Abstract
Additive manufacturing, or 3D printing, has become significantly more commonplace in tissue engineering over the past decade, as a variety of new printing materials have been developed. In extrusion-based printing, materials are used for applications that range from cell free printing to cell-laden bioinks that mimic natural tissues. Beyond single tissue applications, multi-material extrusion based printing has recently been developed to manufacture scaffolds that mimic tissue interfaces. Despite these advances, some material limitations prevent wider adoption of the extrusion-based 3D printers currently available. This progress report will provide an overview of this commonly used printing strategy, as well as provide insight into how this technique can be improved. As such, we hope that our prospective report guides the inclusion of more rigorous material characterization prior to printing, thereby facilitating cross-platform utilization and reproducibility.
Keywords: extrusion-based 3d printing, hydrogels, printing parameters, bioprinting
Graphical Abstract
Extrusion-based 3D printing has demonstrated significant promise for the fabrication of cell-free and cell-laden engineered tissues This progress report discusses extrusion-based 3D printing and recent advances in this field with examples of how they are approaching biomedical engineering problems. These highlights illustrate the advancements that are leading the way for development, characterization, and design of materials for 3D printing.
1. Introduction
3D printing or rapid prototyping has long been an established field for materials processing. Over the past decade, this technology has become increasingly applied to materials for healthcare as the number and diversity of biological printing substrates has increased while the cost associated with fabrication strategies has decreased. Commercial industrial grade printers can cost >$250k USD, but the advent of low cost printing options, including RepRap, MakerBots, BioBots, and others, have greatly expanded accessibility to 3D printers useful for printing biomaterials. Although these printers have limitations, they have spurred the development of novel materials and their applications. As a result, this has provided researchers with new ways to address tissue engineering problems not previously possible via traditional fabrication strategies, e.g. hydrogels.[1–3]
Extrusion-based 3D printing is one of the most common printing methodologies, and thus we will largely focus on the progress made with this type of printing. This method benefits from wide adoption, ease of use, precision printing of complex geometries via computer-aided design (CAD), and multiple solidification methods, despite requiring materials with specific printability characteristics.[4–7] With this report, we will describe how each of these characteristics affects printing. For example, one of the most common solidification strategies is photo-crosslinking using free radical based photoinitiators and UV. [7,8] Scaffolds can be cured using temperature changes, modification of pH or ion concentration, as well as combinations or other means of curing. [8–12] Regardless, crosslinking typically occurs in the presence of cells for bioinks, so methods that minimize conditions that negatively impact survival while also optimizing the mechanical properties, mimicking surface architecture, and maintaining other physical features have become widely adopted.[4,12–24]
After discussing printing parameters, we will next turn to printing cell-laden scaffolds, which adds complexity to successful fabrication and survival.[7,8,10,11,18,25–28] These issues can largely be reduced to: 1) biocompatibility of the curing agent or crosslinking mechanism, 2) mechanical forces which the cells are exposed to during the printing process, and 3) nutrient, waste, and gas exchange once the scaffold is fabricated. Cytotoxicity of the materials, solvents, and printing parameters also need to be accounted for during the design stages as well as during the fabrication process. For example, limited UV exposure to cure photo-crosslinkable hydrogels can have minimal effect on cells embedded within the hydrogel; too much or intense UV exposure can lead to deleterious effects on cell survival, function, and proliferation. Furthermore, shear stresses present when extruding the material can lead to cell death.[25] Therefore, careful considerations must be made to ensure the scaffold is fabricated quickly enough to minimize cell exposure to these harsh environments, but slow enough to minimize cell exposure to these forces to prevent cell death. Once fabricated, the scaffold needs appropriate nutrient exchange for the long-term survival of the scaffold either in vitro or in vivo.
Given the wide range of parameters that can affect extrusion-based 3D printing and the relatively inconsistent characterization of material parameters that result in successful scaffolds discussed above, we will focus the beginning of this report on how scaffolds are designed, modeled, and fabricated using extrusion-based 3D printing. We will then discuss specific variants of extrusion-based 3D printing, how they can be used for both cell-free and cell-laden (bioink) printing, and what the current state-of-the-art applications are using this methodology versus other 3D printing methods. In a third section of this report, we will focus on the major challenge of reproducibility from laboratory to laboratory using different models of extrusion based printers. We will conclude our report with the major current and future challenges that need to be addressed for the advancement of this methodology.
2. Designing, modeling, and fabricating extrusion-based 3D constructs
In the past decade, extrusion-based 3D printing has transitioned from singe component or material printing to a more complex multi-material printing. This transition has been facilitated through the use of a combination of custom and opensource software and printers as well as the continued advancement of 3D printer technologies available such as the use of multiple print heads concurrently.[28–32] For example, in addition to custom built printers there are more off-the-shelf options available with the ability to print with multiple materials and under several different conditions such as the BioBot 1 and 2 by BioBots; the 3D-Bioplotter Starter, Developer, and Manufacturer Series by EnvisionTEC; and 3Dη series by NScrypt as well as the 3DDiscovery by regenHU. Furthermore, this has been augmented by interest in translating acquired images from commercially and clinically available imaging modalities such as MRI, CT, and ultrasound into formats able to be processed and sliced for printing. In this section, we will discuss the fundamentals of extrusion-based 3D printing and discuss how objects are designed, modeled, and fabricated.
First, extrusion-based 3D printing methods typically use a pneumatic actuator or screw device to feed material through a cartridge and into a nozzle or needle for deposition. These common extrusion methods enable compatibility with a large number of materials, but all feature a curing step that is chemical, photoactivated, etc. Material deposition in X, Y, and Z, are controlled by actuators that regulate positioning of the nozzle in three dimensions. Printing complex geometries with this method can require sacrificial supports as each layer is built on top of a previous layer. Multi-head or -nozzle printers can enable seamless printing of both materials to create these models with minimal user input aside from geometry and materials. However, this added complexity requires that materials be carefully chosen for compatibility with printing conditions, and that the printer be carefully calibrated such that there is not a mismatch in the dimensions of the support scaffold and the desired object. Finally when designing a scaffold, one should select the appropriate printing material, as certain materials perform better and have been extensively characterized. For instance, for bone tissue engineering, a common material is polycaprolactone (PCL) due to its relatively low melting point (~60°C), its mechanical strength being similar to native tissue (compressive modulus ~150 to 200 MPa), and its ability to be compounded with bioactive molecules to aid in the deposition of bone.[16,22,32,33] However if the end goal is to fabricate a scaffold out of specific cell types, bioinks are used, which allow for direct printing of cells embedded in hydrogels.[8,23,27,28,31,34–36] These materials tend to have reduced mechanical strength versus thermoplastics, making fabrication with multiple materials necessary to achieve the desired structural integrity.
Secondly to model a specific system using an extrusion-based 3D printer, a user must employ a 3D geometry designed via CAD software. The user can define specific parameters that will interpolate surface features and/or the inner geometry. For constructs eventually requiring perfusion, CAD models can be processed using fluid dynamics simulations to model fluid flow through the design, nutrient exchange and consumption, as well as diffusion into/out of the fabricated object.[37–39] Therefore even with complex geometries, boundary conditions for the culture and cell survival can be established theoretically prior to fabrication to ensure compatibility with systems such as syringe pumps, bioreactors, or other dynamic and static culturing methods. Yet, once an engineered tissue or mimetic system becomes exceedingly large, nutrient exchange and transfer become limiting factors. In vivo vasculature provides a mechanism for delivering nutrients and removing waste from tissue. While that may also be the case for implantable 3D printed devices in vitro, nutrient and waste exchange becomes more complicated by diffusion limits and nutrient consumption. Additionally, host circulation will regulate chemical signaling, maintain homeostasis, and deliver the host’s defenses to protect against infection. Vascular mimetics developed by Miller et al are a good example of this, where they described the generation of larger cell laden hydrogels using sacrificial molds.[40] Aside from vascularity, structure porosity, which can also be analyzed in silico, can modulate appropriate flow rates to ensure adequate nutrient exchange within the device.[41,42] Prior to fabrication, more careful consideration of diffusion and nutrient limitations may improve experimental efficiency by reducing trial and error typically encountered with material modification.
