Abstract
Natural products, an infinite treasure of bioactive chemical entities, persist as an inexhaustible resource for discovery of drugs. This review article intends to emphasize on one of the naturally occurring flavonoids, astragalin (kaempferol 3-glucoside), which is a bioactive constituent of various traditional medicinal plants such as Cuscuta chinensis. This multifaceted compound is well known for its diversified pharmacological applications such as anti-inflammatory, antioxidant, neuroprotective, cardioprotective, antiobesity, antiosteoporotic, anticancer, antiulcer, and antidiabetic properties. It carries out the aforementioned activities by the regulation and modulation of various molecular targets such as transcription factors (NF-κB, TNF-α, and TGF-β1), enzymes (iNOS, COX-2, PGE2, MMP-1, MMP-3, MIP-1α, COX-2, PGE-2, HK2, AChe, SOD, DRP-1, DDH, PLCγ1, and GPX), kinases (JNK, MAPK, Akt, ERK, SAPK, IκBα, PI3K, and PKCβ2), cell adhesion proteins (E-cadherin, vimentin PAR-2, and NCam), apoptotic and antiapoptotic proteins (Beclin-1, Bcl-2, Bax, Bcl-xL, cytochrome c, LC3A/B, caspase-3, caspase-9, procaspase-3, procaspase-8, and IgE), and inflammatory cytokines (SOCS-3, SOCS-5, IL-1β, IL-4, IL-6, IL-8, IL-13, MCP-1, CXCL-1, CXCL-2, and IFN-γ). Although researchers have reported multiple pharmacological applications of astragalin in various diseased conditions, further experimental investigations are still mandatory to fully understand its mechanism of action. It is contemplated that astragalin could be subjected to structural optimization to ameliorate its chemical accessibility, to optimize its absorption profiles, and to synthesize its more effective analogues which will ultimately lead towards potent drug candidates.
1. Introduction
Medicinal plants have been an infinite source of therapeutic agents since millions of years. Most of the discovered drugs either belong to natural products or derivatives of natural compounds [1, 2]. The actual fact is that nature is the creator of seemingly limitless series of molecular structures. These structures can serve as unlimited sources for the development of drugs, robust chemotypes, and pharmacophores which are able to be amplified into scaffolds of novel drugs for the cure of various ailments [3]. Before the advent of the postgenomic era with high throughput screening, approximately 80% of drugs were either pure extracts of medicinal plants or the semisynthetic analogues of various compounds from natural sources [4]. After the second world war, the pharmaceutical research expanded to massive screening of plant extracts in search of new drugs from natural resources [5]. To date, about 61% of anticancer and 49% of anti-infective compounds have been discovered from natural products [6].
The term “natural products” encompasses chemical entities derived from plants, bread molds, microorganisms, terrestrial vertebrates as well as invertebrates, and marine organisms [7]. These chemical entities are known to have immense chemical diversity with outstanding drug-like properties that contribute towards their multitargeted action [8]. A lot of plant-derived bioactive compounds are used for the cure as well as for the prevention of several diseases. Among these compounds are the polyphenols consisting of alcohols with ≥2 benzene rings and ≥1 hydroxyl group. These polyphenols have a range from simple structural molecules (flavonoids and phenylpropanoids) to highly complex compounds (lignins and melanins). Reports have suggested that polyphenols in general and flavonoids in particular exhibit various biological effects like antiallergic, antibacterial, anti-inflammatory, antiviral, antithrombic, hepatoprotective, antibacterial, and antioxidant activities [9].
Flavonoids are structurally diverse and most abundantly found polyphenols in the human diet [10]. They are mostly found in the form of glycosides and acylglycosides. Flavonoids have been divided into various classes such as flavones, flavonols, flavanones, flavanonols, flavanols or catechins, and anthocyanins. They are the essential constituents of our food and are found in onions, parsley, berries including blue berries, black tea, green tea, bananas, red wine, all citrus fruits, sea blackthorns, and dark chocolates with the contents of 70% or more [11].
Astragalin (kaempferol-3-O-β-D-glucoside), a bioactive natural flavonoid, has been well known for its medicinal importance. It has been reported to exhibit multiple pharmacological properties including antioxidant [12, 13], anti-inflammatory [14], anticancer [15], neuroprotective [16], and cardioprotective property [16].
2. Natural Sources of Astragalin
Astragalin, a naturally occurring flavonoid, has been identified in a variety of plants (Figure 1 and Table 1) such as Cuscuta chinensis Lam., a member of the Convolvulaceae family, which consists of about 60 genera and 1,650 species. The seeds of the genus Cuscuta are a rich source of astragalin and are utilized as a traditional folk medicine to cure osteoporosis in various Asian countries including Pakistan [17]. C. chinensis has high contents of astragalin, that is, 29–34% of total phenolics as compared to other species [18]. Cassia alata belongs to the family Fabaceae (the largest family among angiosperms) that comprises of ∼700 genera and 20,000 species. The leaves of C. alata are found to be effective against skin diseases including eczema and chronic skin impurities in tropical regions of the world (Malaysia, Brazil, and Indonesia) [19]. Astragalin has also been isolated from the plants of Ebenaceae, Rosaceae, and Eucommiaceae families. The summary of plants containing astragalin, parts utilized, and biological features are enlisted in Table 1.
Figure 1.
Natural sources of astragalin.
Table 1.
