Abstract
OBJECTIVES:
Rubia cordifolia L. (RC) is a well-known and highly valuable medicinal plant in the Ayurvedic system. The present study involves evaluating antioxidant and cardioprotective property of RC root extract.
MATERIALS AND METHODS:
The characterization of RC root extract was carried out using standard phytochemical and biochemical analysis. The functional groups were analyzed by Fourier transform infrared (FTIR) spectroscopy and phytotherapeutic compounds were identified using high-resolution mass spectrometry (HR-MS). Cardioprotective activity of RC root extract was investigated against cyclophosphamide (CP; 100 mg/kg, i.p)-induced cardiotoxicity in male albino Wistar rats. RC (100, 200, and 400 mg/kg, p.o) or silymarin (100 mg/kg, p.o) was administered immediately after CP on the 1st day and the next consecutive 10 days. Biochemical and histopathological analysis was performed to observe the cardioprotective effects of RC root extract.
RESULTS:
Phytochemical analysis revealed the presence of secondary metabolites that include alkaloids, flavonoids, saponins, and anthraquinones in RC root extract. FTIR analysis revealed the presence of several functional groups. Based on HR-MS analysis, eight major phytotherapeutic compounds were identified in methanol root extract of RC. Biochemical analysis in CP-induced rat model administered with RC extract revealed significantly enhanced levels of antioxidant markers such as superoxide dismutase, catalase, and glutathione S-transferase. Histopathological study showed that the rat model treated with the root extract had reduced the cardiac injury.
CONCLUSION:
Our results have shown that the RC extract contains various antioxidant compounds with cardioprotective effect. Treatment with RC root extract could significantly protect CP-induced rats from cardiac tissue injury by restoring the antioxidant markers.
Keywords: Antioxidants, cardioprotection, cyclophosphamide, Fourier transform infrared, high-resolution mass spectrometry, Rubia cordifolia L.
Introduction
Heart failure is one of the most common cardiovascular diseases in humans across the globe. Coronary heart diseases and stroke are major public health issues in developed as well as developing countries.[1] Several classes of medicines and their combinational drugs are available in the market to treat cardiovascular diseases, but overall treatment is expensive and is known to cause side effects such as allergic reaction, hypotension, arrhythmia, and dizziness with severities ranging from minimal to fatal outcomes. Medicinal plants are considered as an important source of therapeutic agents and inherently have lesser side effects. Hence, herbal medicines may help in lowering the risk of heart failure and improve the normal functioning of the cardiovascular system by multitarget approach. Further, herbal medicines are less expensive and more affordable to all socioeconomic groups of the society.
Rubia cordifolia L. (RC) is a valuable medicinal plant in Ayurvedic system owing to its multiple pharmacological properties and often referred as common Madder or Indian Madder in the coffee family Rubiaceae. RC is also known as Manjistha, an Ayurvedic herb mentioned by Bhatt and Kushwah.[2] RC exhibits several beneficiary roles such as antioxidant, potent blood purifier, calcium channel blocker, diuretic, antiplatelet, antidiabetic, anti-stress, and vasodilating properties in cardiac health.[2,3] RC is known to inhibit platelet activating factor-induced platelet aggregation and may play a beneficial role in coronary artery disease.[4] Further, RC relaxes the spasms of smooth muscles of heart and blood vessels, like standard “calcium channel blocker” drug. This potent spasmolytic activity of RC suggests the presence of calcium channel blocker(s) like phytoconstituents. Therefore, it indicates possibility to treat arrhythmias (irregular heartbeats) resulting ischemia-reperfusion condition due to calcium overload.[5] RC also consists of chemical constituents such as rubiadin, quinine, morphine, aspirin, iridoids, glycosides, bicyclic hexapeptides, triterpenes, and many other bioactive secondary metabolites.[6] The free anthraquinones and combined anthraquinones present in RC exhibits antioxidant properties, which can be used in detoxification of ischemia-induced free-radical generation.[7]
The patients undergoing treatment for cardiac failure, who are overburdened with drug-induced toxic effects may seek remedy using herbal medicine for more efficacious, safer, and affordable option. Therefore, there is room for a novel approach in the treatment of heart failure with compounds having lesser side effect profile and higher efficacy than the existing treatment. Hence, the primary aim is to screen and characterize phytoconstituents in RC extract. Further, secondary aim is to evaluate the potential benefits of RC extract against cyclophosphamide (CP)-induced cardiotoxicity in albino Wistar rats.
Materials and Methods
Chemicals and reagents
The analytical grade chemicals and reagents were supplied by Merck India Ltd. Organic solvents such as petroleum ether, chloroform, ethyl acetate, and methanol (high-pressure liquid chromatography grade, Merck) were used. De-ionized water was obtained from ELGA water purification system (Metrohm, UK). The drugs used were Silymarin (Alrin-Bcap70 mg; Admac Pharma Ltd., India) and CP (Cyclomet 50 mg; BDH Industries Pvt. Ltd., India).