There has been a push to develop more patient-specific approaches to the design of 3D printed scaffolds. One method of generating patient-specific geometries is to acquire images from typical medical imaging technologies such as MRI, CT, and ultrasound scans. These source images can then be imported into a CAD software to develop your model. While image acquisition can be cost prohibitive, online repositories of 3D models (https://3dprint.nih.gov) have been instrumental in the proliferation of 3D printed models as it eliminates the need for direct access to these costly imaging devices and techniques. We believe that the expansion and sharing of 3D model data will provide researchers with the ability to rapidly reproduce models. It should also allow researcher more time to spend on material development and functionalization strategies.
Finally, to fabricate an extrusion-based 3D construct, one must first take their design–after fully vetting the model–and fabricate it with the printer. To accomplish this, slicing programs are used to take the overall geometry produced in CAD software and slice it into layers such that it can be printed using material cylinders created during the extrusion process. During slicing, the spacing between layers set by the printer is critical to ensure adequate contact between layers and prevent delamination. The exact amount of overlap desired is material dependent and determined by user input, but typically Z height change between layers can be 75% to 100% of the strand diameter. Between each layer, it is common to rotate fiber orientation by a specified angle to create different pore sizes and contact angles. Depending on the mechanical properties of the material being deposited, strand-to-strand spacing may be modified to prevent layer sagging, especially in unsupported regions. These properties can be augmented by changing strand diameter via (1) needle diameter, (2) extrusion rate, (3) printer head speed, (4) extruded material viscosity, and (5) temperature of the nozzle. For instance, as the extrusion rate decreases at a fixed temperature, printer head speed, and needle (or nozzle) diameter, the thickness of the resultant strand will decrease. Similarly, as the needle diameter is decreased or the printer head speed is increased, the resultant strand diameter will decrease although this change may need to be accompanied by an increase in applied pressure to result in uniform strand deposition. Material viscosity and temperature of the nozzle are interconnected and can have similar effects of print fidelity and strand diameter. If viscosity is too low, then the material may flow too much after printing and the strand will not be uniform and will typically flatten. Therefore, it is important to carefully select these parameters to ensure the best print fidelity and to help maintain structural integrity.
At the outset of this report and before discussion specific variants of extrusion-based 3D printing, we note some of its general advantages and caveats. Extrusion-based 3D printing is more cost effective and arguably easier to adopt than other printing methods mentioned previously. Secondly geometrical and fabrication parameters can be easily changed to accomplish the user’s scaffold requirements, e.g. high modulus, structural integrity, etc. For example, layer-by-layer fabrication using cylindrical fibers increases structural integrity compared to other 3D printing strategies, e.g. inkjet or droplet based fabrication. By reducing the number of material interfaces, there is decreased need for strong bonds between deposited layers, though these interfaces still require characterization to ensure a strong bond between layers. For materials that are cured using a light source, this can be achieved by purposefully “soft-curing” i.e. partially crosslinking the material at each layer. This will help ensure that there are still reactive groups in the previous layer to form covalent bonds with the newly added layer. Additionally, printing parameters can also be changed to decrease the interface area to volume ratio, which will increase the mechanical strength of the resultant print. Despite these advantages, specific caveats should be and are being addressed by the field. For example, cell exposure to shear forces or harsh curing parameters negatively impact survival. The requirement of multiple materials to mimic a complex tissue or potentially provide the necessary structural support requires the complication of multi-material extrusion.[17,19,32] Multi-component systems, however, can easily create interfacial tissues in bone, muscle, vasculature, and organogenesis. [19,28–31,43,44] Thus while this remains a concern, it can be used to one’s advantage in these applications. As discussed previously, another critical caveat is efficient nutrient, gas, and waste exchange. While the incorporation of channels or embedded microvasculature alleviates this concern, and similar solutions exist for other caveats, these challenges exist nonetheless for basic users of extrusion-based 3D printing systems. While this is certainly not an exhaustive list of the pros and cons to extrusion-based printing, we believe that it represents critical points that one can take into account prior to adopting an extrusion-based 3D printing approach.
3. Variations on extrusion-based 3D printing
Beyond basic approaches to extrusion-based 3D printing, there are several common variants. In this section, we note what these variants are and how they can be used for both cell-free and cell-laden (bioink) printing. We also discuss current state-of-the-art applications using this methodology, especially how they relate to vascularized mimics, multi-material scaffolds, and tissue mimetics.
3.1. Fused deposition modeling (FDM) and extrusion-based printing
FDM uses a heated print head or cartridge to melt a polymer or mixture and uses a feeding mechanism such as a screw or pressure driven system to push the highly viscous melt through the syringe. This process yields structures that have well defined architecture and well controlled geometry. Given the high temperatures usually involved in this form of 3D printing, FDM is used to fabricate designs out of scaffolding materials or to fabricate scaffolds that will be used as sacrificial layers post printing. The following sections illustrate some of the more common and most recent advances in applications of FDM.
3.1.1. Vascular mimetic models
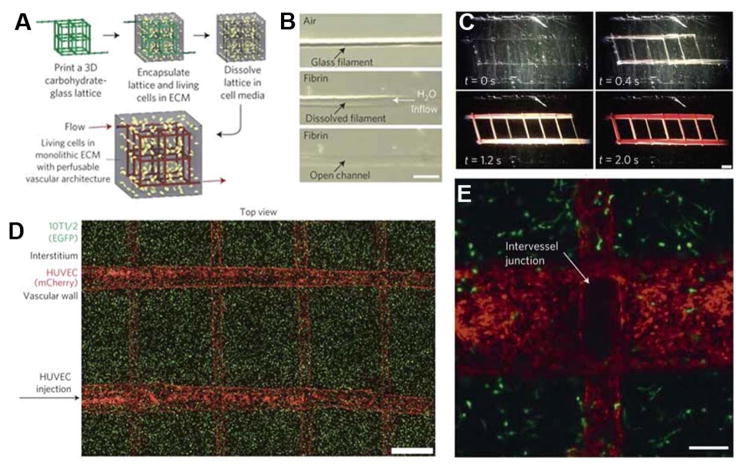
Inadequate nutrient and waste exchange is a significant tissue engineering problem that limits the scaffold size when vasculature is absent. As such, many groups have attempted to 3D print acellular scaffolds to generate a microvasculature that could provide sufficient nutrient flux.[45–47] One of the most common methods to make these models is to print a sacrificial mold, a method pioneered by Miller and coworkers in which liquid sugar was FDM-printed onto a surface, crystallized, and an extracellular matrix deposited into the interstitial spaces (Figure 1). This original concept has been further refined by coating the printed substrate to inhibit solubilization when an aqueous hydrogel containing cells is cast around it. After casting the hydrogel, the substrate is dissolved using warmed water with a perfusion system, resulting in channels that can be seeded with HUVECs to make the artificial vasculature.[40] Although originally described in vitro, a similar method has also been adopted in vivo application.[48] Kolesky and coworkers fabricated similar mimetics using gelatin as a sacrificial layer and expanded to larger tissues.[18,19,49] Lee and colleagues used this same concept, but instead of fabricating an acellular gelatin layer, they exploited the biocompatibility of gelatin and its thermoresponsive gelation/melting to directly seed cells in a cell-laden hydrogel and then dissolved away the gelatin. Gradually dissolving the hydrogel enabled gelatin-embedded HUVECs to adhere to the inner lumen of the vascular mimetic. These examples represent a fraction of the progress in this area, but despite these successes, there remain key fabrication issues that prohibit a wide array of materials to be used in this application. For example, the interdependence of bulk and template material properties reduces the potential combinations available for 3D printing of vascular mimetics. This observation, combined with successful initial approaches, reinforces the need to further develop and more completely characterize biomaterials for this application.