Plants containing astragalin as an important constituent with its biological properties.
| Name of the plant | Parts used/extract | Biological activities | References | |
|---|---|---|---|---|
| Botanical name | Common name | |||
| Acer truncatum | Shantung maple | — | — | [21] |
| Aceriphyllum rossii | Mukdenia | Aerial parts | Antioxidant | [22] |
| Agrimonia pilosa | Hairy agrimony | Aerial parts | Antihemorrhagic, antiplatelet, antioxidant, and acetylcholinesterase inhibitory | [23] |
| Allium ursinum | Wild garlic | Flowers | Antimicrobial | [24] |
| Allium victorialis | Alpine leek | — | Antitumor | [25] |
| Alsophila spinulosa | Hook tryon | Leaves | Antixanthine oxidase | [26] |
| Apocynum venetum | Luobuma | Leaves | Lower blood pressure, antidepressant, antinephritis, and antineurasthenia | [27] |
| Jasminum subtriplinerve Blume | — | Aerial parts | — | [28] |
| Astragalus hamosus | Dwarf yellow milk vetch | Aerial parts | — | [29] |
| Caesalpinia decapetala | Mysore thorn | Leaves | — | [30] |
| Calligonum polygonoides | Phog | Aerial parts | Antiulcer, anti-inflammatory, hypoglycemic, and antioxidant | [31] |
| Camellia sinensis | Tea | Leaves and seeds | Antidysentery, antihyperlipidemia, antihyperglycemia, and anti-inflammatory | [32–35] |
| Carthamus lanatus L. | Downy safflower | Aerial parts | Antioxidant | [35] |
| Cassia alata | Ringworm bush | Leaves | Antioxidant, anti-infectious, and DNA repair | [19] |
| Celastrus gemmatus Loes | Chinese bittersweet | Leaves | — | [36] |
| Centella asiatica | Asiatic pennywort | Leaves | Anti-inflammatory | [37] |
| Clerodendrum philipinum | Chinese glory bower | Roots | — | [38] |
| Conyza filaginoides | Laennecia filaginoides | Aerial parts | Antiprotozoal | [39] |
| Corchorus olitorius L. | Moroheiya | Leaves | Inhibits the histamine | [40] |
| Cuscuta chinensis | Chinese dodder | Seeds | Antiosteoporotic | [17, 41–43] |
| Cuscuta australis | Australian dodder | Seeds | — | [17, 41–43] |
| Diodia teres | Buttonweed | Whole plant | — | [44] |
| Drosera peltata | Sundew | Antitussive | [45] | |
| Dianthus barbatus cv | Sweet William | Aerial parts | Anti-inflammatory | [46] |
| Eucommia ulmoides | Hardy rubber tree | Leaves | Antidiabetic, antioxidant, and hypnotic effect | [47–49] |
| Eupatorium cannabinum L. | Hemp agrimony | Aerial parts | — | [50] |
| Eupatorium lindleyanum | Aerial parts | — | [51] | |
| Exochorda racemosa | Pearlbrush | — | — | [52] |
| Flaveria bidentis (L.) Kuntze | Coastal plain yellow tops | Leaves | — | [53, 54] |
| Flos gossypii | — | Flowers | — | [55] |
| Gladiolus gandavensis | Gladiolus | Aerial parts | — | [56] |
| Glycyrrhiza glabra | European licorice | Leaves | — | [57] |
| Glycyrrhiza uralensis Fisch | Chinese licorice | Leaves | — | [58] |
| Gynura procumbens | Longevity spinach | — | Antidiabetic | [59] |
| Hedera helix | English ivy | — | — | [60] |
| Helianthemum glomeratum | Island rushrose | Aerial parts | — | [61] |
| Hemistepta lyrata Bunge | — | Whole plant | — | [62] |
| Hippophae rhamnoides L. | Sea buckthorn | Leaves | — | [63] |
| Ipomoea batatas | Sweet potato | Leaf | — | [64] |
| Koelreuteria paniculata | Golden rain tree | Flowers | Antioxidant | [65] |
| Allium ampeloprasum | Wild leek | Leaves | Antioxidant | [66] |
| Ligusticum chuanxiong | — | Aerial parts | — | [67] |
| Lindera aggregate | Evergreen lindera | Leaves | — | [68] |
| Litsea coreana | — | Leaves | Antioxidant | [69] |
| Magnolia fargesii | — | Flowers | Anticomplement | [70] |
| Moringa oleifera Lam. | Drumstick tree | Leaves | Antioxidant | [71] |
| Morus alba L. | White mulberry | Leaves | Hypoglycemic and antioxidant | [72–78] |
| Mussaenda arcuate | Forest star | Leaves | [79] | |
| Nelumbo nucifera | Sacred lotus | Leaves | Lipolytic activity | [80–84] |
| Ochradenus baccatus | Taily weed | Aerial parts | — | [85] |
| Orostachys japonica | Rock pine | — | Calpain inhibitory activity | [86] |
| Diospyros kaki | Japanese persimmon | Leaves | Angiotensin converting enzyme activity, and inhibition of atopic dermatitis (AD) | [12, 87–89] |
| Rosa agrestis | Field briar | Leaves | Anti-inflammatory and antioxidant | [13, 90–92] |
| Peucedanum alsaticum | — | Fruits | — | [93] |
| Phaseolus vulgaris L. | Common bean | — | [94] | |
| Phlomis spinidens | — | Aerial parts | Antiallergic | [95] |
| Phyllanthus muellerianus | — | Leaves | Antibacterial and anti-inflammatory | [96] |
| Polygala cyparissias | — | Antiulcer | [97] | |
| Polygonum salicifolium | Knotweed | Aerial parts | DPPH-free radical scavenging activity | [98] |
| Prunus padus L. | European bird cherry | Flowers and leaves | Antioxidant | [99] |
| Prunus serotina Ehrh | Black cherry | Leaves and flowers | — | [100] |
| Pseudotsuga menziesii | Oregon pine | Needles | Cytotoxic | [101] |
| Radix astragali | Milk vetch root | Roots | Antidiabetic | [102–104] |
| Rhus sylvestris | Sumach | Stems and leaves | Antiosteoporotic | [105] |
| Rosa soulieana | Shrub rose | Flowers | Antioxidant | [106] |
| Rubus rigidus var. camerunensis | Ronce blanche | Aerial parts | Antioxidant | [107] |
| Sapium sebiferum | Chinese tallow | Leaves | — | [108] |
| Solenostemma argel | Arghel | Aerial parts | Antibacterial | [109] |
| Solidago canadensis L. | Canada goldenrod | — | Antioxidant | [110] |
| Sorbus aria (L.) | Lutescens | Leaves | — | [111] |
| Tadehagi triquetrum | — | Whole plant | Antimicrobial and anti-inflammatory | [112] |
| Tiarella polyphylla | Foam flower | Whole plant | — | [113] |
| Trachelospermum jasminoides | Confederate jasmine | Leaves | Antifungal | [114] |
| Urtica cannabina | — | Fruits | — | [115] |
| Vahlia capensis | — | — | Antibacterial | [116] |
| Vicia calcarata | Few flowered vetch | Aerial parts | Hepatoprotective | [117] |
| Wedelia chinensis | — | Whole plant | Inhibitor of the complement system | [118] |
Astragalin can also be produced in vivo by glycosylation of kaempferol at the 3C-O position [20]. UDP-dependent glycosyltransferases (UGT) were used as biocatalysts in the synthesis of astragalin. A recombinant strain of Arabidopsis thaliana was used to construct an efficient UDP-glucose synthesis pathway by use of enzymes such as uridylyltransferase, sucrose phosphorylase, and sucrose permease. BL21-II was a recombinant strain designed to scale up the production of astragalin by using a fed-batch fermentator.
