G, Stellaard F. Liver breath tests non-invasively predict higher stages of non-alcoholic steatohepatitis. Clin Sci. (Lond) 2006;111(2):135–43. [DOI] [PubMed] [Google Scholar]
- 82. Linden MA, Sheldon RD, Meers GM, Ortinau LC, Morris EM, Booth FW, Kanaley JA, Vieira-Potter VJ, Sowers JR, Ibdah JA, and others. Aerobic exercise training in the treatment of non-alcoholic fatty liver disease related fibrosis. J Physiol. 2016;594(18):5271–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300(5):G874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gonçalves IO, Passos E, Rocha-Rodrigues S, Diogo CV, Torrella JR, Rizo D, Viscor G, Santos-Alves E, Marques-Aleixo I, Oliveira PJ, and others. Physical exercise prevents and mitigates non-alcoholic steatohepatitis-induced liver mitochondrial structural and bioenergetics impairments. Mitochondrion 2014;15:40–51. [DOI] [PubMed] [Google Scholar]
- 85. Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99(8):1497–502. [DOI] [PubMed] [Google Scholar]
- 86. Irie M, Sohda T, Iwata K, Kunimoto H, Fukunaga A, Kuno S, Yotsumoto K, Sakurai K, Iwashita H, Hirano G, and others. Levels of the oxidative stress marker gamma-glutamyltranspeptidase at different stages of nonalcoholic fatty liver disease. J Int Med Res. 2012;40(3):924–33. [DOI] [PubMed] [Google Scholar]
- 87. Liu S, Shi W, Li G, Jin B, Chen Y, Hu H, Liu L, Xie F, Chen K, Yin D. Plasma reactive carbonyl species levels and risk of non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(6):1010–5. [DOI] [PubMed] [Google Scholar]
- 88. Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37(1):56–62. [DOI] [PubMed] [Google Scholar]
- 89. Yesilova Z, Yaman H, Oktenli C, Ozcan A, Uygun A, Cakir E, Sanisoglu SY, Erdil A, Ates Y, Aslan M, and others. Systemic markers of lipid peroxidation and antioxidants in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100(4):850–5. [DOI] [PubMed] [Google Scholar]
- 90. Oh S, Tanaka K, Warabi E, Shoda J. Exercise reduces inflammation and oxidative stress in obesity-related liver diseases. Med Sci Sports Exerc. 2013;45(12):2214–22. [DOI] [PubMed] [Google Scholar]
- 91. Oh S, Tanaka K, Tsujimoto T, So R, Shida T, Shoda J. Regular exercise coupled to diet regimen accelerates reduction of hepatic steatosis and associated pathological conditions in nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2014;12(5):290–8. [DOI] [PubMed] [Google Scholar]
- 92. Hu X, Duan Z, Hu H, Li G, Yan S, Wu J, Wang J, Yin D, Xie Q. Proteomic profile of carbonylated proteins in rat liver: Exercise attenuated oxidative stress may be involved in fatty liver improvement. Proteomics 2013;13(10–11):1755–64. [DOI] [PubMed] [Google Scholar]
- 93. Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am J Physiol Endocrinol Metab. 2016;311(4):E730–40. [DOI] [PubMed] [Google Scholar]
- 94. Moon HY, Song P, Choi CS, Ryu SH, Suh PG. Involvement of exercise-induced macrophage migration inhibitory factor in the prevention of fatty liver disease. J Endocrinol. 2013;218(3):339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Piguet AC, Saran U, Simillion C, Keller I, Terracciano L, Reeves HL, Dufour JF. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. 2015;62(6):1296–303. [DOI] [PubMed] [Google Scholar]
- 96. Wong VW, Chitturi S, Wong GL, Yu J, Chan HL, Farrell GC. Pathogenesis and novel treatment options for non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol. 2016;1(1):56–67. [DOI] [PubMed] [Google Scholar]
- 97. Tapper EB, Krajewski K, Lai M, Challies T, Kane R, Afdhal N, Lau D. Simple non-invasive biomarkers of advanced fibrosis in the evaluation of non-alcoholic fatty liver disease. Gastroenterol Rep (Oxf.) 2014;2(4):276–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li L, Chen L, Hu L, Liu Y, Sun HY, Tang J, Hou YJ, Chang YX, Tu QQ, Feng GS, and others. Nuclear factor high-mobility group box1 mediating the activation of Toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology 2011;54(5):1620–30. [DOI] [PubMed] [Google Scholar]
