Abstract
Introduction
Dopamine and glutamate are crucial neurotransmitters in Parkinson disease (PD). While recent large meta-analyses reported that genetic variation of dopamine (DRD2, DRD3) and glutamine (NMDA, GRIN2B) neurotransmitter receptors was not associated with PD risk, they could conceivably influence PD phenotype. We studied the association of these receptor polymorphisms relating to PD age of onset.
Methods
There were 664 PD patients and 718 controls, all Caucasian, with stored DNA at Mayo Clinic, Jacksonville, Florida. Genotyping was performed for DRD2 (Taq 1A, rs1800497), DRD3 (rs6280), and NMDA (GRIN2B, rs7301328) polymorphisms with ABI Taqman assays. Single nucleotide polymorphism associations with age of onset were evaluated using dominant, recessive, and additive genotypic models.
Results
DRD3 variant carriers had an approximate 4.4-year decrease in mean age of onset when both copies of the minor allele were present (P = 0.0034) and an approximate 1.5-year decrease in mean age at onset for every additional minor allele (P = 0.023) (recessive and additive models, respectively). There was no association with age of onset for DRD2 or GRIN2B under any statistical model (all P ≥ 0.22).
Conclusions
The DRD3 (rs6280) polymorphism, but not DRD2 (Taq1A) or GRIN2B, influences younger PD age of onset in the US Caucasian population. Validation of these findings in larger studies with other ethnic groups is indicated.
Keywords: Age, Dopamine, Genetics, NMDA, Parkinsonism, Receptor
1. Background
The cardinal motor signs of Parkinson disease (PD) emerge following the loss of a critical mass of dopaminergic neurons within the substantia nigra. The neurotransmitters dopamine and glutamate play a central role in basal ganglia circuitry, and dopamine also modulates glutamatergic influences on the basal ganglia [1,2]. Therefore, inherent genetic variation in dopaminergic or glutamatergic neurotransmission might conceivably influence either the risk of developing PD or its phenotype.
The association of PD risk with dopamine receptor (DRD2, DRD3) and glutamate-N-methyl-D-aspartate (NMDA) receptor polymorphisms has already been examined extensively over the past couple of decades. Several case-control studies have reported variable associations of DRD2 polymorphisms with PD risk [3–8]. Certain DRD3 polymorphisms have been associated with PD risk (e.g. rs2134655) [8], while others (e.g. rs6280) [4,5] and the NMDA GRIN2B receptor polymorphisms were not apparently associated with PD risk [6]. Recently, two very large meta-analysis studies found no association with genetic variation in DRD2, DRD3, or NMDA GRIN2B relating to PD risk [9,10].
However the genetic influence of these dopamine and NMDA receptor polymorphisms on PD phenotype is less well studied. It is logical that phenotypic variability in PD is likely influenced by genetic variability. One such phenotype is the age of onset of PD.
The primary aim of this study was to investigate the association of dopamine (DRD2, DRD3) and glutamate NMDA (GRIN2B) receptor polymorphisms in relation to PD age of onset in a Caucasian population.
2. Methods
The subjects included 664 PD patients evaluated by Mayo Clinic neurologists and 718 normal controls with stored DNA in the Mayo Clinic PD bank in Jacksonville, FL. All subjects provided written consent and the study was approved by the IRB. All subjects were Caucasian. In PD patients, the mean age was 76 ± 11 SD years (Range: 38–101), the mean age at PD onset was 67 ± 12 SD years (Range: 32–94), and 421 patients (63%) were male. The mean age in the control group was 72 ± 13 SD years (Range: 23–96) and 298 subjects (42%) were male. Age at PD onset was defined as the age of the first PD diagnosis by a neurologist.
Genotype polymorphism analysis for DRD2 (Taq 1A, rs1800497), DRD3 (rs6280), and NMDA GRIN2B (rs7301328) was performed with ABI Taqman assays (Life Technologies, Grand Island, NY) on the Sequenom iPLEX ® platform (Agena Bioscience, San Diego, CA). There was no evidence of a departure from Hardy–Weinberg equilibrium in controls (all chi-square P > .05), and pair-wise linkage disequilibrium between variants was very weak (all r2 ≤ 0.0021 in controls).