Figure 1. Examples of 3D printed sacrificial vasculature.
A–C) Workflow and demonstration of fabricating vasculature-like structures using 3D printing and dissolving of the sugar sacrificial channels with the subsequent perfusion of the mimetic. D–E) Demonstration of cell viability within the hydrogel and lining of the artificial vasculature with the formation of intervessel junctions. Reproduced and adapted with permission.[40] Copyright 2012, Nature Publishing Group.
3.1.2. Bone mimetic models
Many groups have re-developed common materials for FDM printing, including polycaprolactone (PCL) and poly(propylene fumarate) (PPF). These materials are used in traditional tissue engineered bone implants due to both their mechanical strength and properties being similar to that of bone, and their slow degradation kinetics which allow for native tissue invasion.[3,5,50,51] Although 3D printing is a relatively recent field, PCL and other thermoplastics are among the most commonly used materials in tissue engineering.[3] This is in part due to their well-defined material properties, low cost, and well-characterized printing parameters.[3,32,50] Most recently, these materials have been modified to mimic native tissue. Bone-derived materials perform very well in vivo and in vitro and provide a platform that performs as well–if not better than–synthetic scaffolds currently in use, e.g. PCL.[52] Importantly, Hung and coworkers and Nyberg and coworkers have independently generated bone tissue mimetics that utilize decellularized ECM components in conjunction with PCL.[16,22] Although these were fabricated using composite materials at a time, the increase in complexity to better mimic the native tissue resulted in an increase in bone formation. With advances to mimic the native tissues, Nicholas and coworkers developed methods for using CAD to model and characterize their printed bone tissue mimetics to better understand the impact of the complex geometry on the physical properties.[32] Materials designed and fabricated in this manner have recently resulted in exciting progress towards fabricating resorbable bone implants made out of PPF and PPF+PCL composites.[4,5,50,51] Not only do these 3D printed scaffolds provide a basis for therapeutics, but recent investigations into the interactions of cancer cells with bone tissue mimetics demonstrate an exciting new platform for assessing cancer cells in vitro. [53]
3.1.3. Soft tissue mimetic models
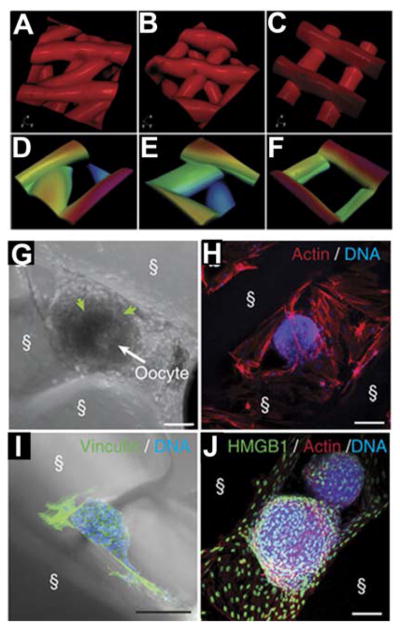
Bioprostheses fabricated via 3D printing show great promise due to the patient-specific capability of 3D printing. Although examples of major soft tissue architecture being replicated using 3D printing are relatively rare, significant progress has been made in tissues where less mechanical support is needed by the implant or where geometry is relatively simple. Given the wide range of tissues that have been attempted, we will focus our discussion on two of the more successful attempts. Laronda and co-workers have recently developed a bioprosthetic ovary using 3D printed microporous scaffolds.[20] The 3D geometry, specifically pore shape, led to an increased interaction between the scaffold and ovarian follicle. Figure 2A–F illustrates the pore geometries investigated, which enabled significant interactions between the material and the follicles depending on fiber orientation (Figure 2G–J). These follicle-scaffold interactions led to an increase in implant survival rate and vascularization. Subsequently implanted follicles restored ovarian function even in sterilized mice. However, evaluation of pore architecture’s influence on cell responses and matrix interactions is limited despite significant exploration of the input hard materials, e.g. PCL. [24] Although similar work has been performed with other hydrogel materials, these attempts are smaller and not 3D printed.[54,55] Additionally, material scale-up to a large animal or a human would not be possible using previous fabrication methods. As such this work demonstrated that pore size and architecture is critical for functional tissue engineering. However, as Laronda and coworkers noted, future endeavors will need to incorporate vasculature or a vasculature mimetic for long-term function.
Figure 2. Soft material fabrication of mimetic structures.
A–F) Illustrations of various inner geometries (30 °, 60 °, and 90° strand orientation between layers) for fabrication with layer-by-layer deposition. G–J) Demonstration of follicle interactions with the scaffolds over 6–8 days of culture showing the preference of follicles for specific geometries. G) scale bar = 50 μm. H–J) scale bar = 100 μm. Reproduced and adapted under creative commons terms.[20] 2017 Nature Publishing Group.
As a second successful example, extrusion-based printing has been used to treat injuries of the eardrum, which is relatively common for children. Current treatments require extended surgical times to implant a scaffold, so recent approaches have attempted to use 3D printed scaffolds. For example, Kuo and coworkers demonstrated that they can image a damaged eardrum, create a CAD model, and fabricate a plug for implantation using methacrylated-gelatin (GelMA) and a sacrificial gelatin layer.[56] These plugs had lips on the inner and outer edges matching the geometry of the defect to allow them to be pressed into place. The scaffolds were strong enough that they maintained structural integrity in vivo in chinchillas until they were degraded and replaced by native tissue. Other groups, using multiple materials including polydimethylsiloxane (PDMS), polylactic acid (PLA), and polycaprolactone (PCL), have mimicked the tympanic membrane with composite materials. [43] These examples demonstrate the progress that has been made in controlling of geometry of soft materials such as hydrogels and how they can impact the future of healthcare.
3.2. Extrusion-based bioprinting
FDM, given its high temperature and other printing properties, is difficult to use in applications where the cells must be embedded in the scaffold struts. On the other hand, bioinks, which are printing materials or resins with cells embedded, have garnered significant interest recently because of their relatively cell permissible printing requirements. Bioprinting materials such as gelatin and GelMA have been widely adopted in 3D printing due to their biocompatibility, ease of fabrication, and relative low cost. However, this simplicity comes at a cost; bioinks do not always provide the necessary cell-substrate interactions and major remodeling needs to occur by the embedded cells for them to behave as they would in vivo. To address this concern, bioink complexity has been steadily increasing to recapitulate major aspects of native ECM. [14,28,44,57] Although we can exert extensive spatial control over material deposition, there are still issues controlling localized print properties. Secondly, the added complexity of modifying the bioinks with additives or manipulation of localized properties can impact the material properties and the printability of the resultant material. Although there has been significant progress in the development of these materials such as in references [14,23,26,27,34,35,58,59], material printability is commonly assessed on a case-by-case basis rather than fully characterized for a range of printing parameters. Therefore any deviation from the published designs and parameters or even printer utilized will result in significant variability. A more generic assessment of material printability could potentially provide a stronger foundation for 3D printing and the adoption of the material for a diverse set of applications.
