Abstract
Citrus aurantium L. (Rutaceae), commonly known as bitter orange, possesses multiple therapeutic potentials. These biological credentials include anticancer, antianxiety, antiobesity, antibacterial, antioxidant, pesticidal, and antidiabetic activities. The essential oil of C. aurantium was reported to display marked pharmacological effects and great variation in chemical composition depending on growing locations but mostly contained limonene, linalool, and β-myrcene. Phytochemically, C. aurantium is rich in p-synephrine, an alkaloid, and many health-giving secondary metabolites such as flavonoids. Animal studies have demonstrated a low affinity of p-synephrine for adrenergic receptors and an even lower affinity in human models. The present review focuses on the different biological activities of the C. aurantium in animal and human models in the form of extract and its pure secondary metabolites. Finally, it is concluded that both the extract and isolated compounds have no unwanted effects in human at therapeutic doses and, therefore, can confidently be used in various dietary formulations.
1. Introduction
Citrus aurantium L. (Rutaceae), commonly known as bitter orange, is usually utilized as a flavoring and acidifying agent for food [1]. Besides the essential oil and its components [2, 3], the fruits of C. aurantium are sources of flavonoid-type compounds with diverse biological effects [4–6]. Additionally, it was reported that flavonoid glycosides were isolated from the plant [7] and the biogenic amine and flavanone contents have been determined [8, 9]. Due to the abundance of health-giving secondary metabolites, C. aurantium is also used for the treatment of several ailments such as anxiety [10], lung and prostate cancers [11], and gastrointestinal disorders and obesity [2, 12]. Due to the prohibition of Ephedra sinica Stapf. in Farw.-containing weight loss products in the market, C. aurantium has found an important place as a preferable agent to replace ephedra, as it contains p-synephrine, a phenylethanolamine type alkaloid, which is chemically similar to adrenergic agents, as appetite suppressants [12–14]. Recently, several scientific studies investigating the potential effects of various parts (including flowers, fruits, and essential oils) of C. aurantium have been conducted [15–17].
In the last years, phytopharmaceuticals have shown an outstanding role in new drug discovery [18–20]. Both in the crude form as well as pure chemical entities, a large population around the globe are getting therapeutic benefits from them [21–23]. In this review article, we have aimed to overview the bioactivity studies performed on C. aurantium revealing its therapeutic potential in the light of isolated molecules and/or essential oils.
2. Chemistry of Citrus aurantium
The chemical composition of C. aurantium is responsible for health-promoting effects. The chemical composition includes vitamins, minerals, phenolic compounds, and terpenoids [21–23]. Among the diverse chemical components in C. aurantium, flavonoids belonging to phenolics have been recognized as important due to their physiological and pharmacological role and their health benefits [21–23].
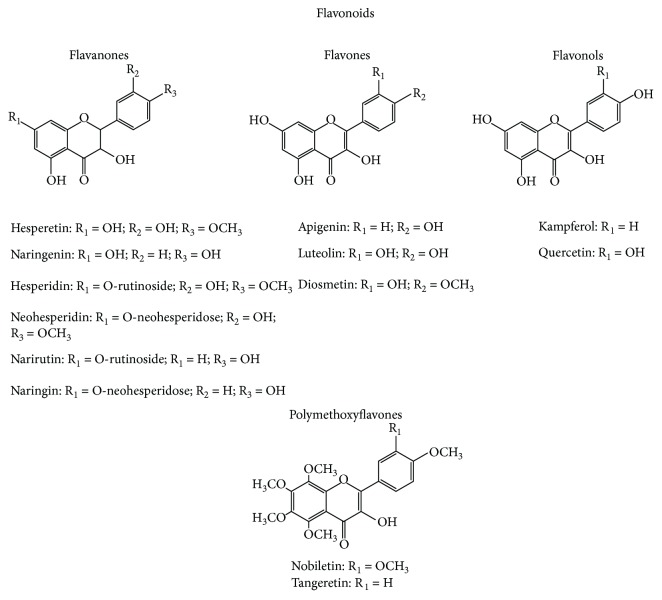
The flavonoids contained in C. aurantium can be divided into four groups, including flavones, flavanones, flavonols, and anthocyanins (only in blood oranges) (Scheme 1)[21–23].
Scheme 1.
Chemical structures of major flavonoids contained in C. aurantium.
Flavonoids are mainly present in Citrus fruits as glycosyl derivatives. Aglycones are mainly present in specific parts of the fruit as peel and seeds, owing to their lipophilic nature and consequently their low solubility in water [21–23]. For glycoside forms, O-glycosides, C-glycosides, rutinosides, glucosides, and neohesperidosides are common [21–23]. The presence of a relatively large number of glycosylated flavonoids in Citrus is a result of many different combinations possible between polyhydroxylated aglycones and a limited number of mono- and disaccharides [24].
Flavanones are the main flavonoids contained in C. aurantium [25]. The most abundant detected free flavanones are hesperetin (4′-methoxy-3′,5,7-trihydroxyflavanone) and naringenin (4′,5,7-trihydroxyflavanone) [26]. Hesperetin and naringenin present a common skeleton: two hydroxyl group in positions C-5 and C-7, respectively, and they can be found as aglycone and/or as glycosides [25]. The most widely distributed glycosides of hesperetin are hesperidin and neohesperidin, which are conjugates with rutinose and neohesperidose, respectively, while, for naringenin, the most abundant glycosyl derivatives are naringin (naringenin-7-neohesperidoside) and narirutin (naringenin-7-rutinoside) [25].
The flavones in aglycon or/and glycosidic form are the second major group of flavonoids in C. aurantium. The most commonly detected free flavones are apigenin, luteolin, and diosmetin. O-Glycosides and C-glycosides are the two main forms of flavone glycosides, and the most common linked sugar moieties include glucose, rutinose, and neohesperidose [26].
In C. aurantium, the flavones may be present also in the methoxylated form, in which all or almost all hydroxyls are capped by methylation, as nobiletin and tangeretin [27]. Furthermore, the C. aurantium may contain low amounts of flavonols, as kaempferol and quercetin, mainly in glycosidic form.
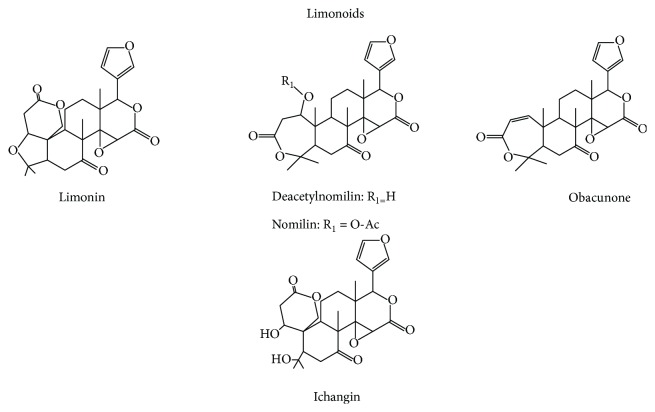
The second class of secondary metabolites found in C. aurantium are the limonoids (Scheme 2). The latter were considered as oxygenated triterpenoids as they contain relatively high numbers of oxygen atoms (7–11) in their structures. All components have a furan ring attached to the D-ring at C-17. Limonoids occur both in glucosidic and aglyconic form. Limonoid aglycones are water-insoluble and responsible for a bitter taste of the Citrus fruits, while limonoid glucosides are water-soluble and tasteless [28]. Among limonoids, the most important one is limonin, known as Citrus constituent since 1841. Limonoid glucosides are more abundant in juices and pulps, such as limonin glucoside, nomilin glucoside, obacunone glucoside, nomilinic acid glucoside, and deacetylnomilinic acid glucoside, because they are water-soluble, while limonoid aglycones such as limonin, nomilin, obacunone, ichangin, and deacetyl nomilin are water-insoluble and are present mainly in seeds and peels [29, 30].
Scheme 2.
Chemical structures of major Limonoids contained in C. aurantium.