Single nucleotide polymorphism (SNP) associations with PD age of onset were examined using linear regression models adjusted for gender. Additive models (effect of each additional minor allele), dominant models (presence vs. absence of the minor allele), and recessive models (presence vs. absence of two copies of the minor allele) were utilized. Regression coefficients and 95% confidence intervals (CIs) were estimated and were interpreted as the increase in mean age at PD onset corresponding to each additional minor allele (additive models), presence of the minor allele (dominant models), or presence of two copies of the minor allele (recessive models).
Associations of each SNP with PD risk were evaluated using logistic regression models adjusted for age and gender. Additive, dominant, and recessive models were again utilized. Odds ratios (ORs) and 95% CIs were estimated. We adjusted for multiple testing using a single-step minP permutation correction separately for each outcome (9 tests for both PD risk and age of onset analysis), after which P values ≤ 0.0073 (age of onset analysis) and ≤ 0.0077 (PD risk analysis) were considered to be statistically significant. All statistical analyses were performed using R Statistical Software (version 2.14.0; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
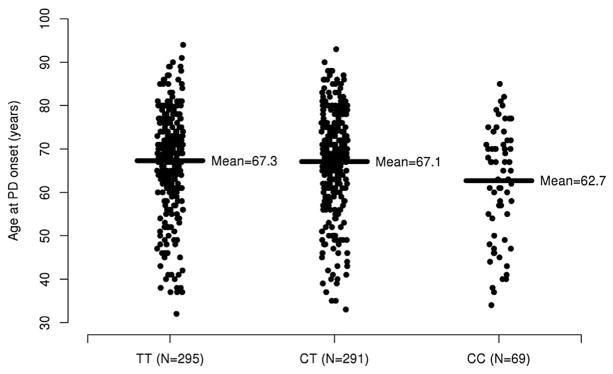
Single-SNP associations with age at onset and PD risk are displayed in the Table 1. Age of onset of PD was significantly associated with the DRD3 (rs6280) variant under a recessive model, with an approximate 4.4 year decrease in mean age of onset when both copies of the minor allele were present (P = 0.0034, Fig. 1). After correcting for multiple testing, the association between the DRD3 (rs6280) variant under an additive model and age at PD onset was not significant (P = 0.023) and there was no notable association under a dominant model (P = 0.22). There was no evidence of an association with PD age of onset for DRD2 (Taq1A) and NMDA GRIN2B under any statistical model (all P ≥ 0.22).
Table 1.
Single-SNP associations with age at PD onset and risk of PD DRD2 (Taq 1A rs1800497), DRD3 (rs6280) and NMDA GRIN2B (rs7301328).
| Association with age at PD onset | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SNP | MA | MAF | Additive model | Dominant model | Recessive model | |||
|
|
|
|
||||||
| Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | Regression coefficient (95% CI) | P-value | |||
| rs1800497 | A | 22.1% | 0.25 (−1.27, 1.78) | 0.75 | 0.07 (−1.78, 1.92) | 0.94 | 1.47 (−2.61, 5.56) | 0.48 |
| rs6280 | C | 32.7% | −1.59 (−2.96, −0.22) | 0.023 | −1.13 (−2.96, 0.70) | 0.22 | −4.42 (−7.38, −1.47) | 0.0034 |
| rs7301328 | C | 40.1% | 0.02 (−1.34, 1.38) | 0.98 | 0.94 (−0.98, 2.86) | 0.34 | −1.63 (−4.22, 0.95) | 0.22 |
|
| ||||||||
| Association with risk of PD | ||||||||
|
| ||||||||
| Additive model | Dominant model | Recessive model | ||||||
|
|
|
|
||||||
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |||
|
| ||||||||
| rs1800497 | A | 20.1% | 1.31 (1.08, 1.59) | 0.0065 | 1.36 (1.08, 1.71) | 0.0096 | 1.54 (0.89, 2.67) | 0.12 |
| rs6280 | C | 33.3% | 0.97 (0.82, 1.15) | 0.73 | 0.97 (0.77, 1.21) | 0.76 | 0.96 (0.68, 1.37) | 0.82 |
| rs7301328 | C | 39.1% | 1.11 (0.95, 1.31) | 0.20 | 1.26 (1.00, 1.59) | 0.048 | 0.97 (0.71, 1.33) | 0.86 |
SNP = single nucleotide polymorphism. MA = minor allele. MAF = minor allele frequency. OR = odds ratio. PD = Parkinson Disease. CI = confidence interval. MAFs in PD patients are given for associations with age at PD onset, while MAFs in the overall sample are given for associations with risk of PD. Single-SNP associations with age at PD onset were evaluated using linear regression models adjusted for gender. Regression coefficients and 95% CIs are interpreted as the increase in mean age at PD onset corresponding to each additional minor allele (additive models), presence of the minor allele (dominant models), or presence of two copies of the minor allele (recessive models). Single-SNP associations with risk of PD were evaluated using logistic regression models adjusted for age and gender. ORs correspond to each additional minor allele (additive models), presence of the minor allele (dominant models), or presence of two copies of the minor allele (recessive models). After applying a single-step minP permutation adjustment for multiple testing, P values ≤ 0.0073 (associations with age at PD onset) and ≤0.0077 (associations with risk of PD) were considered as statistically significant.