Collection of plant material
RC plant material was identified with the help of Senior Scientist Chandrasekhar B.S. presently working in the Institute of Wood Science and Technology, Ministry of Environment and Forests, Government of India. The plant material of RC was collected from Shimoga forest area situated in Karnataka, India. Fresh and healthy leaf, stem, and root parts were collected and thoroughly washed and shade dried for 15–20 days at room temperature (25°C ± 2°C), further pulverized to powdered form.
Preparation of extract and phytochemical screening
Powdered samples of leaf, stem, and root of RC was extracted using petroleum ether, chloroform, ethyl acetate, methanol, and distilled water for 18 h in the order of sequential polarity of solvents. The condensed extract was used for preliminary screening to determine the presence of bioactive compounds using the standard qualitative procedures.[8] The various phytoconstituents in extracts were confirmed by phytochemical tests such as Dragendorff's test, Mayer's test and Wagner's test for alkaloids, Borntrager's test and Modified Borntrager's test for anthraquinones, Anthrone reagent test, Benedict's reagent test and Molish's test for carbohydrates, sodium hydroxide test for coumarins, Shinoda test and Ammonia test for flavonoids, Benedict's reagent test and Fehling solution test for glycosides, ferric chloride test and phosphomolybdic acid test for phenols, sodium hydroxide test for quinines, foam test for saponins, Lieberman–Burchard test and Salkowski test for steroids and terpenoids, and Braemer's test for tannins were tested.
Identification of functional groups by Fourier transform infrared spectroscopy
Fourier transform infrared (FTIR) spectrum was analyzed to characterize the functional groups present in methanolic root extract of RC by potassium bromide (KBr) pellet technique. Dried powder of methanolic solvent extracts of RC was used for FTIR analysis. Ten milligrams of the dried extract powder was mixed along with 100 mg of KBr salt and compressed into a thin pellet and was loaded in FTIR spectroscope (BRUKER-TENSOR 27, Germany) using translucent sample discs. Infrared spectra were recorded on FTIR spectroscopy using OPUS software. The sample of RC was scanned with a spectral range of 4000–400 cm−1 with 64 scans at a resolution of 4 cm−1. Based on absorption frequencies, the functional group of all the spectra was interpreted.
Evaluation by high-resolution mass spectrometry
High-resolution mass spectrometry (HR-MS) analysis of RC methanolic root extract was performed using HR-MS quadrupole time-of-flight (Q-TOF) instrument (WATERS, SYNAPT G2-S, Milford, USA). About 10 mg of sample was dissolved in 1% formic acid in methanol and filtered using 0.45 μ PTFE membranes filter (PTFE, Waters, Milford, USA) and was injected. The sample was analyzed using BEH C18 column (150 mM × 4.6 mM, 5 μm id). A gradient elution of 35:65 1% formic aqueous solution: Acetonitrile (v/v) was used. The flow rate was 1.00 ml/min and the injection volume was 10 μl, and the sample was stored at 4°C in autosampler. HR-MS instrument employs the following conditions; the detection was operated in electron spray ionization mode with capillary voltage of 1 kV.
HR-MS parameters were optimized as follows: The temperature source was 90°C with desolvation gas temperature of 200°C and desolvation gas flow of 350 L/h. For the desired compound, a scan range of m/z of 50–1100 was selected, the automatic gain control was set at 3E6 and the injection period was set to 200 ms. Scan rate was at two scans s-1. External calibration was carried out using a calibration solution in positive and negative modes before each sample series. A targeted MS analysis was performed using the mass inclusion list and molecular weight of the targeted analysis with a 30 s time. The precursor's ions are filtered by the quadrupole which operates at an isolation window of m/z. The fore vacuum, high vacuum, and ultrahigh vacuum were maintained approximately 2 m bar, from 105 and below 1010 m bar, respectively. Collision energy (HCD cell) was operated at 30 kV. The software MassLynx H.I was used for data processing. Molecular name and structure were identified using MassBank database (http://www.massbank.jp) based on the molecular weights of the sample.[9]
Animal experiments
Animal studies were conducted after approval (No: MC/UCMS/IAEC-RRMCH-11/2015) from the Institutional Animal Ethics Committee (IAEC) of Rajarajeswari Medical College and Hospital, Bengaluru. IAEC was formed as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Animal Welfare Division, and Government of India. Male albino Wistar rats weighed between 120 and 150 g were selected and procured from Liveon Biolabs, Karnataka, Bengaluru, for experiments. Wistar rats were maintained under standard laboratory conditions at 25°C ± 2°C and fed with the standard pellet diet and accessed for water throughout the experiment. Totally 51 rats were selected, among this, 15 rats were used for acute toxicity test and remaining 36 rats were used for the further experiments. The dose selection for the drug was based on acute toxicity test. For determining the acute toxicity, each group of five Wistar rats was administered orally with 1000 mg/kg, 1500 mg/kg, and 2000 mg/kg body weight of root extract of RC in water. After 24 h, 40% of death rate was recorded in 2000 mg/kg body weight. LD-50 value of RC methanolic root extract was found to be 1000 mg/kg. Hence, 1/10th (100 mg), 2/10th (200 mg), and 4/10th (400 mg) of dose were considered to be safe for the animals, and same was selected as the therapeutic dose for evaluating cardioprotective activity of RC root extract.