Another class of compounds contained in C. aurantium are phenylethylamine alkaloids with p-synephrine being the most abundant. This compound has a hydroxyl group in the para position on the benzene ring and has some structural similarity to ephedrine. The peel of unripe fruits is the part of the plant which has the highest level of p-synephrine [31].
Zarrad and coworkers [32] explored the chemical composition of Tunisian C. aurantium fruits using gas chromatography (GC) and gas chromatography-mass spectroscopy (GC-MS). 25 compounds were identified in 97.696% exploration, but the most prominent one was limonene with 85.52%, followed by linalool and β-myrcene with 3.365% and 1.628%, respectively. The remaining compounds were in traces. Earlier, Villafane et al. [33] reported the presence of D-limonene and nootkatone in the essential oil of the plant. Similarly, Sanei-Dehkordi et al. [34] identified 21 compounds in peel essential oil of the plant. Of the total explored 98.62% composition, the quantity of limonene was 94.81% while the LC50 was 31.20 ppm against Anopheles stephensi. However, the concentration of limonene was only 0.5–2.5% in C. aurantium from Algeria and linalool and α-terpineol were 18.6% and 15.1%, respectively [35]. Thus, overall considerable variation was observed in the concentration of these major components in C. aurantium grown in different parts of the world [36–41]. Carvalho-Freitas and Costa observed strong anxiolytic and sedative-like effects of the essential oil derived from C. aurantium [42].
3. Pharmacological Profile
3.1. Cytotoxic and Anticancer Effects
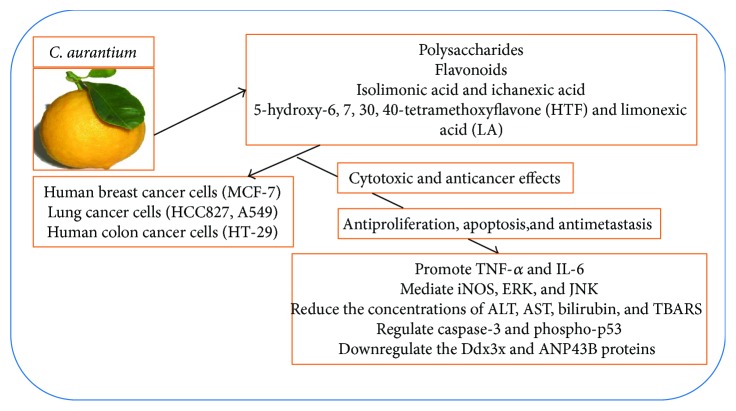
The anticancer activity of the plant has been reported in literature [26, 43]. Cytotoxic properties of the polysaccharides obtained from C. aurantium were examined. Cytotoxic activity on human breast cancer cells (MCF-7) and lung cancer cells (HCC827) and immune-enhancement effect were examined. The results indicated that C. aurantium var. amara polysaccharides displayed good immune-enhancement activity by stimulating the production of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in RAW264.7 cells and by promoting the mRNA expression levels of inducible nitric oxide synthase (iNOS), TNF-α, interleukin-1β (IL-1β), and IL-6. Moreover, phosphorylated extracellular signal-regulated kinase (ERK), phosphorylated c-Jun N-terminal kinase (JNK), phosphorylated p38, and phosphorylated p65 were significantly enhanced in C. aurantium var amara polysaccharides-treated RAW264.7 cells [24, 44]. Isolimonic acid and ichanexic acid from ethyl acetate extract of C. aurantium were isolated by using chromatographic methods. The compounds for their inhibitory action on human colon cancer cells (HT-29) proliferation, apoptosis, and on noncancerous (COS-1 fibroblast) cells were investigated. The compounds displayed an increment in the cell counts in G2/M stage, demonstrating a potential effect in the cell-cycle arrest [25].
Protective activity of the extract obtained from the peels of C. aurantium against apoptosis in cholestatic liver fibrosis-induced mice was investigated. As part of the experiment, cholestatic liver injury was induced by bile duct ligation and the mice were treated with C. aurantium peel extract. According to the histopathological analysis, the administration of C. aurantium peel extract significantly decreased liver fibrosis. Biochemical analysis revealed that the extract reduced the concentrations of alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase, total bilirubin, nitric oxide, and thiobarbituric acid reactive substances (TBARS), suggesting C. aurantium can efficiently regulate bile duct ligation-induced liver injury by displaying antioxidant, anti-inflammatory, and antiapoptotic effects [27].
C. aurantium was shown to possess antimetastasis activities in in vitro assays. A study by Park et al. evaluated the antimetastatic effect of flavonoids, isolated from Korean C. aurantium in mice models. Flavonoids were shown to prevent cancer cell infiltration and localization to the lungs. Moreover, through the regulation of caspase-3 and phospho-p53, the flavonoids induced cancer cell apoptosis. In vitro tests demonstrated that flavonoids inhibited A549 cells metastasis and induced apoptosis and downregulation of the Ddx3x and ANP43B proteins, supporting the use of C. aurantium flavonoids in human lung cancer treatment. According to the bioactivity assays conducted on the major compounds of C. aurantium var. amara, namely, 5-hydroxy-6,7,30,40-tetramethoxyflavone (HTF) and limonexic acid (LA), both compounds showed remarkable antioxidant effects and notable inhibitory action on the B16 and SMCC-7721 cell lines, at a concentration between 6.25 and 50 μg/mL and between 12.5 and 200 μg/mL, respectively [28]. Figure 1 illustrates the cytotoxic effects and mechanisms of C. aurantium extracts.
Figure 1.
Cytotoxic effects and mechanisms of C. aurantium extracts.
Miyazawa et al. [45] isolated three polymethoxy flavonoid-type compounds, namely, tetra-O-methylscutellarein, sinensetin, and nobiletin from C. aurantium. These compounds caused marked downstream regulation of a variety of gene expressions and thus showed strong antimutagenic activity. Wang and coworkers [46] summarized the anticancer effects of polymethoxyflavones with detailed molecular mechanisms. The various amines and flavonoids have been quantitatively analyzed in C. aurantium using liquid chromatography [47, 48]. Hesperidin has been isolated from C. aurantium [49]. It displayed a significant apoptotic effect against liver cell lines, HepG2 cells, by mediating through the upstream regulation of mitogen-activated protein kinase ERK1/2 [50].
3.2. Anxiolytic and Sedative effects
The anxiolytic and sedative effects of C. aurantium by in vivo light-dark box and the marble-burying assays were evaluated. C. aurantium essential oil enhanced the period the mice spent in the light chamber, as well as the number of transitions between the two compartments in the light-dark box test [29]. Moreover, single and repeated treatments with the essential oil were reported to suppress marble-burying behavior [10]. The anxiolytic activity of the blossom of C. aurantium was also evaluated clinically on 60 patients undergoing minor surgical operation with no organic pathology. Two hours before the anesthesia induction, two groups consisting of 30 patients were administered at 1 mL/kg of either C. aurantium distillate or saline solution. Anxiety was assessed by using the Spielberger state-trait anxiety inventory, the Amsterdamd preoperative anxiety, and information scale. The outcome of this study revealed that C. aurantium could be active by reducing preoperative anxiety before minor operation [30].
In traditional Chinese medicine (TCM), the combination of the aqueous extracts of the fruits of Gardenia jasminoides Ellis, C. aurantium, and the bark of Magnolia officinalis Rehd. et Wils. is called Zhi-Zi-Hou-Po (ZZHPD) and has been used to treat depression-like symptoms. Xing et al. [51] explored in vivo antidepressant activity of ZZHPD in rats, by using coat state test, sucrose preference test, forced swimming test, and open-field test. The effects of ZZHPD on hypothalamic-pituitary-adrenal (HPA) axis were determined by measuring the hormone levels. The results showed that ZZHPD improved depressive behaviors and normalized the adrenocorticotropic hormone (ACTH) and corticosterone levels, by restoring the function of HPA axis, and increasing brain-derived neurotrophic factor expression in hippocampus and promoting hippocampal neurogenesis [51]. ACTH/corticotropinis a peptidic hormone secreted from the anterior pituitary, which regulates glucocorticoid cortisol production from the adrenals [52]. Stress increases ACTH release, which induces the stress hormone cortisol, together they cause numerous diseases [53].