Extrusion-based 3D printing of hydrogels have some significant advantages as well as a few drawbacks when compared with other fabrication strategies. One major advantage for extrusion based-printing, is the ability to fabricate designs with high cell densities (e.g. >1×106 cells/mL or even spheroids). [11,60] There are potential issues with shear stress during the fabrication process being one of the leading causes of cell death for cell-laden hydrogel printing.[25,36] However, this has been addressed recently with the use of shear thinning bioinks.[8,61]. These bioinks can lead to an increased cell viability by decreasing the shear stress that the cells are exposed to during the extrusion process and post-extrusion, there is little deformation of the resultant hydrogel, thus leading to a higher fidelity of the resultant print. Additionally, extrusion based printing is compatible with bioinks of a wide range of viscosity, and work particularly well with bioinks of relatively high viscosity (~104 Pa s for the BioBot and 3D-Bioplotter). Thus, this allows for more time for additional curing or crosslinking to help strengthen the final print after the deposition of each layer. However, too much crosslinking during the gelation process can potential inhibit migration, proliferation, and cell spreading. To overcome these challenges, composite bioinks have been used consisting of interpenetrating networks, nanocomposites, or other combinations therein.[8,23,26–28,34–36,59,60] For example, the following types of bioinks are commonly employed to increase cell viability, cell attachment, and cell spreading by reducing the shear stress experienced by the cells and by providing more physiologically relevant binding sites such as RGD: 1) GelMA + Alginate printed into a calcium containing solution, 2) HA-MA, and 3) decellularized ECM.[7,14,23,27,36,60] Additional reviews on specific bioinks, their formulations, and their printibility can be found in the following references.[62–64]
3.2.1. 3D bioprinted soft tissue mimetics for tissue and disease modeling
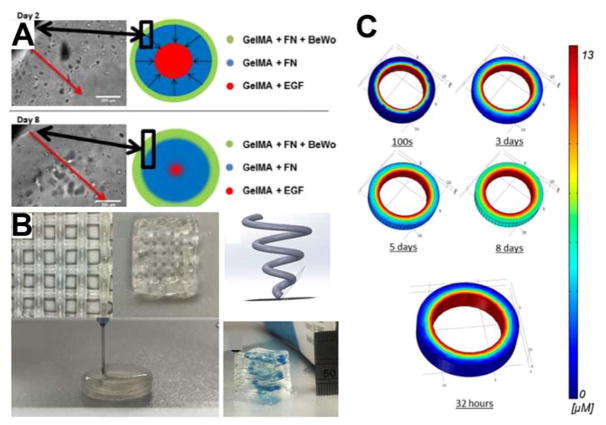
Soft tissue mimetics provide opportunities to better model in vitro conditions. Through the 3D bioprinting process, researchers can spatially control the deposition of cell-laden hydrogels as well as growth factors and cytokines. While a growing number of examples exist–indeed enough for its own report–we will focus on efforts to mimic the spiral arteries of the placenta and explore the origins of diseases such as preeclampsia (Figure 3). Trophoblast migration or lack thereof may be a cause of preeclampsia, but until now there were only very reduced models to interrogate potential mechanisms that cause the life threatening condition during pregnancy. Recapitulating the growth factor gradients present in native tissue will provide a foundation for assessing invasion of trophoblasts (Figure 3A). Furthermore, the ability to fabricate complex spiral geometries mimicking native architecture enables future studies of more complex 3D invasion assays (Figure 3B). As discussed previously, CAD models developed for the printing process can also be analyzed using in silico models. Models can define the growth factor gradients established by printing growth factor laden materials to ensure proper gradients prior to fabrication (Figure 3C). Soft tissue models have also expanded to include liver and have recently been commercialized as a liver mimic to assess toxicity. For example, Organovo has recently demonstrated the ability to fabricate liver tissue using 3D printing, providing a foundation for the fabricating larger tissues.[65] These mimetic tissues are fabricated using a proprietary 3D printer and are directly fabricated onto transwell inserts for in vitro study. The 3D printed tissues were comprised of three different cell types and were used to assess drug induced liver damage.[65] In addition to this work, other researchers have been developing similar technologies using 3D printed microfluidic channels rather than transwell systems to fabricate a liver-on-a-chip to assess drug side effects and cytotoxicity.[66] These soft tissue models will play an integral role in the future design and development of in vitro disease modeling and drug testing.
Figure 3. 3D printed placenta mimetic.
A) Illustration and corresponding micrographs demonstrating the invasion of cells printed onto the outer periphery of the mimetic with an EGF source in the middle of the scaffold at day 2 and day 8 of the study. B) This work demonstrates the ability to fabricate complex scaffold using multiple soft hydrogel materials to create defined regions with different components. C) COMSOL modeling of the EGF gradient established over the time course of the experiment throughout the hydrogel. Models such as this can be readily developed using the material properties and CAD models to theoretically determine the concentration of molecules as a function of time within 3D printed scaffolds. Reproduced and adapted with permission.[41] Copyright 2017, American Chemistry Society.
3.2.2. Interfacial multi-material printing
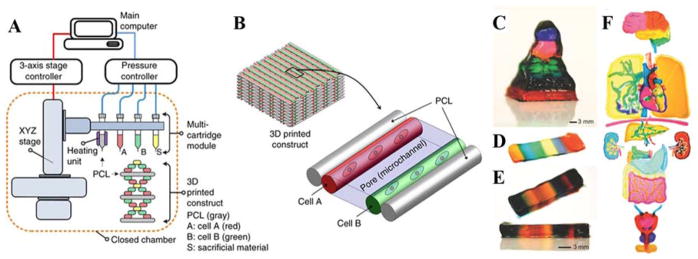
Concurrent utilization of multiple materials is a cutting edge development in 3D printing. Although some custom printers exist with multiple print heads or print nozzles, there are still many that print only one material at a time.[29] Part of this issue is due to the complexity of printing multiple materials at the same time, which requires different print heads calibrated and aligned with each other. If there is mismatch, then this error will propagate as each layer and material is deposited. Kang and coworkers recently developed a novel approach to fabricate a multi-material scaffold.[17] In the method they employed, each layer deposited was built out of one material and the next layer was deposited with some overlap to ensure integrity later. Figure 4A–B shows an exploded view version of 3D printing. After depositing all layers, the scaffold was compressed until the support structures, in this case PCL, came together forming the final structure. Even more recently, Liu et al have demonstrated another method for the rapid deposition of multiple materials to generate complex scaffolds with varying inner geometries and composition.[31] Figure 4C demonstrates the controlled deposition of multiple materials in the Z direction with distinct layering. Figure 4D–E illustrates how this same printing can fabricate scaffolds with up to 7 different inks at the same time with precise control over the spatial deposition of each hydrogel. Furthermore, Figure 4F shows how this can be scaled up to larger, organ-like structures being fabricated with precise control of bioink deposition at a high resolution.[31] Structures such as these have the potential to overcome issues of incorporating in the native vasculature and have great promise for the ability to fabricate scaffolds with multiple cell types concurrently with well-defined spatial parameters.
Figure 4. Examples of more complex multi-material 3D printing.