3. Biological Activities of Astragalin and Their Mechanisms of Action
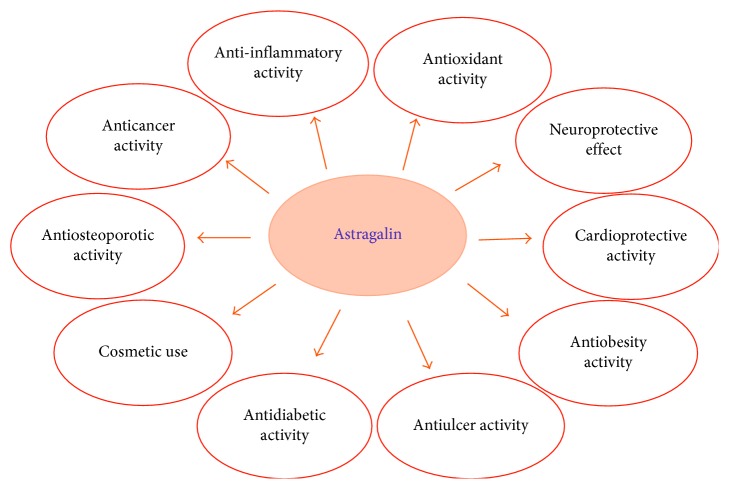
The biologically active and therapeutically effective compound “astragalin” has been known to possess broad spectrum of pharmacological features such as anticancer, anti-inflammatory, antioxidant, neuroprotective, antidiabetic, cardioprotective, antiulcer, and antifibrotic as shown in Figure 2. Various in vivo and in vitro investigations on astragalin have elucidated its medicinal characteristics and mechanism of actions.
Figure 2.
Biological activities of astragalin.
3.1. Anti-inflammatory Activity
Inflammation is an immediate response of a body to tissue damage caused by pathogens and toxic stimuli such as physical or chemical injury. Although inflammatory response is a defense mechanism, but if persistent, it can lead to multiple pathological conditions such as cancer, allergy, atherosclerosis, and autoimmune diseases [119]. Negative after effects associated with nonsteroidal type anti-inflammatory drugs (NSAIDs) arouse a need among researchers to find out effective and safe alternatives [120]. Plant extracts enriched with flavonoids have been known to possess anti-inflammatory activity [121].
Astragalin, a bioactive natural flavonoid, has been known to mitigate inflammation in LPS-induced murine model of mastitis and lung injury model via reducing the activity of myeloperoxidase and the expression of IL-1β, IL-6, and TNF-α. Astragalin's anti-inflammatory response proceeds via inhibition of LPS-induced activation of NF-κB, as it is actively involved in alleviating the deterioration of IkBα and restricting the nuclear translocation of NF-κB [92, 122]. Another investigation on LPS-stimulated expression of inflammatory mediators in macrophages has declared the fact that astragalin actively inhibited the expression of proinflammatory mediators via inhibiting NF-κB signaling pathway [123]. Astragalin has been known to halt the MAPK and NF-κB pathways in leptospira-induced uterine and epithelial inflammation in mice [124]. Astragalin has capability to inhibit the production of prostaglandin E2 (PGE2) in periodontal pathogen-induced periodontitis, a destructive inflammatory pathological condition, in human gingival epithelial cells [125]. Astragalin has been investigated to determine the underlying mechanism for its protective effect against ovalbumin-stimulated allergic reactions in mouse models of allergic asthma. Results have declared that it effectively lowers the eosinophil count in lung tissues and inhibited eosinophilia induced by ovalbumin. As a result, IgE, IL-4, IL-5, and IL-13 were retrieved in bronchoalveolar lavage fluid [126]. Purely prepared astragalin inhibited the activity of PGE2 and downregulated the production of cellular nitrite oxide and IL-6 in LPS-stimulated RAW 264.7 cells [33]. Astragalin treatment leads to the inhibition of alveolar destruction, allergic inflammation, and thickening of airways in the ovalbumin-induced inflammatory mouse model [14]. Anti-inflammatory activities of astragalin in different animal models are recorded in Table 2.
Table 2.