Letie et al. [54] conducted another study in vivo, where rat models were exposed to the essential oil of C. aurantium at 1.0%, 2.5%, and 5.0% concentrations by inhalation, in acrylic boxes before employing elevated plus maze and open-field assays. C. aurantium essential oil increased the time of the animals in the open arms of the elevated plus maze test and the time of active social interaction in the open field [54].
Wolfenbuttel et al. [55] investigated the influence of C. aurantium essential oil on melatonin and corticosterone in mice after inhalation for 30 min. Melatonin provides protective effects on neuronal cells and acts as an antidepressant by restoration of corticosterone levels. After treatment, the hormone levels did not present variation, whereas behavioral tests showed that the inhalation of 10% essential oil causes an anxiolytic-like and sedative effect. From these data, Citrus essential oil represents a valuable tool for the treatment of the anxiety disturbs, apparently without interference with melatonin and corticosterone physiological levels [55].
3.3. Antidiabetic Effects
Several reports have been cited in literature regarding the antidiabetic effects of C. aurantium [56–58]. An in vivo study was performed to evaluate the antidiabetic effect and to reveal the toxicity profile of the aqueous extract of the fruits of C. aurantium and the leaves of Rauwolfia vomitoria. In this study, NMRI lean mice (6-week-old) and C57BL/6J lean mice (6- or 11-week-old) were used. A single dose, which corresponds to seventy-fold of a human daily dose, was detected to be nontoxic to the animals. A significant weight loss was determined when the dosage, which corresponds to tenfold of a human daily dose, was administered to C57BL/KsBom-db (db/db) genetic diabetic mice for 6 weeks. These mice were maintained on the carbohydrate-deficient diet during the treatment period. The food intake was not significantly different from the control group animals; however, the serum triglyceride levels of the treated animals were significantly higher suggesting the lipid mobilization from internal stores. The fatty acid levels of the eyes of the treated mice remarkably reduced along with stearoyl-CoA desaturase activity [59]. A possible effect of the extract obtained from C. aurantium and p-synephrine on liver metabolism was evaluated. In order to measure catabolic and anabolic pathways, an isolated perfused rat liver was used. Both the extract and the compound were found to enhance glycolysis, glycogenolysis, oxygen uptake, and perfusion pressure. p-Synephrine increased the glucose output at 200 μM concentration. C. aurantium extract enhanced gluconeogenesis at low concentrations, however, inhibited at high concentrations. The effects of C. aurantium extract on liver metabolism was found to be similar to those of adrenergic agents, and p-synephrine could be responsible from the activity [14].
3.4. Antiobesity Effects
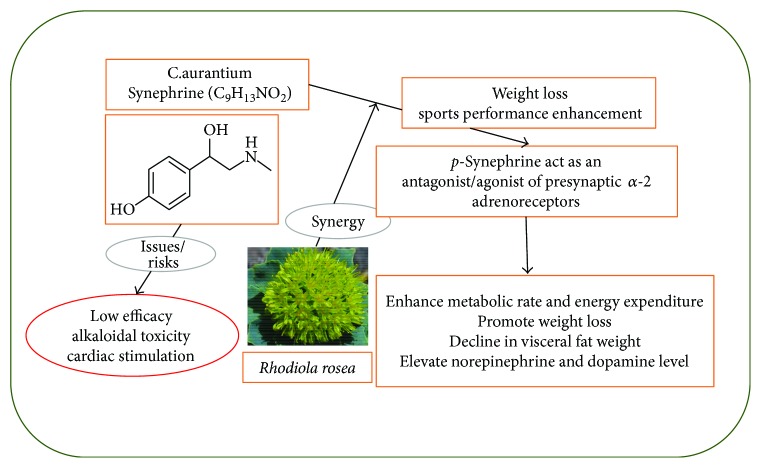
Besides its scientifically proven antimicrobial, antioxidant, cytotoxic, anxiolytic, and antidiabetic effects, C. aurantium extract has been commonly utilized for the weight loss and as sports performance enhancer, in dietary supplements [60–62]. Therefore, the use of C. aurantium extract and its constituent p-synephrine (C9H13NO2), for the treatment of obesity in 360 subjects, was reviewed. More than 50% of the subjects involved in these clinical studies were overweight, and approximately two-thirds of them consumed caffeine (132–528 mg/day) and p-synephrine (10–53 mg/day). Approximately 44% of the subjects used a C. aurantium/p-synephrine product, while the remaining consumed a combination product containing multiple ingredients with p-synephrine. The results showed that C. aurantium extract alone or in combination with other ingredients did not cause significant adverse effects including an increase in heart rate or blood pressure or change in electrocardiographic data, serum chemistry, blood cell counts, or urinalysis. p-Synephrine, alone or in combination products, was demonstrated to enhance metabolic rate and energy expenditure and to promote weight loss when given for six to 12 weeks [63].
The study by Verpeut et al. [64] investigated the effect of the combination of C. aurantium (standardized to 6% p-synephrine) and Rhodiola rosea L. (golden root) (standardized to 3% rosavins and 1% salidroside) on diet-induced obesity in Sprague-Dawley rats. Acute administration of C. aurantium (1–10 mg/kg) or R. rosea (2–20 mg/kg) alone did not decrease food intake in normal weight animals; however, the combination of C. aurantium (5.6 mg/kg) and R. rosea (20 mg/kg) provided a 10.5% feeding suppression. On the other hand, 10 days of treatment with C. aurantium (5.6 mg/kg) or R. rosea (20 mg/kg) alone, or in combination, to the animals fed on a high-fat diet (60% fat) during the 13-week period led to a 30% decline in visceral fat weight, compared with other treatments. Coadministration of C. aurantium and R. rosea also resulted in an elevation in hypothalamic norepinephrine and frontal cortex dopamine, indicating the beneficial role of C. aurantium and R. rosea in the treatment of obesity [64]. Figure 2 illustrates the various pharmacological effects of C. aurantium extracts.
Figure 2.
Pharmacological effects of C. aurantium extracts.
3.5. Effects on the Cardiovascular System
The cardiovascular toxicity of C. aurantium extracts with different concentrations of protoalkaloid p-synephrine (4 and 6%) was reported by Calapai et al. in 1999 in rats. Authors showed an antiobesity effect by the administration of C. aurantium but also possible cardiovascular toxicity. The cardiovascular effects have not been confirmed by studies using much higher doses of p-synephrine [65].
Another study was carried out to evaluate the cardiovascular effects of different doses of C. aurantium and pure p-synephrine in rats. C. aurantium extract and pure p-synephrine enhanced the heart rate and blood pressure. Higher activities were obtained with C. aurantium extract than p-synephrine, suggesting that other compounds in the extract can alter physiological parameters [13]. The effect of p-synephrine on heart rate and blood pressure in female Sprague-Dawley rats was assessed. During 28 days, two types of extracts, one of which contained 6% and other 95% p-synephrine, were administered daily by gavage at 10 or 50 mg/kg, doses for a 60 kg human equal to 600 and 3000 mg/kg. The outcome of the study showed that both p-synephrine and C. aurantium extract resulted in clinically insignificant increases in heart rate and blood pressure at doses many times greater than used in humans. p-Synephrine and bitter orange extract exhibited little or no effect on the cardiovascular effects of caffeine [13].
Safety of p-synephrine was investigated by Ratamess et al. [66] on humans, animals, and in vitro. Authors reported over 30 human studies indicating that the cardiovascular effects of p-synephrine and bitter orange extracts are clinically insignificant. p-Synephrine showed a greater ability to bind adrenergic receptors in rodents than in humans, and the data in literature on its effects on animals cannot be indicative for men at commonly used doses.