- 99. Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126(3):859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. El-Kader SMA, Al-Jiffri OH, Al-Shreef FM. Markers of liver function and inflammatory cytokines modulation by aerobic versus resisted exercise training for nonalcoholic steatohepatitis patients. African Health Sci. 2014;14(3):551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology 2009;50(1):68–76. [DOI] [PubMed] [Google Scholar]
- 102. Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, Choudhuri G. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21(1 Pt 1):191–8. [DOI] [PubMed] [Google Scholar]
- 103. Pugh CJ, Cuthbertson DJ, Sprung VS, Kemp GJ, Richardson P, Umpleby AM, Green DJ, Cable NT, Jones H. Exercise training improves cutaneous microvascular function in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2013;305(1):E50–8. [DOI] [PubMed] [Google Scholar]
- 104. Pugh CJ, Spring VS, Kemp GJ, Richardson P, Shojaee-Moradie F, Umpleby AM, Green DJ, Cable NT, Jones H, Cuthbertson DJ. Exercise training reverses endothelial dysfunction in nonalcoholic fatty liver disease. Am J Physiol Heart Circ Physiol. 2014;307(9):H1298–306. [DOI] [PubMed] [Google Scholar]
- 105. de Piano A, de Mello MT, Sanches Pde L, da Silva PL, Campos RM, Carnier J, Corgosinho F, Foschini D, Masquio DL, Tock L, and others. Long-term effects of aerobic plus resistance training on the adipokines and neuropeptides in nonalcoholic fatty liver disease obese adolescents. Eur J Gastroenterol Hepatol. 2012;24(11):1313–24. [DOI] [PubMed] [Google Scholar]
- 106. Skrypnik D, Ratajczak M, Karolkiewicz J, Madry E, Pupek-Musialik D, Hansdorfer-Korzon R, Walkowiak J, Jakubowski H, Bogdanski P. Effects of endurance and endurance-strength exercise on biochemical parameters of liver function in women with abdominal obesity. Biomed Pharmacother. 2016;80:1–7. [DOI] [PubMed] [Google Scholar]
- 107. Straznicky NE, Lambert EA, Grima MT, Eikelis N, Richards K, Nestel PJ, Dawood T, Masuo K, Sari CI, Dixon JB, and others. The effects of dietary weight loss on indices of norepinephrine turnover: Modulatory influence of hyperinsulinemia. Obesity (Silver Spring) 2014;22(3):652–62. [DOI] [PubMed] [Google Scholar]
- 108. Albano E, Mottaran E, Vidali M, Reale E, Saksena S, Occhino G, Burt AD, Day CP. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut 2005;54(7):987–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, and others. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23(3):554–62. [DOI] [PubMed] [Google Scholar]
- 110. Balducci S, Cardelli P, Pugliese L, D’Errico V, Haxhi J, Alessi E, Iacobini C, Menini S, Bollanti L, Conti FG, and others. Volume-dependent effect of supervised exercise training on fatty liver and visceral adiposity index in subjects with type 2 diabetes The Italian Diabetes Exercise Study (IDES). Diabetes Res Clin Pract. 2015;109(2):355–63. [DOI] [PubMed] [Google Scholar]
- 111. Levinger I, Goodman C, Peake J, Garnham A, Hare DL, Jerums G, Selig S. Inflammation, hepatic enzymes and resistance training in individuals with metabolic risk factors. Diabet Med. 2009;26(3):220–7. [DOI] [PubMed] [Google Scholar]
- 112. Straznicky NE, Lambert EA, Grima MT, Eikelis N, Nestel PJ, Dawood T, Schlaich MP, Masuo K, Chopra R, Sari CI, and others. The effects of dietary weight loss with or without exercise training on liver enzymes in obese metabolic syndrome subjects. Diabetes Obes Metab. 2012;14(2):139–48. [DOI] [PubMed] [Google Scholar]
- 113. Yoshimura E, Kumahara H, Tobina T, Matsuda T, Ayabe M, Kiyonaga A, Anzai K, Higaki Y, Tanaka H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes. 2014;2014:197216. [DOI] [PMC free article] [PubMed] [Google Scholar]