A) Schematic demonstrating the workflow of fabricating a multi-layer 3D printed construct. In these multi-material constructs, scaffolds can be fabricated using support materials such as PCL as well as cell-laden hydrogels, and sacrificial materials as discussed in this review. B) Illustration of how the final multi-material print may look using these materials. Reproduced and adapted with permission.[17] Copyright 2016, Nature Publishing Group. Bottom C–F) examples of more complex multi-material hydrogel printing without a secondary support material. In these examples, up to 7 different bioinks were deposited to create scaffolds with complex spatial designs. In addition, complex features were fabricated even within the organ-like constructs demonstrating the ability to fabricate large hydrogel based scaffolds using multiple materials while maintaining defined spatial control within these scaffolds. Reproduced and adapted with permission.[31] Copyright 2017, John Wiley and Sons.
4. Comparison of other fabrication techniques
Although this progress report focuses mainly on the current state of extrusion-based approaches for tissue engineering applications, there other many other forms of 3D printing in use. There are many reviews devoted to extensive comparison of these technologies, which we recommend to the reader who is unfamiliar with the potential benefits of these other methodologies.[14,15,67–71] However, we will discuss a few key differences between solid laser sintering, stereolithography, inkjet, and extrusion-based fabrication strategies.
Selective laser sintering (SLS) generates scaffolds out of resin beds and a sintering process at the surface. These can be metals, polymers, or composites and can be used to make large acellular prints for implantation or in vitro studies.[72] This approach, when combined with two-photon light sources or micro laser sintering, has among the best resolution of modern 3D printing (~20–100 μm), but one major drawback is the limited fabrication speed.[73–76] See Table 1 for an overview and comparison with other 3D printing methods.[67,68,71,77] Another common mode of manufacturing–and one of the oldest–is stereolithography (SLA). Even though stereolithography based printing has a reduced overall print resolution when compared with SLS, materials in use today can reach sub-100 μm in resolution in all three dimensions, which can be seen as adequate for most applications.[68] Resolution can be maintained even at relatively fast fabrication speeds since entire layers can be exposed using UV or visible light at the same time.[69] This printing method allows object height to be determined by the overall print time rather than object complexity. However, complex objects may need additional support structures to maintain fidelity. Inkjet printing is especially useful for the fabrication of small scale objects out of cell-laden hydrogels. This method has among the best resolution for cell-based printing (pL sized droplets), but it lacks the ability to produce mechanically strong structures, limiting structure size as a result.[78] However, the main focus of this report was extrusion based 3D printing due to its relatively widespread adoption and available materials. This form of printing can be used with either cell-laden or cell-free materials. The overall print resolution depends upon a combination of print parameters, e.g. needle diameter, print head velocity, and extrusion pressure or rate, but is typically around 100 microns.[2,4,5,25,61,70] Such resolution is on par with the other forms of 3D printing, but has the added benefit of being one of the most accessible due to the large investment of materials and equipment in this research space. In summary, a printing method may be more advantageous for a given application due to requirements for fabrication speed, cell compatibility, or complex architecture necessitating a support structure.
Table 1.
Comparison of common 3D printing technologies
| Extrusion-based | Stereolithography | Inkjet | Selective Laser Sintering | |
|---|---|---|---|---|
| Resolution | 100 μm | 20–50 μm | ~30 μm | ~20–100 μm |
| Fabrication Speed | Medium | Fast | Fast | Slow |
| Cell Compatible | Yes | Yes | Yes | No |
| Support Structure Required | No | Yes | Yes | No |
5. Reproducibility
Two of the key selling points of 3D printing and additive manufacturing are reproducibility and repeatability. For a given system and material, many groups have demonstrated their ability to reproducibly fabricate scaffolds and designs with relatively high fidelity as is expected when using 3D printing systems. As with any manufacturing process, there can be issues in the scale up from bench top to industrial scale. In 3D printing, this may also not be limited to a change in production scale, but also can be attributed to the many different 3D printing systems either custom-built or commercially available. For each individual printer, there are specific conditions at which a material will print with high fidelity and the greatest reproducibility from batch-to-batch. One challenge for the development of future materials is establishing not just a specific parameter that works for a given material and application, but defining the materials’ properties and behavior using a range of printing parameters.[4,5] When scientists develop new or modified materials, the adoption of these materials for other systems will require iterative investigation using a factorial design. Other scientists can then select the appropriate conditions to best fit their needs, application, and printer configuration and limitations. Given the rate at which new materials are being developed for specific applications, adopting a more thorough materials characterization will have a long-term impact on the utilization of these materials and may facilitate more rapid progress in identifying materials suitable for tissue engineering applications. This will have the greatest impact on new researchers since the most widely use materials have had their printing parameters thoroughly investigated and can provide initial starting points for scientists to develop their model systems using 3D printing.
6. Current and Future Challenges
Although significant advances have been made with regards to resolution and fabrication speed, challenges remain. The drive towards more complex interfacial systems which require accurate deposition of multiple materials during the fabrication process have complicated these challenges. These issues are (1) characterization of the materials developed in terms of printability, (2) compatibility of printing parameters between materials, and (3) cell viability during and after fabrication. With the rapid expansion of the available material types, there is a need for a more unified and complete approach for the characterization of these materials. Understanding the rheological and thermoresponsive properties of new materials will provide the critical building blocks for others to use these materials with the wide assortment of 3D printers available. Ideally, extensive characterization will result in the need to only optimize the conditions for a specific printer and end material parameters.[6] This could lead to a reduction in time between the publication of a new material and adoption of this material by the community as a whole.
FDM and cell-free printing have the most promise in future applications as structural and sacrificial materials. The strength of materials currently in use with FDM have yet to be matched by hydrogels; therefore, composite prints will become more commonplace. This method of extrusion-based printing, as discussed in this review, will provide the foundation for more complex prints. Although it is expected these will be used to make vascular mimetics and structural components, there are still concerns with printing parameters to ensure cell viability when used as a secondary material alongside bioinks.
For extrusion-based bioprinting, embedded cell viability is still below that of other 3D printing technologies due to the forces to which the cells are exposed.[7,36] For this method of printing, it is a constant balance between fabrication speed and viability; however, another factor that can negatively impact cell viability is the time in the printer and not under culture conditions, i.e. the layer-by-layer fabrication time needs to be reduced. The printing bioinks are formulated for cell survival post-printing, but typically are not conducive to cell survival as culture conditions.
As bioink complexity continues to evolve to mimic tissues, there is also the added consideration that a given material may not be sufficient for a given application. Complex problems either for in vitro mimetics or for in vivo implantation will require a range of features, mechanical properties, surface binding sites, etc to ensure appropriate cell survival as discussed earlier in this review. Cell response on not only a bulk material level, but on a local level, will need to be investigated with an emphasis on the interface between the materials. The interplay of the signaling mechanisms will provide areas of increasing importance as the complexity of the printed objects increase. One avenue to deal with the interfacial problem is to use a multifaceted approach using theoretical modeling in conjunction with experimental observations to facilitate the rapid production of new devices and reduce the number of iterations required to generate a scaffold with desired characteristics. Utilization of 3D models in CAD software for analysis such as COMSOL, SolidWorks, AutoCAD, or other commercially available software will enable the determination of the physical properties based on the building blocks of the final scaffold. In the case of tissues like cartilage where there are known zonal variations in porosity, diffusion, mechanical strength, alignment, and cell density, this sort of modeling can be utilized to help predict ideal fabrication geometries.
These three main issues for future applications add in an additional layer of complexity that needs to be accounted for during the manufacturing process. Otherwise, these solutions developed at the bench side may never make the transition to clinical relevance due to high costs or complexity associated with their implementation. Furthermore, for therapeutic applications to increase in prevalence, there will be a need for a centralized fabrication of these devices and scaffolds. In the future, considerations regarding the establishment of facilities with the appropriate regulatory certifications will need to be undertaken. Given the large cost associated with these endeavors, more centralized facilities may be appropriate.[79,80] Additionally by reducing the number of potential fabrication methodologies, the transition from bench to bedside may be shortened.