This review concluded that C. aurantium extract and p-synephrine are safe for use in dietary supplements and foods at commonly used doses. Also, other authors reported similar conclusions [67, 68]. While p-synephrine alone seems to have low toxicity, when it is formulated in combination with other ingredients such as caffeine (Paullinia cupana, Cola nitida, Cola acuminata, and Camellia sinensis), salicin (Salix sp.), and ephedrine (ma huang, Ephedra sinica, and Ephedra sp.) in weight loss products, the mixture could induce some cardiovascular effects. However, the study did not demonstrate that p-synephrine contributed to cardiovascular effects.
Schmitt et al. [69] reported clear signs of toxicity of this mixture in mice of both sexes as reduction in locomotor activity, ptosis, seizures, salivation, agitation, piloerection, and deaths after acute oral administration of 300, 350, and 400 mg/kg total of p-synephrine, ephedrine, salicin, plus caffeine in a 10 : 4 : 6 : 80 w/w ratio.
The volatile oil obtained from the flowers of C. aurantium var. amara, namely, neroli oil, is used to reduce heart rate and palpitations, to encourage sleep, and to soothe the digestive tract. Kang et al. [15] investigated the activity mechanism of neroli in mouse aorta. Neroli was found to exert vasodilator activity in mice precontracted with prostaglandin (PG)-F2α. Nevertheless, the relaxation was reduced in the endothelium-denuded ring or preincubation with the nitric oxide synthase inhibitor by neroli treatment. Moreover, the relaxation induced by neroli was partially reversed by soluble guanylyl cyclase inhibitor. Neroli also inhibited extracellular Ca2+-dependent and depolarization-induced contraction in a dose-dependent manner. Nonselective cation channel blocker, Ni2+, decreased neroli-induced relaxation. On the other hand, a K+ channel blocker, tetraethylammonium chloride, did not alter the relaxation. In order to prevent Ca2+ influx through the smooth muscle voltage-gated Ca2+ channels, verapamil was added. In this case, the ryanodine (a class of intracellular calcium channels) receptor inhibitor reduced neroli-induced relaxation. All these data indicated that neroli-induced relaxation might be partly mediated by the nitric oxide-soluble guanylyl cyclase, and ryanodine receptor signaling pathway [15].
3.6. Effect on Microorganisms
Antimicrobial activity of C. aurantium was investigated by using several in vitro assays [70–72]. For instance, Karabıyıklı et al. [1] explored the antimicrobial action of C. aurantium juice against Salmonella enterica Typhimurium and Listeria monocytogenes. For this purpose, both neutralized and unneutralized juice samples with various concentrations were tested for the inoculation of the microorganisms at 4°C and 37°C temperatures, during a period of seven days. The results showed that Salmonella enterica Typhimurium and Listeria monocytogenes not only survived but also grown for two days in neutralized juice at 37°C. On the other hand, on day 7, none of them survived. The results also revealed that L. monocytogenes was less resistant than S. enterica Typhimurium. The low pH of C. aurantium juice was suggested to be responsible for its antimicrobial potential along with the duration of the incubation period as well as the temperature [1]. In another research, the antimicrobial potential of C. aurantium was investigated, and high antimicrobial activity was recorded against Bacillus subtilis and Staphylococcus aureus (among 12 microorganisms tested), with the minimum inhibition concentration (MIC) values of 2.7 mg/mL and 4.8 mg/mL. Moderate effects were detected against Saccharomyces cerevisiae and Mucor ramannianus with the values of 9.2 mg/mL and 5 mg/mL, respectively. In the same study, radical scavenging and antibacterial effects of the essential oil obtained from the leaves of C. aurantium were evaluated. Weak antioxidant effect was detected against 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azinobis-3-ethylbenzothiazoline-6-sulphonate (ABTS) radicals [73].
3.7. Pesticidal Effects
Previous in vitro investigations on the essential oil of C. aurantium peels and its isolated component limonene have demonstrated their pest fumigant activity against Bemisia tabaci (silverleaf whitefly). In 24 h exposure, insect mortality between 41.00 and 47.67% was determined at 2.5 and 20.0 μL/L air concentrations. In the same study, the authors conducted another assay, investigating the anticholinesterase effect of the oil itself, as well as that of the pure compound. Both exerted anticholinesterase effect with the IC50 values of 2.94 mM and 3.54 mM, respectively [74–76]. Other studies investigated the larvicidal effect of the essential oils of C. aurantium against Anopheles labranchiae. After 24 h, the mortality counts were carried out, and LC50 and LC90 values were determined. The outcome of this study demonstrated that the essential oils exerted significant larvicidal potential. C. aurantium essential oil was found to be the most active by the LC50 and LC90 values of 22.64 mg/L and 83.77 mg/L, respectively [13, 77]. The essential oil extract from the fresh peeled ripe fruit of Citrus aurantium showed also good larvicidal effect against mosquito vector Anopheles stephensi (LC50 values, 31.20 ppm) The main constituent of the leaf oil was limonene (94.81).
3.8. Antioxidant Effects
DPPH, ABTS, and ferric-reducing antioxidant power assays were used for the determination of the antioxidant potential of the macerate of the albedo layers of C. aurantium fruits obtained by protopectinase-SE (produced by Geotrichum klebahnii and hydrolyzed selectively the intercellular protopectin of plant tissues). Moreover, the levels of total phenols, reducing sugars, vitamin C, total flavonones, naringin, and galacturonic acid and total acidity were detected. Antioxidant activity, vitamin C, and total flavonone levels were found to be the highest with the greatest degree of tissue maceration [78–81]. In vivo and in vitro antioxidant activities of polysaccharide fractions from C. aurantium were evaluated. The most active fraction was subjected to ion exchange and gel-filtration chromatography to obtain four purified polysaccharides. Upon the evaluation of their antioxidant effect, it was found that C. aurantium can be utilized as an antioxidant in the food and medical industries [82, 83].
In a study by Lagha-Benamrouche et al. [84], the antioxidant potentials of the peels and leaves of seven orange varieties obtained from Algeria were investigated by linoleic acid and β-carotene oxidation assays. The presence of phenolic compounds was also investigated. C. aurantium cv. Bigarade was found to have the highest phenol level. The antioxidant activity assay was in accordance with the phytochemical findings. C. aurantium displayed the highest action on slowing down the rate of linoleic acid and β-carotene oxidation [84, 85]. An antioxidant study was performed on the C. aurantium fruits on different ripening stages. According to the outcome obtained from DPPH free radical-scavenging and β-carotene/linoleic acid systems, the antioxidant activity was found to be varied related to the amount of the phenolic components [86]. Another antioxidant activity study revealed that during dehydration, different air-drying temperature affected the antioxidant effect of the C. aurantium by-products, including peel and pulp remaining after juice extraction [79]. C. aurantium peel and juice were suggested as a new potential source of natural antioxidants; although, they were found to be less effective than butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and ascorbic acid, used as antioxidant standards [87, 88]. Anti-inflammatory activity of the flavonoid-type compounds of Korean C. aurantium, namely, nobiletin, naringin, and hesperidin was investigated. Inhibition of proinflammatory mediators by blocking nuclear factor-kappa B (NF-ƘB) and mitogen-activated protein kinase (MAPK) signaling in lipopolysaccharide- (LPS-) stimulated RAW 264.7 macrophages was assessed [89]. The flavonoids were found to have the capacity to suppress the mRNA and protein expression of COX-2 and iNOS, by clarifying their anti-inflammatory action [4]. A polymethoxy flavonoid-rich C. aurantium extract was shown to have a protective effect on alcohol-induced liver injury in an animal study via AMPK and Nrf2-related signal regulation [90].
3.9. Antiulcer Effects
In order to assess the effect of C. aurantium essential oil and its main compound limonene on gastric mucosa, Moraes et al. [2] conducted a study in vivo. The essential oil and its compound limonene were found to possess protective activity in the gastrointestinal system against lesions, which were induced by ethanol and nonsteroidal anti-inflammatory drugs in rats, at doses of 250 mg/kg and 245 mg/kg, respectively. The essential oil and limonene increased the production of gastric mucus. The findings revealed that C. aurantium essential oil and its main compound limonene can be used as a promising target for the development of a novel gastroprotective drug [2]. In another study, the gastroprotective effect of β-myrcene, a monoterpene-type compound of C. aurantium, was evaluated. Experimental models of ulcer, induced by ethanol, NSAID stress, Helicobacter pylori, ischemia-reperfusion injury (I/R), and cysteamine (a drug used to treat cystinosis) was used to assess the ameliorative activity. β-Myrcene was administered at dose of 7.5 mg/kg. The results showed a potential role for β-myrcene against peptic ulcer disease. β-Myrcene contributed to the maintenance of integrity of the gastric mucosa with a significant decrease of ulcerative lesions, attenuating lipid peroxidative damage and preventing depletion of GSH, GR, and GPx [91].