Anti-inflammatory activities of astragalin in vitro and in vivo.
| Assay | Organism tested | Dose/concentration | Molecular targets | References |
|---|---|---|---|---|
| LPS-induced mouse mastitis | Mouse mastitis | 10, 25, and 50 mg/kg | TNF-α↓, IL-1β↓, IL-6↓, p65┴, and IκBα┴ | [92] |
| LPS-induced endotoxemia and lung injury in mice | Mice (lung) | 25, 50, and 75 mg/kg | TNF-α┴, IL-1β┴, and IL-6┴ | [122] |
| LPS-induced macrophages in mice | Mouse cells | 1–100 µg/mL | iNOS↓, COX-2↓,TNF-α↓, IL-1β↓, IL-6↓, MIP-1α↓, MCP-1↓, NF-κB p65┴, IκBα┴, and NO┴ | [127] |
| LPS-induced RAW 264.7 cells. | Mice (RAW 264.7 cells) | 1, 10, and 100 μM | NO↓ and TNF-α↓ | [37] |
| Inhibitory activity on the histamine release by KU812 cells | KU812 cells | 10 to 30 μmol/L | IL-4↓, IL-13↓, and (IFN-γ) no effect | [12] |
| LPS-induced inflammation in RAW 264.7 cells | Mice (RAW 264.7 cells) | NO┴, IL-6┴, and PGE2┴ | [33] | |
| P. gingivalis-induced human gingival epithelial (HGE) cells | Human gingival epithelial cells | COX-2┴, IL-6┴, IL-8┴, MMP-1┴, MMP-3┴, PGE-2┴, and IL-4┴ | [125] | |
| Anti-inflammatory effects on Leptospira interrogans-induced inflammatory response | Uterine and endometrial epithelial cells of mice | 100 μg/mL | TNF-α┴, IL-1β┴, IL-6┴, NF-κB↓, p38┴, p-p38 MAPK↓, ERK┴, JNK┴, and p-p65↓ | [124] |
| Protective effects against ovalbumin- (OVA-) induced allergic inflammation | Mouse model of allergic asthma | 0.5 mg/kg and 1 mg/kg | SOCS-3┴, SOCS-5┴, and IFN-γ↑ | [126] |
| Alleviation in hepatic fibrosis function | Diabetic rats and nondiabetic rats | PAR2┴, IL-1β↓, IL-6↓, TNF-α↓, and TGF-β1┴ | [128] | |
| Prevention from atopic dermatitis | NC/Nga mice | 1.5 mg/kg | IgE↓ | [87] |
↑Upregulation; ↓downregulation; ┴inhibition.
3.2. Antioxidant Activity
In living systems, free radicals such as hydroxyl radicals (OH·), superoxide anion (O2·−), singlet oxygen (1O2), and ROS are reported to have deleterious impacts on cellular functions. Excessive production of free radicals may affect the balance of prooxidant and antioxidant systems in the body, thus causing various pathological conditions such as arterial hypertension, rheumatism, inflammation, diabetes, cancer, neurodegenerative disorders, and genetic mutations [120]. Researchers have affirmed various plant extracts as natural and infinite treasure of antioxidants. These antioxidants act as free radical scavengers, electron donors, and chelating agents for free catalytic metals in biological systems [75].
Astragalin also inhibits the endotoxin-induced oxidative stress, which can lead to epithelial apoptosis and eosinophilia. It can also act as an antagonizing agent against endotoxin-induced oxidative stress via modulation of LPS-TLR signaling network [129]. Astragalin causes the suppression of 6-hydroxydopamine-stimulated neurotoxicity in Caenorhabditis elegans via modulation of apoptosis-related pathways and alleviation of oxidative stress [130]. Astragalin has capability to improve neural function in the ischemia brain injury model of rats via blocking the apoptosis in the hippocampus region by enhancing the expression of NCam [131] (Table 3).
Table 3.
Antioxidant activity of astragalin in vitro and in vivo.
| Assay | Organism tested | Dose/concentration | Molecular targets | References |
|---|---|---|---|---|
| Free radical-scavenging activity | 1, 3, 10, 30, 100, or 300 µg/mL | [107] | ||
| Inhibitory activity against autophagy-associated airway epithelial fibrosis | Mice | 1–20 μM | E-cadherin┴, vimentin┴, Beclin-1↓, LC3A/B↓, EMT↓, and TGF-β1┴ | [132] |
| Apoptotic and eosinophilia amelioration | BEAS-2B cells | 1–20 μM | TLR-4↓, Eotaxin-1↓, PLCγ1↓, PKCβ2↓, p-p22↓, p-47↓, JNK↓, p38 MAPK↓, Akt↑, and ERK↑ | [129] |
| Suppression of 6-hydroxydopamine-induced neurotoxicity in Caenorhabditis elegans | C. elegans | 2.0 mg/mL | egl-1 ↓, SOD↑, GPX↑, AChe↑, and p38 MAPK↓ | [130] |
| Neuroprotective effect against ischemic brain injury | Wister rats | 5 mg/kg and 15 mg/kg | NCam↑ | [131] |
↑Upregulation; ↓downregulation; ┴inhibition.
3.3. Neuroprotective Activity
Disturbance in cerebral redox homeostasis is the main cause of neurodegenerative diseases in humans. Cerebral oxidative stress leads to dopaminergic neuronal cell death and dysfunction. Neuroprotective mechanism of naturally occurring bioactive entities is associated with their free radical scavenging capability generated by neurotoxins and oxidative stress-induced processes in neuronal cells of the brain [133].