Fig. 1.
Age at PD onset according to DRD3 rs6280 genotype. The sample mean is shown with a solid horizontal line for each genotype.
Additionally, we assessed the variants for association with PD risk (Table 1). We observed a significant association between the DRD2 variant and risk of PD under an additive model (OR: 1.31, P = 0.0065). No other significant associations with PD risk were observed after multiple testing correction, though nominally significant associations were noted for the DRD2 variant under an additive model (P = 0.0096) and the NDMA GRIN2B variant under a dominant model (P = 0.048) (Table 1). Allele and genotype frequencies for PD patients and controls are displayed for each variant in the Supplemental Table.
4. Discussion
We investigated dopamine and glutamate candidate gene variants for association with age of onset of PD. In this Caucasian population sample, the DRD3 (rs6280) variant was associated with earlier PD age of onset (recessive model), and the DRD2 and NMDA GRIN2B polymorphisms were not. Risk of PD was associated with the DRD2 variant alone.
The risk of earlier age of onset of PD associated with the DRD3 (rs6280) polymorphism does not imply direct causation but may simply reflect other unexamined variations in compensatory reserve during nigrostriatal neurodegeneration. A clinical diagnosis of PD is based on parkinsonian motor features, which are secondary to nigrostriatal loss. Receptors wired in series with the dopaminergic nigrostriatal system might vary in their capacity to withstand or be influenced by such insults. As motor signs are necessary to make the diagnosis, patients with better receptor compensatory reserve might have minimized clinical findings compared to the degree of nigrostriatal degeneration. This would influence the appearance of motor signs and therefore the age of onset of PD.
DRD3 (rs6280) corresponds to a Ser9Gly variant, located in the extracellular N-terminus of DRD3, a region postulated to affect receptor glycosylation and trafficking [11]. Thus, genetic variation may conceivably affect dopaminergic transmission. The DRD3 receptors are widely expressed throughout the basal ganglia, including ventral striatum, and the neocortex [12]. There is age-related decline in both DRD3 and DRD2 receptors [13], so genetic variants could potentially influence the rate and severity of network-based neurodegeneration.
Our findings for DRD3 (rs6280) and risk of earlier PD age of onset requires validation in both a larger cohort study, and within other ethnic groups. Similarly, the lack of association of DRD2 or NMDA GRIN2B with PD age of onset warrants confirmation. This is cautioned by our findings of PD risk associated with the DRD2 polymorphism, which agrees with previous case-control studies of similar size, but was not borne out in the large GWAS studies [9,10]. Large unbiased genome-wide association study efforts have characterized common genetic variation that influences susceptibility to PD. This approach may help to move the field closer to teasing out the genetic factors that influence phenotypic variability, perhaps coupled with Next-Generation sequencing in populations or families.
Other phenotypes within PD, besides age of onset, have been subject to genetic investigation including drug-induced traits such as dyskinesia and impulse control disorders. For example, DRD3 (rs6280) has been associated with diphasic dyskinesia [14], and both DRD3 (rs6280) and GRIN2B were associated with impulse control behaviors [15]. This genetic influence on phenotypic traits may not only identify individuals at risk but may also generate novel therapeutic intervention targets and inform biomarker discovery programs.
Supplementary Material
Acknowledgments
Funding sources for study
OAR is supported in part by the Michael J. Fox Foundation, NINDS R01# NS078086, and The Mayo Clinic Florida Morris K. Udall Parkinson’s Disease Research Center of Excellence (NINDS P50 #NS072187).
We would like to thank the patients and families who donated DNA samples for this work.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.parkreldis.2015.11.016.