Polo et al. confirmed the healing properties of C. aurantium essential oil on gastric ulcers after treatment in middle-aged Wistar rats. They showed a significant reduction of the lesion area (76%) within the gastric mucosa that appears regenerated (59%) when compared to the negative control group [92].
On the other hand, Hamdan et al. assessed the effects of hesperidin and neohesperidin, important flavonoid-type components of C. aurantium. For this purpose, indomethacin-induced ulcer models of rats were used. Omeprazole was administered as a reference standard for the comparison. The parameters analyzed in the present study were ulcer index, gastric cyclooxygenase-2 (COX-2) gene expression, TNF-α, MDA, and GSH levels. Histopathalogical analysis was also performed. The findings of the study revealed that hesperidin and neohesperidin notably aggravated indomethacin-induced gastric damage as evidenced by increased ulcer index and histopathological alteration [93]. Recently, hesperidin showed strong inhibition against ovarian cancer cell viability and caused apoptosis mediated through endoplasmic reticulum stress signaling pathways [94]. Hafidh et al. [95] observed the potent anticancer effects of limonene in hepatocellular carcinoma and HepG2, and the cell line caused the modulation of cancer-inducing genes. These data confirmed the antioxidant and prooxidant behavior of flavonoids showing the potential benefits and adverse effects of these opposing events.
4. Adverse Effects
Because of the possible adverse effects of C. aurantium in the cardiovascular system, the use of C. aurantium-containing products has been questioned mainly due to the p-synephrine content [96]. As a fat decreasing agent, it was used in traditional medications, but it has been banned by the National Collegiate Athletic Association (NCAA), due to its risks such as cardiovascular hazards and alkaloid toxicity [97]. However, the cardiovascular effects of p-synephrine have been opposed by several studies [98–101]. The study by Rodrigues et al. [102] was designed to investigate the herb-drug interactions between a standardized extract of C. aurantium and amiodarone, an antiarrhythmic medication, in rats. In the first step of the study, a single-dose of C. aurantium and amiodarone was administered orally to the rats at 164 mg/kg and 50 mg/kg doses, respectively. In the second step, the rats were pretreated with C. aurantium for 14 days, and on the 15th day, the amiodarone was administered at the same dose. On the other hand, the control group rats received vehicle only. The results demonstrated a significant enhancement of the peak plasma concentration of amiodarone in rats pretreated with C. aurantium extract, indicating a potential herb-drug interaction between C. aurantium extract and amiodarone [102]. Most recent clinical studies on 16 individuals have indicated that C. aurantium extract and its principal alkaloid, p-synephrine, had no adverse effects on the cardiovascular system after 15 days of treatment [103]. Moreover, Stohs et al. [67] reported approximately 30 human studies indicating that p-synephrine and bitter orange extracts do not result in cardiovascular effects; the effects in rodents cannot be directly extrapolated to humans at commonly used doses.
Regarding the adverse effects of formulations for body weight loss containing Ephedra sinica and Citrus aurantium, Arbo et al. used theuterotrophic assay to test the antiestrogenic activity of ephedrine, p-synephrine, E. sinica, and C. aurantium in immature female rats. Only ephedrine at 0.5 mg/kg/day presented a significative antiestrogenic effect [104].
5. Conclusion
In conclusion, studies regarding the bioactivity potential of C. aurantium have revealed that flowers, fruits, essential oils, and phytoconstituents of this plant exerted several biological effects including antimicrobial, antioxidant, cytotoxic, anxiolytic, antidiabetic, antiobesity, and anti-inflammatory activities. However, further long-term studies are needed in order to affirm its safety, especially regarding the plant-drug interactions and the proper dosage. p-Synephrine is a very poor adrenergic agonist and, therefore, is not expected to produce cardiovascular effects. It should be noted that it is banned by the NCAA which was assumed without evidence that it possesses cardiovascular risks and potential toxicity. It is not banned by the World Anti-Doping Agency (WADA) which deals with Olympic athletes.
Conflicts of Interest
The authors of this article have no conflict of interest.
Authors' Contributions
Haroon Khan designed the overall review. Ipek Suntar, Haroon Khan, and Seema Patel wrote the initial draft of the review while Rita Celano and Luca Rastrelli finalized the review.
References
- 1.Karabıyıklı Ş., Değirmenci H., Karapınar M. Inhibitory effect of sour orange (Citrus aurantium) juice on Salmonella Typhimurium and Listeria monocytogenes. 2014;55(2):421–425. doi: 10.1016/j.lwt.2013.10.037. [DOI] [Google Scholar]
- 2.Moraes T. M., Kushima H., Moleiro F. C., et al. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: role of prostaglandins and gastric mucus secretion. 2009;180(3):499–505. doi: 10.1016/j.cbi.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Barceloux D. G. Hoboken, NJ, USA: John Wiley & Sons, Inc; 2008. Citrus Oil and Limonene; pp. 635–643. [DOI] [Google Scholar]
- 4.Kang S. R., Park K. I., Park H. S., et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. 2011;129(4):1721–1728. doi: 10.1016/j.foodchem.2011.06.039. [DOI] [Google Scholar]
- 5.Hamada Y., Nakajima M., Tsuzuki K., et al. Heptamethoxyflavone reduces phosphodiesterase activity and T-cell growth in vitro. 2017;174(3-4):113–120. doi: 10.1159/000481094. [DOI] [PubMed] [Google Scholar]
- 6.Liu L., Shan S., Zhang K., Ning Z.-Q., Lu X.-P., Cheng Y.-Y. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. 2008;22(10):1400–1403. doi: 10.1002/ptr.2504. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X.-L., Xu W.-F., Chen G., Wang H.-F., Pei Y.-H. Two new phenolic glycosides isolated from the fruits of Citrus aurantium. 2017;15(1):41–44. doi: 10.1016/S1875-5364(17)30006-7. [DOI] [PubMed] [Google Scholar]
- 8.Pellati F., Benvenuti S., Melegari M. High-performance liquid chromatography methods for the analysis of adrenergic amines and flavanones in Citrus aurantium L. var. amara. 2004;15(4):220–225. doi: 10.1002/pca.771. [DOI] [PubMed] [Google Scholar]
- 9.Bagatela B. S., Lopes A. P., Cabral E. C., Perazzo F. F., Ifa D. R. High-performance thin-layer chromatography/desorption electrospray ionization mass spectrometry imaging of the crude extract from the peels of Citrus aurantium L.(Rutaceae) 2015;29(16):1530–1534. doi: 10.1002/rcm.7246. [DOI] [PubMed] [Google Scholar]
- 10.de Moraes Pultrini A., Almeida Galindo L., Costa M. Effects of the essential oil from Citrus aurantium L. in experimental anxiety models in mice. 2006;78(15):1720–1725. doi: 10.1016/j.lfs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Park K.-I., Park H.-S., Kim M.-K., et al. Flavonoids identified from Korean Citrus aurantium L. inhibit non-small cell lung cancer growth in vivo and in vitro. 2014;7:287–297. doi: 10.1016/j.jff.2014.01.032. [DOI] [Google Scholar]
- 12.Fugh-Berman A., Myers A. Citrus aurantium, an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. 2004;229(8):698–704. doi: 10.1177/153537020422900802. [DOI] [PubMed] [Google Scholar]