Astragalin has been reported to decrease the neurodegeneration in C. elegans stimulated by 6-OHDA and increase lifespan of astragalin-treated nematode. It also reduces the ROS levels, inhibits lipid peroxidation, and increases SOD and GPx activities. Furthermore, it is capable of enhancing AChE and reducing the transcript level of proapoptotic gene egl-1 associated with neuronal cell death [130]. In another attempt, the effects of astragalin on CNS were assessed by the application of the leaves extract of Eucommia ulmoides. The extract with high percentage of astragalin had a significant effect on metabolism of mice. Moreover, it effectively prolonged the convulsion latency and diminished the convulsion rate. These results strengthen the fact that E. ulmoides has a very good hypnotic effect on CNS [49]. Astragalin also suppressed carrageenan-stimulated paw edema in rats. Neural function is also reported to be improved by the use of astragalin in ischemic brain injury rat models [131].
3.4. Cardioprotective Activity
Myocardial infarction and ischemic heart failure are the leading causes of mortality in the developing countries, and their number is increasing day-by-day. They may result in reperfusion arrhythmias, myocardial stunning, and similar other cardiovascular disorders [16]. An enhanced perception of ischemia reperfusion (I/R) damage provides an innovative approach for new cardioprotective administrations [134]. Regulation of bradykinin, adenosine, opioid, adrenergic, and other G-protein connected receptors have been known to be associated with myocardial protection [135].
Certain epidemiological studies have confirmed that flavonoids stimulate cardioprotective effects against myocardial ischemia [136]. Astragalin, a bioactive flavonoid, was proved to be effective against acute I/R injury in Sprague-Dawley rats as its mechanism of action precedes via diminishing intracellular oxidative stress and apoptosis. The associated mechanism involves decreased expression of MDA, TNF-α, IL-6, ROS, and Bax along with the increased ratio of GSH/GSSG, respectively [137].
3.5. Antiobesity Activity
The term “obesity” can be defined as impaired energy balance that usually results from either enhanced caloric intake and/or reduced energy consumption. Currently, much attention has been given to several nutritional aspects that may be useful for inhibiting body fat accumulation and decreasing the risk of diseases related to obesity. In case of mammals, energy metabolism is maintained by lipolytic action in adipose tissues which is generally stimulated by some pharmacologically important lipolytic hormones such as nor-epinephrine, epinephrine, and catecholamines [80]. Many cellular investigations have determined that dietary polyphenols decrease viability of adipocytes and growth of preadipocytes, downregulate triglyceride accumulation and adipocyte differentiation, and induce fatty acid beta-oxidation and lipolysis [138].
Astragalin along with other known flavonoids isolated from N. nucifera showed inhibitory effect on diet-induced obesity and also activated β-adrenergic receptor pathway, but additional experimentation is required to fully elucidate its possible mechanism of action [80].
3.6. Antiulcer Activity
Ulcer is a chronic lesion which usually develops due to an imbalance between numerous protective and aggressive factors. Gastric ulcers being represented by repeated incidents of healing and reexacerbation contribute towards chronic inflammation which may persist for 10–20 years. It is a well-known fact that naturally occurring phenolic entities have capability to shield gastric mucosa from injury due to their cytoprotective and antioxidant features. Furthermore, flavonoids stimulate mucus secretion, block pepsinogen, prohibit Ca2+ influx, and also change GSH metabolism. Astragalin, a pharmacologically active flavonoid isolated from C. cyparissias, has been examined for its antiulcer activity. Results demonstrated that 30 mg/kg dosage of astragalin effectively decreases percentage of lesion area, total area of lesion, and ulcer index in the mice model of gastric secretion [97].
3.7. Antidiabetic Activity
Diabetes mellitus is characterized by hyperglycemia which is caused by deficit in insulin action or production [139]. Currently available antidiabetic therapeutics such as hypoglycemic drugs and insulin have limitations of their own. Natural products and herbal medicines have been suggested as one of the treatment options for diabetes since ancient times. Naturally occurring bioactive chemical entities such as flavonoids, terpenoids, alkaloids, and phenolics have been reported as antidiabetic agents [140].
Diabetic retinopathy (DR) arises due to diabetes mellitus and is one of the most common causes of vision loss. Hyperglycemia leads to overexpression of many biological effectors such as vascular endothelial growth factor (VEGF) which is very crucial for the development of DR. Astragalin derived from A. membranaceus has beneficial effects against hyperglycemia. It helps to prevent DR by decreasing the overexpression of VEGF in cultured muller cells and alleviating the effects caused by high concentration of glucose in the blood [141].
3.8. Antifibrotic Activity
Environmental factors like air pollutants may result in considerable production of reactive oxygen species in the airways. Astragalin isolated from leaves of persimmon and green tea can be effectual in allaying ROS-prompted bronchial fibrosis as it has capability to inhibit auto phagosome formation in the airways [132]. It also alleviates hepatic fibrosis by regulating PAR2 (protease-activated receptor 2) mechanism. AGS regulates proinflammatory cytokines namely IL-6, IL-1β, and TNF-α. It also attenuates the PAR2 signaling expression, and its protective effects are especially prominent in diabetic animal models [128].
3.9. Cosmetic Use
Astragalin glucosides can be used as valuable agents in cosmetics due to their important chemical characteristics. First of all, it inhibits collagenase activity. Collagenase is involved in the hydrolyzation of dermal matrix protein formation as well as wrinkle formation. Secondly, astragalin has an antioxidant activity as it alleviates the free radical species. Thirdly, astragalin controls the pigmentation in the skin caused by melanin [142]. Melanin pigment causes darkening of complexion in skin, eyes, and hair in humans. Nelumbo nucifera (lotus) contains bioactive compounds astragalin and hyperoside in the receptacles which are known to be the melanogenesis inhibitor, thus possibly decreasing the skin darkness [143]. Astragalin along with quercetin is known to possess protective effect against the UV radiations. UV radiations can make the skin of animals prone to various biological responses such as DNA damage, formation of sunburn cells, melanogenesis, photoaging, skin cancer, hyperplasia, immune suppression, and edema. UV radiations from the sun can also damage macromolecules in the epidermal layer of animals creating specific changes in the skin, for example, mutations in genes and changes in the immune system. Expression of major CXC chemokines, that is, chemokine ligand 1 (CXCL1) and chemokine ligand 2 (CXCL2), at sites of inflammation within the skin are upregulated after the exposure of skin to UV radiations. These chemokines are the potent stimulators of neutrophil activation which later on produce ROS and leads to oxidative stress. Astragalin, a major flavonoid, can be used as a barrier against UV-induced damage as it is associated with downregulation of CXCL-1 and CXCL-2 in the skin and thus can be used as a photoprotective agent [144] (Table 4).