7. Conclusion
Over the past decade, 3D printing research at the interface between biology, tissue engineering, and materials science has made significant progress towards fabricating complex in vitro model systems and in vivo therapeutics. This progress report highlights some of the most recent advances that are leading the way in which researchers approach the development, characterization, and design of materials to impart improved printability, long-term cell survival, and clinical relevance. Significant challenges remain for the widespread adoption of 3D printing for many healthcare applications, but continual material and printing improvements can address these issues and provide an avenue for broader utilization of extrusion-based 3D printing as a transformative technology.
Acknowledgments
The authors acknowledge support from National Institutes of Health grants R01AG045428 (to A.J.E.), R01CA206880 (to A.J.E.), and F32HL126406 (to J.K.P.), as well as support from the National Science Foundation grant 1463689 (to A.J.E.).
Biographies

Jesse Placone received his Bachelor of Science in Biomedical Engineering (2008) from Johns Hopkins University and transitioned to the Johns Hopkins Materials Science and Engineering department where he received his PhD (2013). His first postdoctoral experience was at University of Maryland with Dr. John P. Fisher in the field of 3D printing for regenerative medicine. Currently, he is working at University of California, San Diego as a Postdoctoral Fellow on the development of micropatterns for 3D printed vascular grafts.

Adam J. Engler is an Associate Professor of Bioengineering at the University of California, San Diego, having joined the faculty there in 2008. He is also resident scientist at the Sanford Consortium for Regenerative Medicine, where his lab studies how extracellular cues, including the matrix, regulates cell fate. He has published more than 80 peer-reviewed manuscripts in this field and holds 2 patents on novel polymer hydrogel applications. Prior to San Diego, Dr. Engler performed his undergraduate and doctoral training at the University of Pennsylvania and his postdoctoral training at Princeton University.
References
- 1.Alssabbagh M, Tajuddin A, Abdulmanap M, Zainon R. Evaluation of 3D printing materials for fabrication of a novel multi-functional 3D thyroid phantom for medical dosimetry and image quality. Radiation Physics and Chemistry. 2017;135:106–112. doi: 10.1016/j.radphyschem.2017.02.009. [DOI] [Google Scholar]
- 2.Ersumo N, Witherel CE, Spiller KL. Differences in time-dependent mechanical properties between extruded and molded hydrogels. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/3/035012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht LD, Sawyer SW, Soman P. Developing 3D Scaffolds in the Field of Tissue Engineering to Treat Complex Bone Defects. 3d Printing and Additive Manufacturing. 2016;3:106–112. doi: 10.1089/3dp.2016.0006. [DOI] [Google Scholar]
- 4.Ting G, Timothy RH, Casey GL, Feng G, Ankit G, Jordan ET, Antonios GM, John PF. 3D printing PLGA: a quantitative examination of the effects of polymer composition and printing parameters on print resolution. Biofabrication. 2017;9:024101. doi: 10.1088/1758-5090/aa6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trachtenberg JE, Placone JK, Smith BT, Piard CM, Santoro M, Scott DW, Fisher JP, Mikos AG. Extrusion-Based 3D Printing of Poly(propylene fumarate) in a Full-Factorial Design. ACS Biomaterials Science & Engineering. 2016;2:1771–1780. doi: 10.1021/acsbiomaterials.6b00026. [DOI] [PubMed] [Google Scholar]
- 6.Diamantides N, Wang L, Pruiksma T, Siemiatkoski J, Dugopolski C, Shortkroff S, Kennedy S, Bonassar LJ. Correlating rheological properties and printability of collagen bioinks: the effects of riboflavin photocrosslinking and pH. Biofabrication. 2017;9 doi: 10.1088/1758-5090/aa780f. [DOI] [PubMed] [Google Scholar]
- 7.Ahn G, Min K-H, Kim C, Lee J-S, Kang D, Won J-Y, Cho D-W, Kim J-Y, Jin S, Yun W-S, Shim J-H. Precise stacking of decellularized extracellular matrix based 3D cell-laden constructs by a 3D cell printing system equipped with heating modules. Scientific Reports. 2017;7:8624. doi: 10.1038/s41598-017-09201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu WJ, Heinrich MA, Zhou YX, Akpek A, Hu N, Liu X, Guan XF, Zhong Z, Jin XY, Khademhosseini A, Zhang YS. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Advanced Healthcare Materials. 2017;6 doi: 10.1002/adhm.201601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai YC, Li SM, Hu SG, Chang WC, Jeng US, Hsu SH. Synthesis of Thermoresponsive Amphiphilic Polyurethane Gel as a New Cell Printing Material near Body Temperature. Acs Applied Materials & Interfaces. 2015;7:27613–27623. doi: 10.1021/acsami.5b10697. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z, Su X, Xu Y, Kong B, Sun W, Mi S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. 2016;6:24474. doi: 10.1038/srep24474. https://www.nature.com/articles/srep24474#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atabak Ghanizadeh T, Miguel AH, Nicholas RL, Wenmiao S. Three-dimensional bioprinting of complex cell laden alginate hydrogel structures. Biofabrication. 2015;7:045012. doi: 10.1088/1758-5090/7/4/045012. [DOI] [PubMed] [Google Scholar]
- 12.Hong S, Sycks D, Chan HF, Lin S, Lopez GP, Guilak F, Leong KW, Zhao X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Advanced materials (Deerfield Beach, Fla) 2015;27:4035–4040. doi: 10.1002/adma.201501099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mironov AV, Grigoryev AM, Krotova LI, Skaletsky NN, Popov VK, Sevastianov VI. 3D printing of PLGA scaffolds for tissue engineering. Journal of Biomedical Materials Research Part A. 2017;105:104–109. doi: 10.1002/jbm.a.35871. [DOI] [PubMed] [Google Scholar]
- 14.Gao G, Lee JH, Jang J, Lee DH, Kong J-S, Kim BS, Choi Y-J, Jang WB, Hong YJ, Kwon S-M, Cho D-W. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Advanced Functional Materials. 2017;27 doi: 10.1002/adfm.201700798. 1700798-n/a. [DOI] [Google Scholar]
- 15.Guo T, Lembong J, Zhang LG, Fisher JP. Three-Dimensional Printing Articular Cartilage: Recapitulating the Complexity of Native Tissue. Tissue Engineering Part B: Reviews. 2016;23:225–236. doi: 10.1089/ten.teb.2016.0316. [DOI] [PubMed] [Google Scholar]
- 16.Hung BP, Naved BA, Nyberg EL, Dias M, Holmes CA, Elisseeff JH, Dorafshar AH, Grayson WL. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomaterials Science & Engineering. 2016;2:1806–1816. doi: 10.1021/acsbiomaterials.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotech. 2016;34:312–319. doi: 10.1038/nbt.3413. http://www.nature.com/nbt/journal/v34/n3/abs/nbt.3413.html#supplementary-information. [DOI] [PubMed] [Google Scholar]