- 13.Hansen D. K., George N. I., White G. E., et al. Physiological effects following administration of Citrus aurantium for 28 days in rats. 2012;261(3):236–247. doi: 10.1016/j.taap.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Peixoto J. S., Comar J. F., Moreira C. T., et al. Effects of Citrus aurantium (bitter orange) fruit extracts and p-synephrine on metabolic fluxes in the rat liver. 2012;17(5):5854–5869. doi: 10.3390/molecules17055854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang P., Ryu K.-H., Lee J.-M., Kim H.-K., Seol G. H. Endothelium- and smooth muscle-dependent vasodilator effects of Citrus aurantium L. var. amara: focus on Ca2+ modulation. 2016;82:467–471. doi: 10.1016/j.biopha.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 16.Kostopoulou Z., Therios I., Roumeliotis E., Kanellis A. K., Molassiotis A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. 2015;86:155–165. doi: 10.1016/j.plaphy.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Pellati F., Benvenuti S. Erratum to “chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium” [J. Chromatogr. A 1161 (2007) 71–88] 2007;1164(1-2):p. 334. doi: 10.1016/j.chroma.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 18.Amin S., Khan H. Revival of natural products: utilization of modern technologies. 2016;12(2):103–106. doi: 10.2174/1573407212666160314195845. [DOI] [Google Scholar]
- 19.Khan H., Amin S. ACE inhibition of plant alkaloids. Targeted approach for selective inhibition. 2017;14(1):85–89. doi: 10.2174/1570193X14666161201124705. [DOI] [Google Scholar]
- 20.Khan H., Khan M. A., Hussain S., Gaffar R., Ashraf N. In vivo antinociceptive and anticonvulsant activity of extracts of Heliotropium strigosum. 2016;32(5):860–865. doi: 10.1177/0748233713513489. [DOI] [PubMed] [Google Scholar]
- 21.Khan H., Nabavi S. M., Sureda A., et al. Therapeutic potential of songorine, a diterpenoid alkaloid of the genus Aconitum. 2017 doi: 10.1016/j.ejmech.2017.10.065. In press. [DOI] [PubMed] [Google Scholar]
- 22.Nabavi S. F., Khan H., D'Onofrio G., et al. Apigenin as neuroprotective agent: of mice and men. 2018;128:359–365. doi: 10.1016/j.phrs.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Marya, Khan H., Nabavi S. M., Habtemariam S. Anti-diabetic potential of peptides: future prospects as therapeutic agents. 2018;193:153–158. doi: 10.1016/j.lfs.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Han M. H., Lee W. S., Lu J. N., et al. Citrus aurantium L. exhibits apoptotic effects on U937 human leukemia cells partly through inhibition of Akt. 2012;40(6):2090–2096. doi: 10.3892/ijo.2012.1350. [DOI] [PubMed] [Google Scholar]
- 25.Jayaprakasha G. K., Mandadi K. K., Poulose S. M., Jadegoud Y., Nagana Gowda G. A., Patil B. S. Novel triterpenoid from Citrus aurantium L. possesses chemopreventive properties against human colon cancer cells. 2008;16(11):5939–5951. doi: 10.1016/j.bmc.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 26.Lee S. H., Yumnam S., Hong G. E., et al. Flavonoids of Korean Citrus aurantium L. induce apoptosis via intrinsic pathway in human hepatoblastoma HepG2 cells. 2015;29(12):1940–1949. doi: 10.1002/ptr.5488. [DOI] [PubMed] [Google Scholar]
- 27.Lim S. W., Lee D. R., Choi B. K., et al. Protective effects of a polymethoxy flavonoids-rich Citrus aurantium peel extract on liver fibrosis induced by bile duct ligation in mice. 2016;9(12):1158–1164. doi: 10.1016/j.apjtm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H.-Y., Yang L., Wei J., Huang M., Jiang J.-G. Bioactivity evaluations of ingredients extracted from the flowers of Citrus aurantium L. var. amara Engl. 2012;135(4):2175–2181. doi: 10.1016/j.foodchem.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Costa C. A. R. A., Cury T. C., Cassettari B. O., Takahira R. K., Flório J. C., Costa M. Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT 1A-receptors and reduces cholesterol after repeated oral treatment. 2013;13(1):p. 42. doi: 10.1186/1472-6882-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akhlaghi M., Shabanian G., Rafieian-Kopaei M., Parvin N., Saadat M., Akhlaghi M. Citrus aurantium blossom and preoperative anxiety. 2011;61(6):702–712. doi: 10.1016/S0034-7094(11)70079-4. [DOI] [PubMed] [Google Scholar]
- 31.Rossato L. G., Costa V. M., Limberger R. P., Bastos M. d. L., Remião F. Synephrine: from trace concentrations to massive consumption in weight-loss. 2011;49(1):8–16. doi: 10.1016/j.fct.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Zarrad K., Hamouda A. B., Chaieb I., Laarif A., Jemâa J. M.-B. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. 2015;76:121–127. doi: 10.1016/j.indcrop.2015.06.039. [DOI] [Google Scholar]
- 33.Villafane E., Tolosa D., Bardon A., Neske A. Toxic effects of Citrus aurantium and C. limon essential oils on Spodoptera frugiperda (Lepidoptera: Noctuidae) 2011;6(9):1389–1392. [PubMed] [Google Scholar]
- 34.Sanei-Dehkordi A., Sedaghat M. M., Vatandoost H., Abai M. R. Chemical compositions of the peel essential oil of Citrus aurantium and its natural larvicidal activity against the malaria vector Anopheles stephensi (Diptera: Culicidae) in comparison with Citrus paradisi. 2016;10(4):577–585. [PMC free article] [PubMed] [Google Scholar]
- 35.Abderrezak M., Abaza I., Aburjai T., Kabouche A., Kabouche Z. Comparative compositions of essential oils of Citrus aurantium growing in different soils. 2014;5:1913–1918. [Google Scholar]
- 36.Hosni K., Hassen I., M'Rabet Y., Sebei H., Casabianca H. Genetic relationships between some Tunisian Citrus species based on their leaf volatile oil constituents. 2013;50:65–71. doi: 10.1016/j.bse.2013.03.035. [DOI] [Google Scholar]
- 37.Hosni K., Zahed N., Chrif R., et al. Composition of peel essential oils from four selected Tunisian Citrus species: evidence for the genotypic influence. 2010;123(4):1098–1104. doi: 10.1016/j.foodchem.2010.05.068. [DOI] [Google Scholar]
- 38.McKay D. L., Blumberg J. B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.) 2006;20(8):619–633. doi: 10.1002/ptr.1936. [DOI] [PubMed] [Google Scholar]
- 39.Sarrou E., Chatzopoulou P., Dimassi-Theriou K., Therios I. Volatile constituents and antioxidant activity of peel, flowers and leaf oils of Citrus aurantium L. growing in Greece. 2013;18(9):10639–10647. doi: 10.3390/molecules180910639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang M.-H., Yang L., Zhu L., Piao J.-H., Jiang J.-G. Comparative GC/MS analysis of essential oils extracted by 3 methods from the bud of Citrus aurantium L. var. amara Engl. 2011;76(9):C1219–C1225. doi: 10.1111/j.1750-3841.2011.02421.x. [DOI] [PubMed] [Google Scholar]
- 41.Kirbaslar F. G., Kirbaslar S. I. Composition of cold-pressed bitter orange peel oil from Turkey. 2003;15(1):6–9. doi: 10.1080/10412905.2003.9712247. [DOI] [Google Scholar]
- 42.Carvalho-Freitas M. I. R., Costa M. Anxiolytic and sedative effects of extracts and essential oil from Citrus aurantium L. 2002;25(12):1629–1633. doi: 10.1248/bpb.25.1629. [DOI] [PubMed] [Google Scholar]
- 43.Pimenta F. C. F., Alves M. F., Pimenta M. B. F., et al. Anxiolytic effect of Citrus aurantium L. on patients with chronic myeloid leukemia. 2016;30(4):613–617. doi: 10.1002/ptr.5566. [DOI] [PubMed] [Google Scholar]
- 44.Shen C.-Y., Yang L., Jiang J.-G., Zheng C.-Y., Zhu W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. 2017;8(2):796–807. doi: 10.1039/c6fo01545j. [DOI] [PubMed] [Google Scholar]