Table 4.
Cosmetic uses of astragalin.
| Assay | Organism tested | Dose/concentration | Molecular targets | References |
|---|---|---|---|---|
| Inhibition of melanin secretion | Leuconostoc mesenteroides | 10 mM | MMP-1┴ | [142] |
| Protection against UV damage | Mice (BalB/c) and human keratinocyte cells (HaCaT cells) | 2.5 mg/kg and 0.25 µM/ml | CXCL-1↓ and CXCL-2↓ | [144] |
↓Downregulation; ┴inhibition.
3.10. Antiosteoporotic Activity
Osteoporosis is characterized by structural deterioration of tissues in the bone along with lower bone mass and bone fragility. The main causes of osteoporosis include estrogen deficiency, excess of glucocorticoids, and oxidative stress. Astragalin, an active compound, isolated from crude methanolic extract of the seeds of C. chinensis showed estrogenic activity against osteoporosis, and it is responsible for significant osteoblastic cell proliferation in UMR-106 osteoblastic cells [17].
3.11. Anticancer Activity
Currently, cancer is the second leading cause of mortality worldwide. In spite of advances in the development of new therapeutic preferences for cancer, its ratio is increasing day by day. Every year, almost 7 million people die due to cancer. Lung cancer particularly non-small cell lung cancer (NSCLC) accounts for more than 80% of deaths all around the world today. Therefore, it is necessary to discover new cheap and inexpensive drugs that can ameliorate the antitumor effects and reduce the side effects of generally recommended chemotherapy drugs [145].
Natural phytochemicals that are active constituents of medicinal plants, seeds, fruits, and herbs including polyphenols (flavonoids, terpenoids, and carotenoids) have gained significant recognizance for their potential value as therapeutic agents [146, 147]. Much research work has been conducted towards the assessment of phenolic phytochemicals as potent prophylactic agents as they can act on multiple cellular targets. The mechanistic insight into chemoprevention incorporates induction of apoptosis and cell cycle arrest or prohibition of certain cell signaling pathways mostly protein kinases C (PKC), glycogen synthase kinase (GSK), mitogen-activated protein kinases (MAPK), and phosphoinositide 3-kinase (PI3K) leading to abnormal AP-1, COX-2, and NF-κB expressions. Efficacy of chemopreventive agents revert their capacity to counteract with certain up-stream signals that leads to redox imbalances, genotoxic injury, and other situations of cellular stress. Thus, targeting damaged molecules along with interrupted signal transduction pathways in cancer epitomize a rational strategy for chemoprevention, and phenolic compounds seem to be auspicious in this aspect [147, 148]. In recent years, flavonoids have drawn developing consideration as powerful anticancer agents against various cancer types [149].
Several investigations on astragalin have explained its anticancer effect due to its promising competency to inhibit proliferation in different cancer cell lines including leukemia (HL-60) [15], hepatocellular (HepG2, Huh-7, and H22) [150], skin (HaCaT, A375P, and SK-MEL-2) [151], and lung (A549 and H1299) cancerous cells [145].
Astragalin heptaacetate (AHA), a therapeutically active flavonoid, induces apoptosis in HL-60 cells through release of cytochrome c into the cytosol. The associated mechanism involves activation of Bax, caspase-3/-7, and p38MAPK and intracellular ROS generation along with inhibition of cell signaling pathways JNK/SAPK and ERK ½ [15]. Astragalin also prohibits TNF-α-induced NF-κB activation in A549 and H1299 cells. Moreover, AG-triggered cell death is affiliated with increased Bax : Bcl-2 ratio and enhanced cleavage of caspase-3/-9 and PARP in conjunction with blockage of PI3K/Akt, MAPK, and ERK 1/2 signaling cascades in a time- and dose-related manner [145]. In hepatocellular carcinoma cells, astragalin (AG) significantly suppressed proliferation both in vitro in HepG2 cells and in vivo in Huh-7 (nude mice) and H22 (Kunming mice) cells via mechanistically inhibiting hexokinase 2 and upregulating miR-125b expression, respectively [150].
Astragalin can be a novel anticancer agent for the cure and prevention of UVB-stimulated actinic keratosis skin lesion by suppressing phospho-MSK1, γ-H2AX, and p38MAPK activation in a time-and dose-related manner in human HaCaT cells in vitro and Babl/c mice in vivo. In another report, astragalin strongly exerted cytotoxic effects in A375P and SK-MEL-2 cancerous cells in a concentration-dependent way through induction of apoptosis. The underlying cell death mechanism involves activation of Bax and caspase-3/-9, cleavage of PARP, and downregulation of cyclin D1 and Mcl-1 along with inhibition of Sry-related HMg-Box Gene 10 (SOX10) signaling cascade [151, 152]. The reported data recommend astragalin's multitargeted activity in preference to single effect that may perform an imperative role towards developing astragalin into potential anticancer drug in future (Table 5).
Table 5.