Footnotes
- Conception and design of the study, or acquisition of data, or analysis and interpretation of data – A.H., J.E.A, M.G.H., D.J.S., R.J.U, Z.K.W., M.S.O., J.V., S.R., O.A.R
- Drafting the article or revising it critically for important intellectual content- A.H., J.E.A, M.G.H., D.J.S., R.J.U, Z.K.W., M.S.O., J.V., S.R., O.A.R
- Final approval of the version to be submitted. – A.H., J.E.A, M.G.H., M.S.O., O.A.R
Competing interests
AH, RJU, JAV, MGH, DJS, JEA, SR and OAR have no financial disclosures. ZKW is supported by the Udall National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke P50NS40256, NIH/National Institute on Aging (NIA) P01AG017216, NIH/NIA R01AG015866, and Pacific Alzheimer Research Foundation C06-01 grants, and holds a patent on LRRK2 genetic variability. MSO has received consultant fees for the National Parkinson Foundation; and research grants from NIH, NPF, Michael J. Fox Foundation, The Parkinson Alliance, Smallwood Foundation, Bachmann-Strauss Foundation, Tourette Syndrome Association, and the UF Foundation. MSO has received royalties for publications with Demos, Manson, Amazon, Smashwords, Book-s4Patients, and Cambridge. MSO is associate editor for New England Journal of Medicine Journal Watch Neurology. MSO has participated in CME and educational activities on movement disorders sponsored by PeerView, Prime, Quantia, Henry Stewart, and Vanderbilt University.
References
- 1.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 2.Greenamyre JT. Glutamatergic influences on the basal ganglia. Clin Neuropharmacol. 2001;24:65–70. doi: 10.1097/00002826-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Dick FD, De Palma G, Ahmadi A, Osborne A, Scott NW, Prescott GJ, et al. Gene-environment interactions in parkinsonism and Parkinson’s disease: the geoparkinson study. Occup Environ Med. 2007;64:673–680. doi: 10.1136/oem.2006.032078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeling BH, Vilarino-Guell C, Ross OA, Wszolek ZK, Uitti RJ, Farrer MJ. DRD3 Ser9Gly and HS1BP3 Ala265Gly are not associated with Parkinson disease. Neurosci Lett. 2009;461:74–75. doi: 10.1016/j.neulet.2009.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGuire V, Van Den Eeden SK, Tanner CM, Kamel F, Umbach DM, Marder K, et al. Association of DRD2 and DRD3 polymorphisms with Parkinson’s disease in a multiethnic consortium. J Neurol Sci. 2011;307:22–29. doi: 10.1016/j.jns.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SJ, Liu HC, Liu TY, Cheng CY, Hong CJ. Association analysis for genetic variants of the NMDA receptor 2b subunit (GRIN2B) and Parkinson’s disease. J Neural Transm. 2002;109:483–488. doi: 10.1007/s007020200039. [DOI] [PubMed] [Google Scholar]
- 7.Tan EK, Tan Y, Chai A, Tan C, Shen H, Lum SY, et al. Dopamine D2 receptor TaqIA and TaqIB polymorphisms in Parkinson’s disease. Mov Disord. 2003;18:593–595. doi: 10.1002/mds.10406. [DOI] [PubMed] [Google Scholar]
- 8.Dai D, Wang Y, Wang L, Li J, Ma Q, Tao J, et al. Polymorphisms of and genes and Parkinson’s disease: A meta-analysis. Biomed Rep. 2014;2:275–281. doi: 10.3892/br.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lill CM, Roehr JT, McQueen MB, Kavvoura FK, Bagade S, Schjeide BM, et al. Comprehensive research synopsis and systematic meta-analyses in Parkinson’s disease genetics: The PDGene database. PLoS Genet. 2012;8:e1002548. doi: 10.1371/journal.pgen.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeanneteau F, Funalot B, Jankovic J, Deng H, Lagarde JP, Lucotte G, et al. A functional variant of the dopamine D3 receptor is associated with risk and age-at-onset of essential tremor. Proc Natl Acad Sci USA. 2006;103:10753–10758. doi: 10.1073/pnas.0508189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- 13.Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging. 2000;21:683–688. doi: 10.1016/s0197-4580(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Cho J, Lee EK, Park SS, Jeon BS. Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Mov Disord. 2011 Jan;26(1):73–79. doi: 10.1002/mds.23400. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, Jeon BS. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009 Sep 15;24(12):1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.