Experimental protocol
The experimental animals were randomly grouped into six groups, having six rats in each group as follows:
Group 1: Control rats received distilled water (1 ml/kg bwt), orally for 10 days
Group 2: Rats were injected intraperitoneally with a single drug concentration of CP (100 mg/kg bwt, ip) dissolved in saline, on the initial day of the experimental period
Group 3: Rats received CP as in Group 2, quickly after that supplemented with RC extract (100 mg/kg bwt, po) through gavages for 10 continuous days
Group 4: Rats received CP as in Group 2, quickly after that supplemented with RC extract (200 mg/kg bwt, po) through gavages for 10 continuous days
Group 5: Rats received CP as in Group 2, quickly after that supplemented with RC extract (400 mg/kg bwt, po) through gavages for 10 continuous days
Group 6: Rats received CP as in Group 2, quickly after that supplemented with silymarin (100 mg/kg b.wt, p.o) through gavages for 10 continuous days.
Serum collection and organ preparation
Collection of blood sample from rats was done adhering to Good Laboratory Practices. Rats were anesthetized by ether and approximately 3–4 ml of blood was collected by cardiac puncture. Serum was separated and stored at −80°C until use. The hearts were dissected instantly and perfused with chilled saline. After washing with chilled saline, the hearts were gently patted dry, weighed, and each heart was divided longitudinally to produce two equal left and right halves. One half was homogenized in 10 volumes of (w/v) of phosphate buffer (50 mM, pH 7.4). The homogenates were centrifuged at 7000 ×g for 10 min at 4°C, and the supernatants were analyzed for catalase (CAT), superoxide dismutase (SOD), glutathione S-transferase (GST), and lipid peroxidation (LPO) levels.
Other half of heart tissue was subjected to histopathological study. The heart tissue was fixed with 10% buffered formalin solution. The tissue was embedded in paraffin; sections were cut at 5 μm and stained with hematoxylin and eosin and examined for histoarchitectural changes under light microscope.
Determination of superoxide dismutase, catalase, glutathione S-transferase, and LPO levels
SOD assay was performed as per Kakkar et al.; The assay reaction mixture contained 1.2 ml of 0.052 M sodium pyrophosphate buffer (pH 8.3), 0.1 ml of 186 μM phenazine methosulphate, 0.3 ml of 300 μM nitroblue tetrazolium, and 0.2 ml of 780 μM NADH. Required dilutions of enzyme extract of cardiac tissue and water were added to make up to total volume 3 ml. The reaction was started by adding NADH and incubated at 30°C for 90 s. The reaction was stopped by adding 1 ml glacial acetic acid and was stirred vigorously with 4 ml of n-butonal and allowed to stand for 10 min. The mixture was centrifuged at 4000 rpm for 10 min and butonal layer was taken out for spectrophotometer measurements at 560 nm. The unit of enzyme activity was defined and expressed in units/mg protein.[10] CAT activity was measured as specified by Aebi. The total volume of assay reaction mixture was 3 ml, containing a 0.1 ml enzyme extract of cardiac tissue, 1.9 ml of phosphate buffer (50 mM, pH 7), and 1.0 ml of 30 mM H2O2. The reaction was started by adding H2O2 and initial and final absorbance was recorded at 240 nm using ultraviolet-visible Spectrophotometer for 3 min with 30 s intervals. Controls were prepared using 0.1 ml phosphate buffer (0.1 M, pH 7.4). Finally, the CAT activity expressed as μmoles of H2O2 metabolized/mg protein/min.[11] GST was assessed as per Habig et al. The assay reaction volume was 3 ml containing 0.1M potassium phosphate buffer pH 6.5, 1 mM 1-chloro-2, 4-dinitrobenzene (CDNB), 1 mM GSH, and 0.1 ml of enzyme extract. The change in the optical density was read at 340 nm for 3 min and absorbance change per min was recorded. A complete assay mixture without enzyme was used as control. Finally, activity was expressed as nmol of CDNB conjugated/mg protein/min.[12] Lipid peroxidation (LPO) levels were determined by thiobarbituric acid (TBA) method as per Ohkawa et al. The assay mixture contained 0.1 ml of the enzyme extract of cardiac tissue, 0.2 ml of sodium dodecyl sulfate (8.1%), 1.5 ml of acetic acid solution (20%, pH 3.5), and 1.5 ml of 0.8% aqueous solution of TBA. The mixture was made up to 4 ml with distilled water and heated at 95°C for 60 min. After cooling, 1 ml of distilled water and 5 ml of n-butanol: Pyridine (15:1, v/v) were added and the mixture was shaken briskly followed by centrifugation at 4000 rpm for 10 min. The absorbance of the organic layer was measured at 532 nm and the standard curve was developed using tetraethoxypropane. Finally, results were expressed as TBA reactive substances in nmol/g wet weight.[13] For estimation of lipid peroxide (LPO) levels in serum, 50 μl of sample was treated with 4.0 ml of 0.083 N H2SO4 and 0.5 ml of 10% phosphotungstic acid. It was mixed well and allowed to stand for 5 min at room temperature, centrifuged at 4000 rpm for 10 min. Supernatant was discarded and repeated above steps with 2.0 ml of 0.083N H2SO4 and 0.3 ml of 10% phosphotungstic acid. The obtained pellet was further resuspended in 4 ml of sterile distilled water and 1 ml of TBA reagent and incubated at 95°C for 60 min. After cooling, 5 ml of n-butanol was added and centrifuged at 4000 rpm for 15 min. Organic layer was taken for absorbance at 553 nm, and results were expressed as TBA reactive substances in nmol/ml serum.[14]
Statistical analysis
Data were analyzed using the SPSS version 17 (SPSS Inc., Chicago, IL, USA). All the in vivo experiments were analyzed, and the results were expressed as mean ± standard error of mean for six rats in each group was analyzed using one-way analysis of variance followed by Student's paired t-test. The P < 0.05 is considered as statistically significant.