Anticancer activities of astragalin in vitro and in vivo.
| Type of cancer | Cell line | Dose/concentration | Molecular targets | References |
|---|---|---|---|---|
| Leukemia | HL-60 | 6 ± 1 µM | Bax↑, Bcl-2↓, caspase-3/-7Act, JNK/SAPK┴, and ERK 1/2┴ | [15] |
| Hepatocellular | HepG2, Huh-7, and H22 | — | HK2┴ and miR-125b↑ | [150] |
| Skin | HaCaT, A375P, and SK-MEL-2 | 50 and 100 μM/mL | p38 MAPK↓, phospho-MSK1↓, γ-H2AX↓, caspase-9/-3Act, BaxAct, PARP cleavage, cyclin D1↓, Mcl-1↓, and SOX10┴ | [151, 152] |
| Lung | A549, H1299, H226, H838, H23, H1437, H125, H2009, and H2087 | 5, 40 µg/mL (A549) and 20 µg/mL (H1299) | Bax:Bcl-2↑, caspase-9/-3↑, p-IKK-β↓, NF-κB p65┴, TNF-α┴, ERK-1/2┴, JNK↑, PI3K/Akt┴, DDH┴, DRP-1↓, pro-caspase-3/-8↑, and Bax↑ | [145, 153] |
| Breast | ZR-75-1, T47D, BT20, MCF-1, and MCF-7 | — | DDH┴, DRP-1↓, pro-caspase-3/-8↑, and Bax↑ | [153] |
| Gastric | AGS, SC-M1, NUGC-1, NUGC-3, and KOTA-III | — | DDH┴, DRP-1↓ pro-caspase-3/-8↑, and Bax↑ | [153] |
↑Upregulation; ↓downregulation; ┴inhibition.
4. ADMET Profiles of Astragalin
ADMET profiles along with biological activity spectra were performed for astragalin based on in-silico tools. The results indicate that astragalin is a potential anticancer agent which is unlikely to present any acute hazard or toxicity. Furthermore, astragalin can be absorbed by human intestines, but it is incapable of penetration to Caco-2 cells. Astragalin has been validated as a novel substrate of p-glycoprotein which is crucial for the metabolism and clearance of the compounds and for the efflux of drugs [154].
5. Conclusions and Future Perspectives
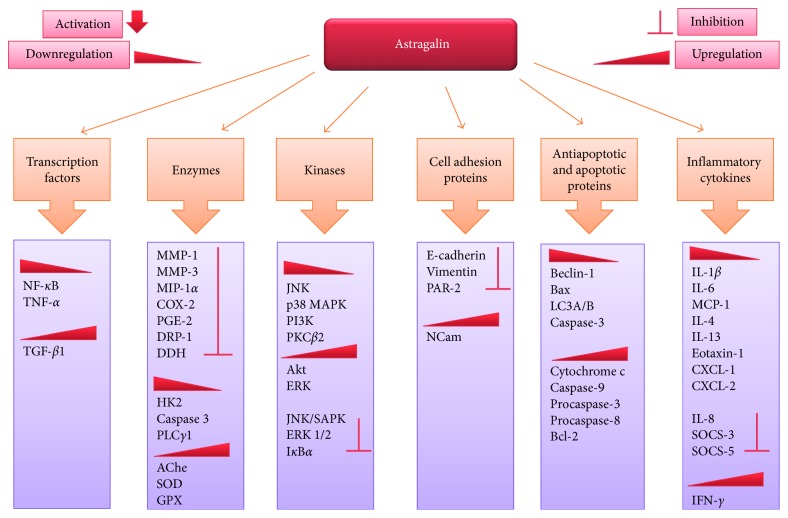
Astragalin, a natural flavonoid, has been isolated from various traditional medicinal plants such as Cassia alata, Moringa oleifera, Nelumbo nucifera, Cuscuta spp., Radix astragali, Morus alba, and Eucommia ulmoides. Astragalin has been reported to modulate inflammatory responses by regulating the expression of NF-κB, iNOS, cytokines/chemokines (COX-2, TNF-α, IL-10, and IL-6), MAPK signaling pathways (PGE2, IgE, IL-4, IL-5, IL-13, IL-1β, and IL-6), and PAR2 signaling expression. It also has the capability to alleviate the production of ROS and inhibit the endotoxin-induced oxidative stress (Figure 3). Astragalin is also known to be an inhibitor of ERK-1/2 and Akt signaling; therefore, it is a significant compound against cancer proliferation. In this review paper, we have emphasized on various pharmacological properties of astragalin such as anti-inflammatory, antioxidant, neurological, cardioprotective, antidiabetic, and anticancer. Although several in vitro and in vivo investigations have demonstrated its diversified pharmacological applications, further experimentation along with medicinal chemistry approaches and preclinical trials is still obligatory to uncover the knowledge of its biological and pharmacological applications and their associated mechanisms of actions for the treatment and prevention of several diseases.
Figure 3.
A diagrammatic representation of molecular targets and mechanism of action of astragalin. Astragalin has capability to modulate various transcriptional factors, enzymes, protein kinases, cell adhesion molecules, apoptotic and antiapoptotic proteins, and inflammatory cytokines resulting in anticancer, anti-inflammatory, antioxidant, and cardioprotective activities.
Acknowledgments
This study was supported by the research grant from The Nagai Foundation, Tokyo, Japan (NFT-R4-2017 and NFT-R4-2018) and TWAS-COMSTECH Research Grant (no. 17-180 RG/PHA/AS_C). The authors would also like to thank Higher Education Commission (HEC), Pakistan, for providing access to related papers from various journals.