- 18.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 20.Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8 doi: 10.1038/ncomms15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotech. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 22.Nyberg E, Rindone A, Dorafshar A, Grayson WL. Comparison of 3D-Printed Poly-ε-Caprolactone Scaffolds Functionalized with Tricalcium Phosphate, Hydroxyapatite, Bio-Oss, or Decellularized Bone Matrix. Tissue Engineering Part A. 2016;23:503–514. doi: 10.1089/ten.tea.2016.0418. [DOI] [PubMed] [Google Scholar]
- 23.Pati F, Cho DW. Bioprinting of 3D Tissue Models Using Decellularized Extracellular Matrix Bioink. Methods Mol Biol. 2017:7021–7026_7027. doi: 10.1007/978-1-4939-7021-6_27. [DOI] [PubMed] [Google Scholar]
- 24.Sobral JM, Caridade SG, Sousa RA, Mano JF, Reis RL. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011;7:1009–1018. doi: 10.1016/j.actbio.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Blaeser A, Duarte Campos DF, Puster U, Richtering W, Stevens MM, Fischer H. Controlling Shear Stress in 3D Bioprinting is a Key Factor to Balance Printing Resolution and Stem Cell Integrity. Adv Healthc Mater. 2016;5:326–333. doi: 10.1002/adhm.201500677. [DOI] [PubMed] [Google Scholar]
- 26.Colosi C, Shin SR, Manoharan V, Massa S, Costantini M, Barbetta A, Dokmeci MR, Dentini M, Khademhosseini A. Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28:677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A, Gatenholm P, Enejder A, Simonsson S. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Scientific Reports. 2017;7:658. doi: 10.1038/s41598-017-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Advanced Materials. 2015;27:1607–1614. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung JW, Lee J-S, Cho D-W. Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. 2016;6:21685. doi: 10.1038/srep21685. https://www.nature.com/articles/srep21685#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Kim KE, Bang S, Noh I, Lee C. A desktop multi-material 3D bio-printing system with open-source hardware and software. International Journal of Precision Engineering and Manufacturing. 2017;18:605–612. doi: 10.1007/s12541-017-0072-x. [DOI] [Google Scholar]
- 31.Liu W, Zhang YS, Heinrich MA, De Ferrari F, Jang HL, Bakht SM, Alvarez MM, Yang J, Li Y-C, Trujillo-de Santiago G, Miri AK, Zhu K, Khoshakhlagh P, Prakash G, Cheng H, Guan X, Zhong Z, Ju J, Zhu GH, Jin X, Shin SR, Dokmeci MR, Khademhosseini A. Rapid Continuous Multimaterial Extrusion Bioprinting. Advanced Materials. 2017;29 doi: 10.1002/adma.201604630. 1604630-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas S, Prachi D, Michael W, Elizabeth CH. Fabrication of biomimetic bone grafts with multi-material 3D printing. Biofabrication. 2017;9:025020. doi: 10.1088/1758-5090/aa7077. [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Shor L, Darling A, Khalil S, Sun W, Güçeri S, Lau A. Precision extruding deposition and characterization of cellular poly-ε-caprolactone tissue scaffolds. Rapid Prototyping Journal. 2004;10:42–49. doi: 10.1108/13552540410512525. [DOI] [Google Scholar]
- 34.Ahlfeld T, Cidonio G, Kilian D, Duin S, Akkineni AR, Dawson JI, Yang S, Lode A, Oreffo ROC, Gelinsky M. Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication. 2017;9:034103. doi: 10.1088/1758-5090/aa7e96. [DOI] [PubMed] [Google Scholar]
- 35.Myung Gu Y, Geun Hyung K. A cell-printing approach for obtaining hASC-laden scaffolds by using a collagen/polyphenol bioink. Biofabrication. 2017;9:025004. doi: 10.1088/1758-5090/aa6997. [DOI] [PubMed] [Google Scholar]
- 36.Ouyang LL, Yao R, Zhao Y, Sun W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication. 2016;8 doi: 10.1088/1758-5090/8/3/035020. [DOI] [PubMed] [Google Scholar]
- 37.Boetker J, Water JJ, Aho J, Arnfast L, Bohr A, Rantanen J. Modifying release characteristics from 3D printed drug-eluting products. European Journal of Pharmaceutical Sciences. 2016;90:47–52. doi: 10.1016/j.ejps.2016.03.013. doi: https://doi.org/10.1016/j.ejps.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Gu Q, Tomaskovic-Crook E, Lozano R, Chen Y, Kapsa RM, Zhou Q, Wallace GG, Crook JM. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Advanced Healthcare Materials. 2016;5:1429–1438. doi: 10.1002/adhm.201600095. [DOI] [PubMed] [Google Scholar]
- 39.Lozano R, Stevens L, Thompson BC, Gilmore KJ, Gorkin R, Stewart EM, in het Panhuis M, Romero-Ortega M, Wallace GG. 3D printing of layered brain-like structures using peptide modified gellan gum substrates. Biomaterials. 2015;67:264–273. doi: 10.1016/j.biomaterials.2015.07.022. doi: https://doi.org/10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered 3D tissues. Nature materials. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo CY. Development of a 3D printed, bioengineered placenta model to evaluate the role of trophoblast migration in preeclampsia. ACS Biomater Sci Eng. 2016;2:1817. doi: 10.1021/acsbiomaterials.6b00031. [DOI] [PubMed] [Google Scholar]
- 42.Ball O, Nguyen B-NB, Placone JK, Fisher JP. 3D Printed Vascular Networks Enhance Viability in High-Volume Perfusion Bioreactor. Annals of Biomedical Engineering. 2016;44:3435–3445. doi: 10.1007/s10439-016-1662-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clevers H. Modeling Development and Disease with Organoids. Cell. 2016;165:1586–1597. doi: 10.1016/j.cell.2016.05.082. doi: https://doi.org/10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 44.Tao X, Kyle WB, Mohammad ZA, Dennis D, Weixin Z, James JY, Anthony A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5:015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 45.Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, Atluri P. In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks. Tissue Engineering Part C: Methods. 2015;22:1–7. doi: 10.1089/ten.tec.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bégin-Drolet A, Dussault M-A, Fernandez SA, Larose-Dutil J, Leask RL, Hoesli CA, Ruel J. Design of a 3D printer head for additive manufacturing of sugar glass for tissue engineering applications. Additive Manufacturing. 2017;15:29–39. doi: https://doi.org/10.1016/j.addma.2017.03.006. [Google Scholar]
- 47.Sazer D, Miller J. In: 3D Printing and Biofabrication. Ovsianikov Aleksandr, Yoo James, Mironov Vladimir., editors. Springer International Publishing; 2017. pp. 1–27. [Google Scholar]
- 48.Sooppan R, Paulsen SJ, Han J, Ta AH, Dinh P, Gaffey AC, Venkataraman C, Trubelja A, Hung G, Miller JS, Atluri P. In Vivo Anastomosis and Perfusion of a Three-Dimensionally-Printed Construct Containing Microchannel Networks. Tissue Eng Part C Methods. 2016;22:1–7. doi: 10.1089/ten.tec.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee VK, Kim DY, Ngo H, Lee Y, Seo L, Yoo SS, Vincent PA, Dai G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials. 2014;35:8092–8102. doi: 10.1016/j.biomaterials.2014.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buyuksungur S, Endogan Tanir T, Buyuksungur A, Bektas EI, Torun Kose G, Yucel D, Beyzadeoglu T, Cetinkaya E, Yenigun C, Tonuk E, Hasirci V, Hasirci N. 3D printed poly(epsilon-caprolactone) scaffolds modified with hydroxyapatite and poly(propylene fumarate) and their effects on the healing of rabbit femur defects. Biomater Sci. 2017;7 doi: 10.1039/c7bm00514h. [DOI] [PubMed] [Google Scholar]