Results
Phytochemical screening
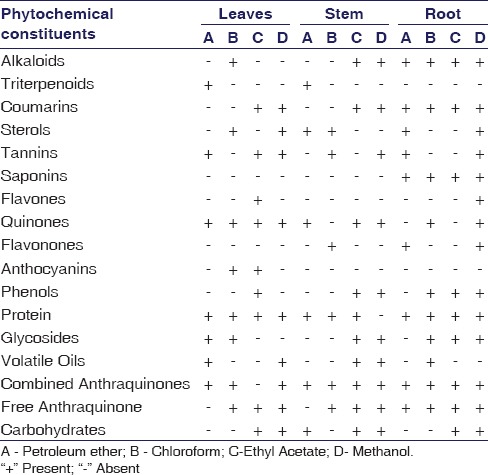
The yields of different extracts of RC are given in Table 1. It was observed that methanolic root extract produced maximum yield of phytochemicals and was about 14.18%. The major phytochemicals such as combined anthraquinones, free anthraquinones, alkaloids, steroids, flavones, flavonoids, phenols, saponins, tannins, proteins, and glycosides were found in solvent root extract. The result of phytochemical screening of root extract is summarized in Table 2.
Table 1.
Yield obtained from leaves, stem and root extract of R.cordifolia
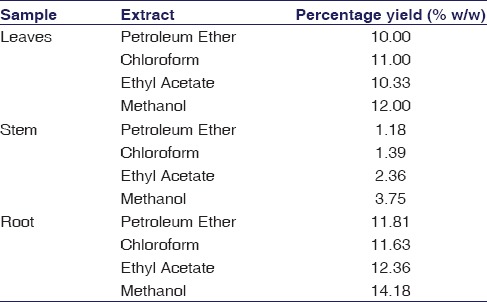
Table 2.
Phytochemical screening of leaves, stem and root extract of R.cordifolia
Fourier transform infrared spectroscopy and functional group analysis
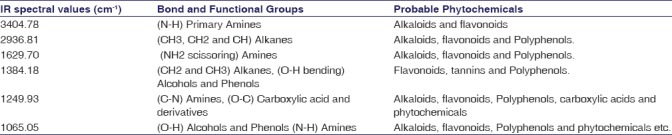
The FTIR spectroscopy was used to identify the functional groups of the active components present in methanolic root extract based on the peak values in the IR region. In the current investigation, FTIR analysis of RC has confirmed the presence of alcohol, phenols, amines, and carboxylic acids. The NH2 scissoring stretching frequency at 1629 cm−1 shows the presence of unsaturated amine group. The major IR stretching frequency at 3404 cm−1 was due to primary amines. The frequency at 2936 cm−1 was due to hydroxyl and aromatic C-H stretching frequency. The band at 1249 cm−1 and 1384 cm−1 were due to the >C=C< and CH2 groups, respectively. This together indicates the presence of a carboxylic acid group. The absorbance at 1065 cm−1 was due to OH stretching which indicates the presence of alcohols and phenols functional groups. There was no absorbance between the regions of 1800–2200 cm−1 indicates that there was no cyanide group in this extract. This result shows that RC does not contain any fatal toxic substances. The occurrence of functional groups in RC was shown in Figure 1 and IR absorption frequencies are tabulated in Table 3.
Figure 1.
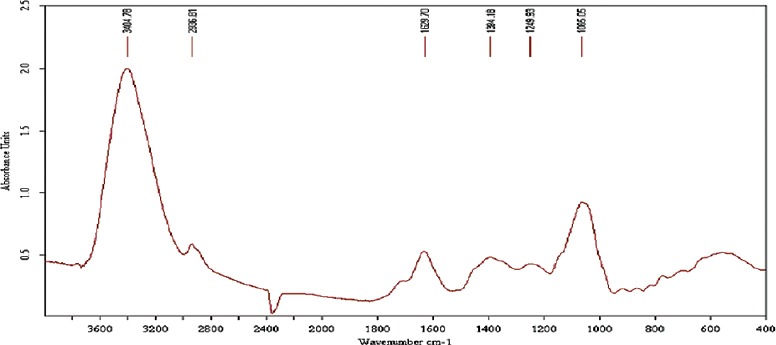
Fourier transform infrared spectrum of methanolic root extract of Rubia cordifolia
Table 3.