- 45.Miyazawa M., Okuno Y., Fukuyama M., Nakamura S.-i., Kosaka H. Antimutagenic activity of polymethoxyflavonoids from Citrus aurantium. 1999;47(12):5239–5244. doi: 10.1021/jf990176o. [DOI] [PubMed] [Google Scholar]
- 46.Wang L., Wang J., Fang L., et al. Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. 2014;2014:10. doi: 10.1155/2014/453972.453972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Avula B., Upparapalli S. K., Navarrete A., Khan I. A. Simultaneous quantification of adrenergic amines and flavonoids in C. aurantium, various Citrus species, and dietary supplements by liquid chromatography. 2005;88(6):1593–1606. [PubMed] [Google Scholar]
- 48.Benavente-Garcia O., Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. 2008;56(15):6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 49.Lee D.-H., Park K.-I., Park H.-S., et al. Flavonoids isolated from Korea Citrus aurantium L. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. 2012;2012:11. doi: 10.1155/2012/515901.515901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yumnam S., Park H. S., Kim M. K., et al. Hesperidin induces paraptosis like cell death in hepatoblatoma, HepG2 cells: involvement of ERK1/2 MAPK. 2014;9(6, article e101321) doi: 10.1371/journal.pone.0101321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xing H., Zhang K., Zhang R., Shi H., Bi K., Chen X. Antidepressant-like effect of the water extract of the fixed combination of Gardenia jasminoides, Citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mild stress. 2015;22(13):1178–1185. doi: 10.1016/j.phymed.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Vanhorebeek I., Van den Berghe G. The neuroendocrine response to critical illness is a dynamic process. 2006;22(1):1–15. doi: 10.1016/j.ccc.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Tsigos C., Chrousos G. P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. 2002;53(4):865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 54.Leite M. P., Fassin Jr J., Baziloni E. M. F., Almeida R. N., Mattei R., Leite J. R. Behavioral effects of essential oil of Citrus aurantium L. inhalation in rats. 2008;18:661–666. doi: 10.1590/S0102-695X2008000500003. [DOI] [Google Scholar]
- 55.Wolffenbüttel A. N., Zamboni A., Becker G., et al. Citrus essential oils inhalation by mice: behavioral testing, GCMS plasma analysis, corticosterone, and melatonin levels evaluation. (160).2018;32(1):160–169. doi: 10.1002/ptr.5964. [DOI] [PubMed] [Google Scholar]
- 56.Alarcon-Aguilara F. J., Roman-Ramos R., Perez-Gutierrez S., Aguilar-Contreras A., Contreras-Weber C. C., Flores-Saenz J. L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. 1998;61(2):101–110. doi: 10.1016/S0378-8741(98)00020-8. [DOI] [PubMed] [Google Scholar]
- 57.Jia S., Hu Y., Zhang W., et al. Hypoglycemic and hypolipidemic effects of neohesperidin derived from Citrus aurantium L. in diabetic KK-A y mice. 2015;6(3):878–886. doi: 10.1039/C4FO00993B. [DOI] [PubMed] [Google Scholar]
- 58.Xiao-Ping Y., Song C.-Q., Yuan P., Mao R.-G. α-Glucosidase and α-amylase inhibitory activity of common constituents from traditional Chinese medicine used for diabetes mellitus. 2010;8(5):349–352. doi: 10.3724/sp.j.1009.2010.00349. [DOI] [Google Scholar]
- 59.Campbell J. I., Mortensen A., Mølgaard P. Tissue lipid lowering-effect of a traditional Nigerian anti-diabetic infusion of Rauwolfia vomitoria foilage and Citrus aurantium fruit. 2006;104(3):379–386. doi: 10.1016/j.jep.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 60.Colker C. M., Kaiman D. S., Torina G. C., Perlis T., Street C. Effects of Citrus aurantium extract, caffeine, and St. John’s wort on body fat loss, lipid levels, and mood states in overweight healthy adults. 1999;60(3):145–153. doi: 10.1016/S0011-393X(00)88523-9. [DOI] [Google Scholar]
- 61.Stohs S. J., Preuss H. G., Shara M. Issues regarding a FACT review paper on the efficacy of herbal supplements containing Citrus aurantium and synephrine alkaloids for the management of overweight and obesity. 2013;18(1):45–47. doi: 10.1111/fct.12005. [DOI] [Google Scholar]
- 62.Haaz S., Fontaine K. R., Cutter G., Limdi N., Perumean-Chaney S., Allison D. B. Citrus aurantium and synephrine alkaloids in the treatment of overweight and obesity: an update. 2006;7(1):79–88. doi: 10.1111/j.1467-789X.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 63.Stohs S. J., Preuss H. G., Shara M. A review of the human clinical studies involving Citrus aurantium (bitter orange) extract and its primary protoalkaloid p-synephrine. 2012;9(7):527–538. doi: 10.7150/ijms.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verpeut J. L., Walters A. L., Bello N. T. Citrus aurantium and Rhodiola rosea in combination reduce visceral white adipose tissue and increase hypothalamic norepinephrine in a rat model of diet-induced obesity. 2013;33(6):503–512. doi: 10.1016/j.nutres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calapai G., Firenzuoli F., Saitta A., et al. Antiobesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. 1999;70(6):586–592. doi: 10.1016/S0367-326X(99)00093-3. [DOI] [Google Scholar]
- 66.Ratamess N. A., Bush J. A., Stohs S. J., et al. Acute cardiovascular effects of bitter orange extract (p-synephrine) consumed alone and in combination with caffeine in human subjects: a placebo-controlled, double-blind study. 2018;32(1):94–102. doi: 10.1002/ptr.5953. [DOI] [PubMed] [Google Scholar]
- 67.Stohs S. J. Safety, efficacy, and mechanistic studies regarding Citrus aurantium (bitter orange) extract and p-synephrine. 2017;31(10):1463–1474. doi: 10.1002/ptr.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Penzak S. R., Jann M. W., Cold J. A., Hon Y. Y., Desai H. D., Gurley B. J. Seville (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. 2001;41(10):1059–1063. doi: 10.1177/00912700122012652. [DOI] [PubMed] [Google Scholar]
- 69.Schmitt G. C., Arbo M. D., Lorensi A. L., et al. Toxicological effects of a mixture used in weight loss products: p-synephrine associated with ephedrine, salicin, and caffeine. 2012;31(2):184–191. doi: 10.1177/1091581811435708. [DOI] [PubMed] [Google Scholar]
- 70.Fabio A., Cermelli C., Fabio G., Nicoletti P., Quaglio P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. 2007;21(4):374–377. doi: 10.1002/ptr.1968. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira S. A. C., Zambrana J. R. M., Di Iorio F. B. R., Pereira C. A., Jorge A. O. C. The antimicrobial effects of Citrus limonum and Citrus aurantium essential oils on multi-species biofilms. 2014;28(1):22–27. doi: 10.1590/S1806-83242013005000024. [DOI] [PubMed] [Google Scholar]
- 72.Meléndez P. A., Capriles V. A. Antibacterial properties of tropical plants from Puerto Rico. 2006;13(4):272–276. doi: 10.1016/j.phymed.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 73.Ellouze I., Abderrabba M., Sabaou N., Mathieu F., Lebrihi A., Bouajila J. Season’s variation impact on Citrus aurantium leaves essential oil: chemical composition and biological activities. 2012;77(9):T173–T180. doi: 10.1111/j.1750-3841.2012.02846.x. [DOI] [PubMed] [Google Scholar]
- 74.Wang J.-J., Tsai J. H., Ding W., Zhao Z.-M., Li L.-S. Toxic effects of six plant oils alone and in combination with controlled atmosphere on Liposcelis bostrychophila (Psocoptera: Liposcelididae) 2001;94(5):1296–1301. doi: 10.1603/0022-0493-94.5.1296. [DOI] [PubMed] [Google Scholar]