- 51.Walker JM, Bodamer E, Kleinfehn A, Luo Y, Becker M, Dean D. Design and mechanical characterization of solid and highly porous 3D printed poly(propylene fumarate) scaffolds. Progress in Additive Manufacturing. 2017;2:99–108. doi: 10.1007/s40964-017-0021-3. [DOI] [Google Scholar]
- 52.Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, Cannizzaro C, Wan LQ, Liu XS, Guo XE, Vunjak-Novakovic G. Engineering anatomically shaped human bone grafts. Proceedings of the National Academy of Sciences. 2010;107:3299–3304. doi: 10.1073/pnas.0905439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trachtenberg JE, Santoro M, Williams C, Piard CM, Smith BT, Placone JK, Menegaz BA, Molina ER, Lamhamedi-Cherradi S-E, Ludwig JA, Sikavitsas VI, Fisher JP, Mikos AG. Effects of Shear Stress Gradients on Ewing Sarcoma Cells Using 3D Printed Scaffolds and Flow Perfusion. ACS Biomaterials Science & Engineering. 2017 doi: 10.1021/acsbiomaterials.6b00641. [DOI] [PubMed] [Google Scholar]
- 54.Shikanov A, Zhang Z, Xu M, Smith RM, Rajan A, Woodruff TK, Shea LD. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng Part A. 2011;17:3095–3104. doi: 10.1089/ten.tea.2011.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kniazeva E, Hardy AN, Boukaidi SA, Woodruff TK, Jeruss JS, Shea LD. Primordial Follicle Transplantation within Designer Biomaterial Grafts Produce Live Births in a Mouse Infertility Model. Sci Rep. 2015;5 doi: 10.1038/srep17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuo C-Y, Wilson E, Fuson A, Gandhi N, Monfaredi R, Jenkins A, Romero M, Santoro M, Fisher JP, Cleary K, Reilly B. Repair of Tympanic Membrane Perforations with Customized Bioprinted Ear Grafts Using Chinchilla Models. Tissue Engineering Part A. 2017 doi: 10.1089/ten.tea.2017.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyler KM, Morgan B, Young-Joon S, Hyun-Wook K, Sang Jin L, James JY, Anthony A. A 3D bioprinted complex structure for engineering the muscle–tendon unit. Biofabrication. 2015;7:035003. doi: 10.1088/1758-5090/7/3/035003. [DOI] [PubMed] [Google Scholar]
- 58.Bakirci E, Toprakhisar B, Zeybek MC, Ince GO, Koc B. Cell sheet based bioink for 3D bioprinting applications. Biofabrication. 2017;9:1758–5090. doi: 10.1088/1758-5090/aa764f. [DOI] [PubMed] [Google Scholar]
- 59.Lorson T, Jaksch S, Lübtow MM, Jüngst T, Groll J, Lühmann T, Luxenhofer R. A Thermogelling Supramolecular Hydrogel with Sponge-Like Morphology as a Cytocompatible Bioink. Biomacromolecules. 2017;18:2161–2171. doi: 10.1021/acs.biomac.7b00481. [DOI] [PubMed] [Google Scholar]
- 60.Skardal A, Devarasetty M, Kang HW, Mead I, Bishop C, Shupe T, Lee SJ, Jackson J, Yoo J, Soker S, Atala A. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs. Acta Biomater. 2015;25:24–34. doi: 10.1016/j.actbio.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 61.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv Mater. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 62.Chimene D, Lennox KK, Kaunas RR, Gaharwar AK. Advanced Bioinks for 3D Printing: A Materials Science Perspective. Ann Biomed Eng. 2016;44:2090–2102. doi: 10.1007/s10439-016-1638-y. [DOI] [PubMed] [Google Scholar]
- 63.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33:6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 64.Kim BS, Kim H, Gao G, Jang J, Cho DW. Decellularized extracellular matrix: a step towards the next generation source for bioink manufacturing. Biofabrication. 2017;9:1758–5090. doi: 10.1088/1758-5090/aa7e98. [DOI] [PubMed] [Google Scholar]
- 65.Nguyen DG, Funk J, Robbins JB, Crogan-Grundy C, Presnell SC, Singer T, Roth AB. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLOS ONE. 2016;11:e0158674. doi: 10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee H, Cho DW. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab Chip. 2016;16:2618–2625. doi: 10.1039/c6lc00450d. [DOI] [PubMed] [Google Scholar]
- 67.Peltola SM, Melchels FPW, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Annals of Medicine. 2008;40:268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 68.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. Journal of Biological Engineering. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mondschein RJ, Kanitkar A, Williams CB, Verbridge SS, Long TE. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials. 2017;140:170–188. doi: 10.1016/j.biomaterials.2017.06.005. doi: https://doi.org/10.1016/j.biomaterials.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Kyle S, Jessop ZM, Al-Sabah A, Whitaker IS. ‘Printability’ of Candidate Biomaterials for Extrusion Based 3D Printing: State-of-the-Art. Advanced Healthcare Materials. 2017;6 doi: 10.1002/adhm.201700264. 1700264-n/a. [DOI] [PubMed] [Google Scholar]
- 71.Jose RR, Rodriguez MJ, Dixon TA, Omenetto F, Kaplan DL. Evolution of Bioinks and Additive Manufacturing Technologies for 3D Bioprinting. ACS Biomaterials Science & Engineering. 2016;2:1662–1678. doi: 10.1021/acsbiomaterials.6b00088. [DOI] [PubMed] [Google Scholar]
- 72.Hatamleh MM, Bhamrah G, Ryba F, Mack G, Huppa C. Simultaneous Computer-Aided Design/Computer-Aided Manufacture Bimaxillary Orthognathic Surgery and Mandibular Reconstruction Using Selective-Laser Sintered Titanium Implant. Journal of Craniofacial Surgery. 2016;27:1810–1814. doi: 10.1097/scs.0000000000003039. [DOI] [PubMed] [Google Scholar]
- 73.Provaggi E, Kalaskar DM. 3D Printing in Medicine. Woodhead Publishing; 2017. pp. 21–42. [Google Scholar]
- 74.Sears NA, Seshadri DR, Dhavalikar PS, Cosgriff-Hernandez E. A Review of Three-Dimensional Printing in Tissue Engineering. Tissue Engineering Part B: Reviews. 2016;22:298–310. doi: 10.1089/ten.teb.2015.0464. [DOI] [PubMed] [Google Scholar]
- 75.Gittard SD, Narayan RJ. Laser direct writing of micro- and nano-scale medical devices. Expert review of medical devices. 2010;7:343–356. doi: 10.1586/erd.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. doi: https://doi.org/10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 77.Vaezi M, Seitz H, Yang S. A review on 3D micro-additive manufacturing technologies. The International Journal of Advanced Manufacturing Technology. 2013;67:1721–1754. doi: 10.1007/s00170-012-4605-2. [DOI] [Google Scholar]
- 78.Kim YK, Park JA, Yoon WH, Kim J, Jung S. Drop-on-demand inkjet-based cell printing with 30-μm nozzle diameter for cell-level accuracy. Biomicrofluidics. 2016;10:064110. doi: 10.1063/1.4968845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hollister SJ, Flanagan CL, Zopf DA, Morrison RJ, Nasser H, Patel JJ, Ebramzadeh E, Sangiorgio SN, Wheeler MB, Green GE. Design Control for Clinical Translation of 3D Printed Modular Scaffolds. Annals of Biomedical Engineering. 2015;43:774–786. doi: 10.1007/s10439-015-1270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hourd P, Medcalf N, Segal J, Williams DJ. A 3D bioprinting exemplar of the consequences of the regulatory requirements on customized processes. Regenerative Medicine. 2015;10:863–883. doi: 10.2217/rme.15.52. [DOI] [PubMed] [Google Scholar]