Major functional groups observed in the Fourier transform infrared spectra of methanolic root extract
High resolution-mass spectrometry analysis
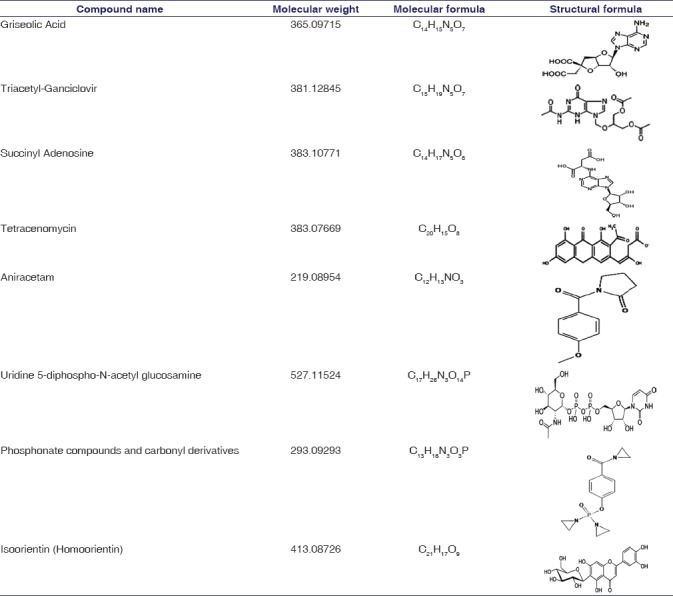
HR-MS was used for identifying large number of phytoconstituents in root extract of RC. Methanolic root extract of RC sample was subjected to HR-MS and analysis of MS spectra lead to identification of 42 different peaks in RC sample. The result of different peaks indicating the presence of phytocompounds was shown in the Figure 2. In this study, the samples were analyzed and spectra were acquired using high-resolution tandem Q-TOF mass spectrometer, which enables the precise measurements of m/z values. Based on molecular weight, respective phytocompounds were identified using MassBank database. In total, 42 unique mass signals were noted in methanolic root extract RC sample. Among these, eight major bioactive components were identified and were found to be griseolic acid (Mol. Wt: 365.09715), triacetyl-ganciclovir (Mol. Wt: 381.12845), succinyl adenosine (sAdo) (Mol. Wt: 383.10771), tetracenomycin (Mol. Wt: 383.07669), aniracetam (Mol. Wt: 219.08954), uridine 5-diphospho-N-acetylglucosamine (Mol. Wt: 527.11524), phosphonate compounds and carbonyl derivatives (Mol. Wt: 293.09293), and isoorientin (Mol. Wt: 413.08726). In our present study, the major eight compounds having a different molecular formula were tabulated in Table 4.
Figure 2.
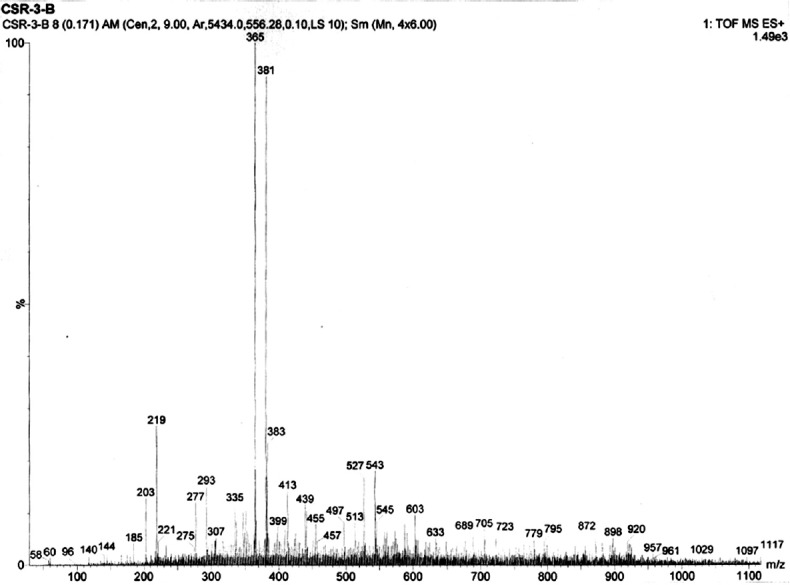
Mass spectrum of isolated compounds from methanolic root extract of Rubia cordifolia
Table 4.
Phytocompounds predicted in methanolic root extract of R.cordifolia by high-resolution mass spectrometry
Animal experiment
During acute toxicity test, we did not observe any behavioral changes such as shivering, spasm, salivation or hypersalivation, loose bowels, dormancy, or sleep for the duration of initial 4 h with RC extract (1000, 1500, and 2000 mg/kg b.wt) administration. There was no death observed after 24 h in 1000 mg/kg and 1500 mg/kg body weight. The 40% mortality rate was observed in 2000 mg/kg body weight. Hence, the LD-50 value of 1000 mg/kg dose was finalized.