- 75.Siskos E. P., Konstantopoulou M. A., Mazomenos B. E., Jervis M. Insecticidal activity of Citrus aurantium fruit, leaf, and shoot extracts against adult olive fruit flies (Diptera: Tephritidae) 2007;100(4):1215–1220. doi: 10.1093/jee/100.4.1215. [DOI] [PubMed] [Google Scholar]
- 76.El-Akhal F., Lalami A. E. O., Guemmouh R. Larvicidal activity of essential oils of Citrus sinensis and Citrus aurantium (Rutaceae) cultivated in Morocco against the malaria vector Anopheles labranchiae (Diptera: Culicidae) 2015;5(6):458–462. doi: 10.1016/S2222-1808(15)60815-5. [DOI] [Google Scholar]
- 77.da Camara C. A. G., Akhtar Y., Isman M. B., Seffrin R. C., Born F. S. Repellent activity of essential oils from two species of Citrus against Tetranychus urticae in the laboratory and greenhouse. 2015;74:110–115. doi: 10.1016/j.cropro.2015.04.014. [DOI] [Google Scholar]
- 78.Zapata Zapata A. D., Gaviria Montoya C. A., Cavalitto S. F., Hours R. A., Rojano B. A. Enzymatic maceration of albedo layer from sour orange (Citrus aurantium L.) with protopectinase-se and measurement of antioxidant activity of the obtained products. 2012;45(2):289–294. doi: 10.1016/j.lwt.2011.08.009. [DOI] [Google Scholar]
- 79.Garau M. C., Simal S., Rossello C., Femenia A. Effect of air-drying temperature on physico-chemical properties of dietary fibre and antioxidant capacity of orange (Citrus aurantium v. Canoneta) by-products. 2007;104(3):1014–1024. doi: 10.1016/j.foodchem.2007.01.009. [DOI] [Google Scholar]
- 80.Karimi E., Oskoueian E., Hendra R., Oskoueian A., Jaafar H. Z. E. Phenolic compounds characterization and biological activities of Citrus aurantium bloom. 2012;17(2):1203–1218. doi: 10.3390/molecules17021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y., Qiao L., Shen Y., Jiang P., Chen J., Ye X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. 2013;78(1):C37–C42. doi: 10.1111/j.1750-3841.2012.03002.x. [DOI] [PubMed] [Google Scholar]
- 82.Wang Q. H., Shu Z. P., Xu B. Q., et al. Structural characterization and antioxidant activities of polysaccharides from Citrus aurantium L. 2014;67:112–123. doi: 10.1016/j.ijbiomac.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 83.Rekha C., Poornima G., Manasa M., et al. Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. 2012;1(2):303–310. doi: 10.7598/cst2012.182. [DOI] [Google Scholar]
- 84.Lagha-Benamrouche S., Madani K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: peels and leaves. 2013;50:723–730. doi: 10.1016/j.indcrop.2013.07.048. [DOI] [Google Scholar]
- 85.Ghasemi K., Ghasemi Y., Ebrahimzadeh M. A. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. 2009;22(3):277–281. [PubMed] [Google Scholar]
- 86.Moulehi I., Bourgou S., Ourghemmi I., Tounsi M. S. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. 2012;39:74–80. doi: 10.1016/j.indcrop.2012.02.013. [DOI] [Google Scholar]
- 87.Jabri karoui I., Marzouk B. Characterization of bioactive compounds in Tunisian bitter orange (Citrus aurantium L.) peel and juice and determination of their antioxidant activities. 2013;2013:12. doi: 10.1155/2013/345415.345415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tundis R., Loizzo M. R., Bonesi M., et al. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. peel essential oils. 2012;77(1):H40–H46. doi: 10.1111/j.1750-3841.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 89.Kim J.-A., Park H.-S., Kang S.-R., et al. Suppressive effect of flavonoids from Korean Citrus aurantium L. on the expression of inflammatory mediators in L6 skeletal muscle cells. 2012;26(12):1904–1912. doi: 10.1002/ptr.4666. [DOI] [PubMed] [Google Scholar]
- 90.Choi B. K., Kim T. W., Lee D. R., et al. A polymethoxy flavonoids-rich Citrus aurantium extract ameliorates ethanol-induced liver injury through modulation of AMPK and Nrf2-related signals in a binge drinking mouse model. 2015;29(10):1577–1584. doi: 10.1002/ptr.5415. [DOI] [PubMed] [Google Scholar]
- 91.Bonamin F., Moraes T. M., dos Santos R. C., et al. The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. 2014;212:11–19. doi: 10.1016/j.cbi.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Polo C. M., Moraes T. M., Pellizzon C. H., Marques M. O., Rocha L. R. M., Hiruma-Lima C. A. Gastric ulcers in middle-aged rats: the healing effect of essential oil from Citrus aurantium L. (Rutaceae) 2012;2012:8. doi: 10.1155/2012/509451.509451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamdan D. I., Mahmoud M. F., Wink M., El-Shazly A. M. Effect of hesperidin and neohesperidin from bittersweet orange (Citrus aurantium var. bigaradia) peel on indomethacin-induced peptic ulcers in rats. 2014;37(3):907–915. doi: 10.1016/j.etap.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 94.Zhao J., Li Y., Gao J., De Y. Hesperidin inhibits ovarian cancer cell viability through endoplasmic reticulum stress signaling pathways. 2017;14(5):5569–5574. doi: 10.3892/ol.2017.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hafidh R., Hussein S., MalAllah M., Abdulamir A., Abu Bakar F. A high-throughput quantitative expression analysis of cancer-related genes in human HepG2 cells in response to limonene, a potential anticancer agent. 2017;18 doi: 10.2174/1568009617666171114144236. [DOI] [PubMed] [Google Scholar]
- 96.Bent S., Padula A., Neuhaus J. Safety and efficacy of citrus aurantium for weight loss. 2004;94(10):1359–1361. doi: 10.1016/j.amjcard.2004.07.137. [DOI] [PubMed] [Google Scholar]
- 97.Arbo M. D., Schmitt G. C., Limberger M. F., et al. Subchronic toxicity of Citrus aurantium L.(Rutaceae) extract and p-synephrine in mice. 2009;54(2):114–117. doi: 10.1016/j.yrtph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 98.Bouchard N. C., Howland M. A., Greller H. A., Hoffman R. S., Nelson L. S. Ischemic stroke associated with use of an ephedra-free dietary supplement containing synephrine. 2005;80(4):541–545. doi: 10.4065/80.4.541. [DOI] [PubMed] [Google Scholar]
- 99.Arbo M. D., Larentis E. R., Linck V. M., et al. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. 2008;46(8):2770–2775. doi: 10.1016/j.fct.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 100.Stohs S. J., Preuss H. G., Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. 2011;25(10):1421–1428. doi: 10.1002/ptr.3490. [DOI] [PubMed] [Google Scholar]
- 101.Shara M., Stohs S. J., Mukattash T. L. Cardiovascular safety of oral p-synephrine (bitter orange) in healthy subjects: a randomized placebo-controlled cross-over clinical trial. 2016;30(5):842–847. doi: 10.1002/ptr.5590. [DOI] [PubMed] [Google Scholar]
- 102.Rodrigues M., Alves G., Falcão A. Investigating herb–drug interactions: the effect of Citrus aurantium fruit extract on the pharmacokinetics of amiodarone in rats. 2013;60:153–159. doi: 10.1016/j.fct.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 103.Shara M., Stohs S. J., Smadi M. M. Safety evaluation of p-synephrine following 15 days of oral administration to healthy subjects: a clinical study. 2018;32(1):125–131. doi: 10.1002/ptr.5956. [DOI] [PubMed] [Google Scholar]
- 104.Arbo M. D., Franco M. T., Larentis E. R., et al. Screening for in vivo (anti) estrogenic activity of ephedrine and p-synephrine and their natural sources Ephedra sinica Stapf.(Ephedraceae) and Citrus aurantium L.(Rutaceae) in rats. 2009;83(1):95–99. doi: 10.1007/s00204-008-0324-8. [DOI] [PubMed] [Google Scholar]