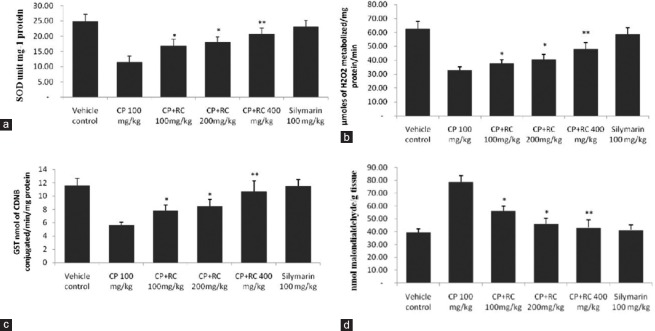
In the current study, intraperitoneal injection of single dose of CP alone in Group 2 showed extreme biochemical changes as well as oxidative injury in the cardiac tissue. Biochemical activities of glutathione-dependent antioxidant enzymes (GST) and antiperoxidative enzymes such as SOD and CAT were reduced significantly (P < 0.05) in heart tissue of Group 2 when compared to Group 1 control rats [Figure 3a–c]. Further, Group 3 to 5 showed an improvement in the levels of these enzymes as compared to Group 2 (P < 0.05). Group 5 rats receiving extract of RC at 400 mg/kg bwt showed almost same enzyme activity levels as compared to Group 6 reference control. SOD, CAT, and GST activity in tissue showed a dose-dependent increase with increasing dose of RC extract. This may be due to the protective property rendered by RC, against oxidative-induced tissue injury by CP.
Figure 3.
Effect of Rubia cordifolia on tissue superoxide dismutase (a), catalase (b), glutathione S-transferase (c), and lipid peroxidation (d) levels in cyclophosphamide-induced cardiotoxicity in rats. The data are expressed as the means ± standard error mean of six rats per group and analyzed using one-way analysis of variance followed by Student's paired t-test. *P < 0.05, **P < 0.01 when compared to cyclophosphamide-induced group. Note: Rubia cordifolia, cyclophosphamide
CP-treated rats showed a significant (P < 0.05) increase in the LPO levels when compared to vehicle control rats. The administration of RC extract after CP treatment in Groups 3 to 5 showed a significant decrease in the LPO levels compared to Group 2 [Figures 3d and 4], with maximum statistical difference found in Group 5 (P < 0.01). RC root extract 400 mg/kg group significantly decreased LPO levels as compared to CP-induced group.
Figure 4.
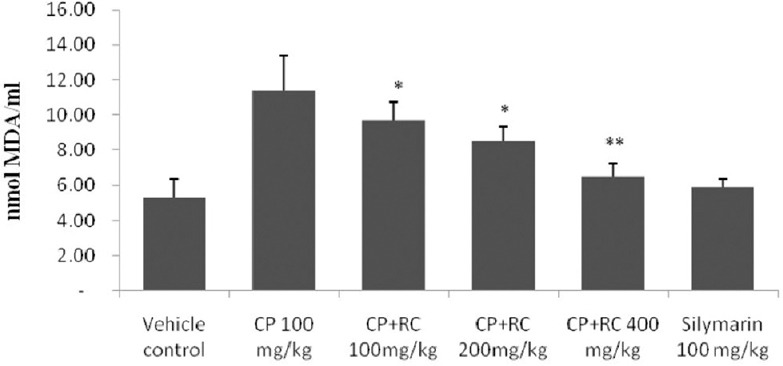
Effect of Rubia cordifolia on lipid peroxidation in cyclophosphamide-induced cardiotoxicity in rats. The data are expressed as the means ± standard error mean of six rats per group and analyzed using one-way analysis of variance followed by Student's paired t-test. *P < 0.05, **P < 0.01 when compared to cyclophosphamide-induced group. Note: Rubia cordifolia, cyclophosphamide
Histopathological examination
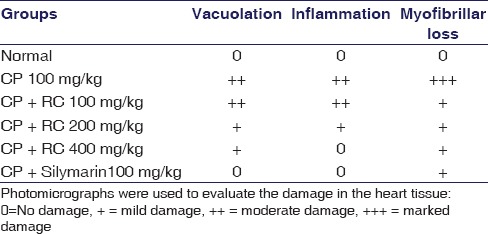
CP-induced changes in the myocardium showing significant level of vacuolar changes in cardiac muscle fibers [Figure 5 and Table 5]. Disintegration of myocardial tissue, vacuolization of cardiomyocytes, invasion of inflammatory cells, and myofibrillar loss were prominent observations in CP-treated rats [Figure 5b] when compared to vehicle control [Figure 5a] and silymarin treated rats [Figure 5f]. RC root extract provided dose-dependent protection against CP-induced myocardial damage as shown in Figure 5c–e.
Figure 5.
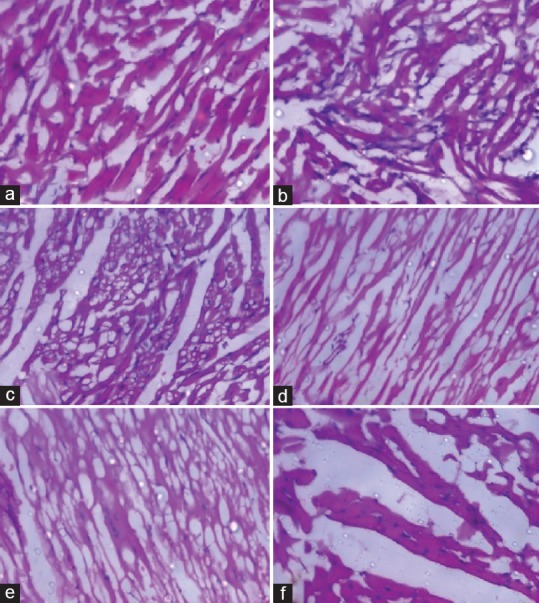
Histopathological evaluation of heart tissue in (a) Normal (H and E, 200×); (b) Cyclophosphamide 100 mg/kg treated (H and E, 200×); (c) Cyclophosphamide + Rubia cordifolia 100 mg/kg treated (H and E, 100×); (d) Cyclophosphamide + Rubia cordifolia 200 mg/kg treated (H and E, 100×); (e) Cyclophosphamide + Rubia cordifolia 400 mg/kg treated (H and E, 100×); (f) Cyclophosphamide + silymarin 100 mg/kg treated rats (H and E, 400×)
Table 5.
Histological changes in myocardial tissues
Discussion
Phytochemical screening and identification of compounds
Therapeutic properties of medicinal plants are due to the presence of diverse secondary metabolites such as flavonoids, alkaloids, saponins, glycosides, and sterols.[15] In our study, we characterized phytoconstituents of RC extract using various phytochemical techniques. Based on our analysis, methanolic root extract of RC has shown higher yield of phytochemicals as compared to leaves and stem [Table 1]. Hence, the methanolic root extract was selected for further characterization. Earlier studies have reported that the presence of anthraquinones in this plant could be responsible for its antioxidant and cardioprotective properties.[2,7] Our results also have shown the presence of cardioprotective phytoconstituents that includes alkaloids, flavonoids, saponins, tannins, combined anthraquinones, and free anthraquinones, which are tabulated in Table 2. Hence, our observation in RC is critical due to the presence of all above-mentioned vital classes of phytochemicals in methanolic root extract. Additional evidence generated from the FTIR analysis of RC root extract has shown the presence of functional groups such as alcohols, phenols, aldehydes, carboxylic acids, amines, and alkanes, thus revealing the presence of vital phytoconstituents [Table 3]. Studies have reported that these phytochemicals exhibits antioxidant, antidiabetic and anti-inflammatory properties.[3,16]
HR-MS analysis revealed the presence of 42 constituents including eight major bioactive components uridine-5-diphospho-N-acetylglucosamine, phosphonate compounds and carbonyl derivatives, griseolic acid, ganciclovir, sAdo, tetracenomycin, aniracetam, and isoorientin [Figure 2 and Table 4]. Among these, uridine-5-diphospho-N-acetylglucosamine and phosphonate compounds and carbonyl derivatives are of particular importance due to their free-radical scavenging property involved in the endogenous mechanism of cardioprotection.[17,18,19,20]
Studies have shown that O-linked N-acetyl glucosamine levels change in response to nutrition and stress, as well as their signaling is shown to have cardioprotection by direct modification of nuclear and cytoplasmic proteins.[17,18] In the present study, uridine-5-diphospho-N-acetylglucosamine has been identified in RC, which might play an important role in cardioprotection by acting against free-radical injury of cardiac myocytes. We also identified the presence of (N)-methanocarbaphosphonate, an analog of 5'-AMP, acting as cardioprotective agent through cardiac P2X receptors, which is a trimeric ligand-gated ion channel explored as a target for the treatment of heart failure.[19,21,22] Other six compounds, griseolic acid, ganciclovir, s-Ado, tetracenomycin, aniracetam, and isoorientin are used as therapeutic agents in antiasthmatic, psychotropic, neurotrophic, anti-inflammatory, and Type-II diabetes.[23,24,25,26,27,28] Hence, the study on RC containing above-mentioned compounds is of particular interest in medicine owing to its wide array of potential pharmacological effects.
Cardioprotective effect of Rubia cordifolia extract in Wistar rats
We treated CP-induced rat models with RC extract and monitored for cardioprotective effect. These rats were monitored for LPO levels, SOD, CAT, and GST activity to evaluate the degree of myocardial injury. Enzyme activity levels of SOD, CAT, and GST increased, whereas LPO levels decreased significantly in CP-induced rats treated with RC extract indicating cardioprotection by scavenging of free radicals. Histopathological observation in CP-induced rats showed varying degree of vacuolation, inflammatory infiltrate, and myofibrillar damage in the myocardium. However, treatment with RC root extract effectively inhibited CP-induced cardiac damage by preventing the destructive pathological processes [Figure 5 and Table 5]. Our study revealed the presence of uridine-5-diphospho-N-acetylglucosamine, phosphonate compounds, and carbonyl derivatives, which are active ingredients in RC root extract might be responsible for the abrogation of CP-elicited cardiotoxicity. Studies suggest that uridine-5-diphospho-N-acetyl glucosamine, phosphonate compounds, and carbonyl derivatives possess cardioprotective properties.[16,18] Hence, these phytoconstituents in RC root extract might be responsible for the protective effect against CP-induced cardiotoxicity.
Conclusion
Based on phytoscreening, FTIR, and HR-MS analysis, we observed the presence of different compounds including uridine-5-diphospho-N-acetylglucosamine, phosphonate compounds, and carbonyl derivatives in root extract of RC. These components may be involved in antioxidant, anti-inflammatory, and cardioprotective properties. Our study demonstrates that root extract of RC augmented the myocardial SOD, CAT, and GST enzyme activity levels and preserved histoarchitecture in CP-induced rats. This cardioprotective property of RC root extract might be due to synergetic effect of bioactive compounds present in them making it good source for the production of cardioprotective herbal medicines. These compounds may provide new directions for identification of cardioprotective agents, which may be given concomitantly with existing drugs used for heart failure. However, further investigations are essential to elucidate the precise molecular mechanism of these bioactive components present in RC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fuster V, Kelly BB, editors. Institute of Medicine (US) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenge to Achieve Global Health. Washington (DC): National Academies Press (US); 2010. [Last accessed on 2017 Aug 16]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK45693/ [PubMed] [Google Scholar]
- 2.Bhatt P, Kushwah AS. Rubia cordifolia overview: A new approach to treat cardiac disorders. Int J Drug Dev Res. 2013;5:47–54. [Google Scholar]
- 3.Divakar K, Chandrasekar SB, Goli D. Diuretic activity of root extract of Rubia cordifolia linn. Pharmacologyonline. 2009;1:597–603. [Google Scholar]
- 4.Tripathi YB, Pandey S, Shukla SD. Anti-platelet activating factor property of Rubia cordifolia linn. Indian J Exp Biol. 1993;31:533–5. [PubMed] [Google Scholar]
- 5.Gilani AH, Janbaz KH, Zaman M, Lateef A, Suria A, Ahmed HR, et al. Possible presence of calcium channel blocker(s) in Rubia cordifolia: An indigenous medicinal plant. J Pak Med Assoc. 1994;44:82–5. [PubMed] [Google Scholar]
- 6.Rao GM, Rao CV, Pushpangadan P, Shirwaikar A. Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia linn. J Ethnopharmacol. 2006;103:484–90. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 7.Yen GC, Duh PD, Chuang DY. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000;70:437–41. [Google Scholar]
- 8.Harborne AJ. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. Netherlands: Springer Science & Business Media; 1998. [Google Scholar]
- 9.Horai H, Arita M, Nishioka T. Comparison of ESI-MS Spectra in MassBank Database. In BioMedical Engineering and Informatics, 2008; BMEI 2008. Vol. 2. International Conference on; 2008. pp. 853–7. [Google Scholar]
- 10.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 11.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 12.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 13.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 14.Yagi K. Free Radical and Antioxidant Protocols. Totowa, NJ: Humana Press; 1998. Simple assay for the level of total lipid peroxides in serum or plasma; pp. 101–6. [DOI] [PubMed] [Google Scholar]
- 15.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med. 2012;7:26. doi: 10.1186/1749-8546-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, et al. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–82. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 18.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Shen JB, Cronin C, Sonin D, Joshi BV, Gongora Nieto M, Harrison D, et al. P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy: Implications for the treatment of heart failure. Am J Physiol Heart Circ Physiol. 2007;292:H1077–84. doi: 10.1152/ajpheart.00515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine: Peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990;265:2563–8. [PubMed] [Google Scholar]
- 21.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–83. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen JB, Pappano AJ, Liang BT. Extracellular ATP-stimulated current in wild-type and P2X4 receptor transgenic mouse ventricular myocytes: Implications for a cardiac physiologic role of P2X4 receptors. FASEB J. 2006;20:277–84. doi: 10.1096/fj.05-4749com. [DOI] [PubMed] [Google Scholar]
- 23.Tulshian DB, Czarniecki M. Total synthesis of griseolic acid. J Am Chem Soc. 1995;117:7009–10. [Google Scholar]
- 24.Drew WL, Miner RC, Busch DF, Follansbee SE, Gullett J, Mehalko SG, et al. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163:716–9. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 25.Yue S, Motamedi H, Wendt-Pienkowski E, Hutchinson CR. Anthracycline metabolites of tetracenomycin C-nonproducing Streptomyces glaucescens mutants. J Bacteriol. 1986;167:581–6. doi: 10.1128/jb.167.2.581-586.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CR, Benfield P. Aniracetam. An overview of its pharmacodynamic and pharmacokinetic properties, and a review of its therapeutic potential in senile cognitive disorders. Drugs Aging. 1994;4:257–73. doi: 10.2165/00002512-199404030-00007. [DOI] [PubMed] [Google Scholar]
- 27.Krijt J, Kmoch S, Hartmannová H, Havlícek V, Sebesta I. Identification and determination of succinyladenosine in human cerebrospinal fluid. J Chromatogr B Biomed Sci Appl. 1999;726:53–8. doi: 10.1016/s0378-4347(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 28.Anilkumar K, Reddy GV, Azad R, Yarla NS, Dharmapuri G, Srivastava A, et al. Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of Pueraria tuberosa. Oxid Med Cell Longev 2017. 2017:5498054. doi: 10.1155/2017/5498054. [DOI] [PMC free article] [PubMed] [Google Scholar]