Abbreviations
- Ache:
Acetylcholinesterase
- Bax:
Bcl-2 associated protein
- Bcl-2:
B-cell lymphoma-2
- COX-2:
Cyclooxygenase-2
- CXCL-1:
Chemokine-1
- CXCL-2:
Chemokine-2
- DAF-16:
Abnormal dauer formation
- DDH:
Dihydrodiol dehydrogenase
- DRP-1:
Dynamin-related protein-1
- E-cadherin:
Epithelial cadherin
- EMT:
Epithelial to mesenchymal transition
- Eotaxin-1:
Eosinophil chemotactic protein
- ERK:
Extracellular signal-regulated kinase
- GPX:
Glutathione peroxide
- GSH:
Glutathione
- HK2:
Human kallikrein-related peptidase-2
- IFN-γ:
Interferon gamma
- IgE:
Immunoglobin E
- IL-13:
Interleukin-13
- IL-1β:
Interleukin-1 beta
- IL-4:
Interleukin-4
- IL-6:
Interleukin-6
- IL-8:
Interleukin-8
- iNOS:
Inducible nitric oxide synthase
- IκBα:
Inhibitor of kappa B alpha
- JNK:
c-Jun N-terminal kinase
- LC3A/B:
Microtubule-associated protein 1 light chain 3A/B
- MAPK:
Mitogen-activated protein kinase
- Mcl-1:
Myeloid cell leukemia 1
- MCP-1:
Monocyte chemoattractant protein-1
- MIP-1α:
Macrophage inflammatory protein 2-alpha
- miR-125:
MicroRNA-125
- MMP:
Mitochondrial membrane potential
- MMP-1:
Matrix metalloproteinase-1
- MMP-3:
Matrix metalloproteinase-3
- NCam:
Neutral cell adhesion molecule
- NO:
Nitric oxide
- PAR2:
Protease-activated receptor 2
- PGE2:
Prostaglandin E2
- PI3K:
Phosphoinositide-3
- PKCβ2:
Protein kinase C beta-2
- PLCγ1:
Phosphoinositide phospholipase C γ1
- SAPK:
Stress-activated protein kinase
- SOCS-3:
Suppressor of cytokine signaling 3
- SOCS-5:
Suppressor of cytokine signaling 5
- SOD:
Superoxide dismutase
- SOD:
Superoxide dismutase
- SOX10:
Sry-related HMg-Box gene 10
- TGF-β1:
Transforming growth factor beta 1
- TLR-4:
Toll-like receptor 4
- TNF-α:
Tumor necrosis factor alpha.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Newman D. J., Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. 2012;75(75):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingston D. G. Modern natural products drug discovery and its relevance to biodiversity conservation. 2011;74(74):496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veeresham C. Natural products derived from plants as a source of drugs. 2012;3(3):200–201. doi: 10.4103/2231-4040.104709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katiyar C., Gupta A., Kanjilal S., Katiyar S. Drug discovery from plant sources: an integrated approach. 2012;33(33):10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harvey A. L. Natural products in drug discovery. 2008;13(13):894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Luo Y., Cobb R. E., Zhao H. Recent advances in natural product discovery. 2014;30(30):230–237. doi: 10.1016/j.copbio.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasul A., Millimouno F. M., Ali Eltayb W., Ali M., Li J., Li X. Pinocembrin: a novel natural compound with versatile pharmacological and biological activities. 2013;2013:9. doi: 10.1155/2013/379850.379850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong J. Role of natural product diversity in chemical biology. 2011;15(15):350–354. doi: 10.1016/j.cbpa.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aruoma O. I., Grootveld M., Bahorun T. Free radicals in biology and medicine: from inflammation to biotechnology. 2006;27(27):1–3. doi: 10.1002/biof.5520270101. [DOI] [PubMed] [Google Scholar]
- 10.Prasain J. K., Barnes S. Metabolism and bioavailability of flavonoids in chemoprevention: current analytical strategies and future prospectus. 2007;4(4):846–864. doi: 10.1021/mp700116u. [DOI] [PubMed] [Google Scholar]
- 11.Spencer J. P. Flavonoids: modulators of brain function? 2008;99(1):ES60–ES77. doi: 10.1017/s0007114508965776. [DOI] [PubMed] [Google Scholar]
- 12.Kotani M., Matsumoto M., Fujita A., et al. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. 2000;106(106):159–166. doi: 10.1067/mai.2000.107194. [DOI] [PubMed] [Google Scholar]
- 13.Bitis L., Kultur S., Melikoglu G., Ozsoy N., Can A. Flavonoids and antioxidant activity of Rosa agrestis leaves. 2010;24(24):580–589. doi: 10.1080/14786410903075507. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y. H., Choi Y. J., Kang M. K., et al. Astragalin inhibits allergic inflammation and airway thickening in ovalbumin-challenged mice. 2017;65(65):836–845. doi: 10.1021/acs.jafc.6b05160. [DOI] [PubMed] [Google Scholar]
- 15.Burmistrova O., Quintana J., Diaz J. G., Estevez F. Astragalin heptaacetate-induced cell death in human leukemia cells is dependent on caspases and activates the MAPK pathway. 2011;309(309):71–77. doi: 10.1016/j.canlet.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Bainey K. R., Armstrong P. W. Clinical perspectives on reperfusion injury in acute myocardial infarction. 2014;167(167):637–645. doi: 10.1016/j.ahj.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Yang L., Chen Q., Wang F., Zhang G. Antiosteoporotic compounds from seeds of Cuscuta chinensis. 2011;135(135):553–560. doi: 10.1016/j.jep.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 18.Donnapee S., Li J., Yang X., et al. Cuscuta chinensis Lam.: a systematic review on ethnopharmacology, phytochemistry and pharmacology of an important traditional herbal medicine. 2014;157(157):292–308. doi: 10.1016/j.jep.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Saito S., Silva G., Santos R. X., Gosmann G., Pungartnik C., Brendel M. Astragalin from Cassia alata induces DNA adducts in vitro and repairable DNA damage in the yeast Saccharomyces cerevisiae. 2012;13(13):2846–2862. doi: 10.3390/ijms13032846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei J., Dong P., Wu T., et al. Metabolic engineering of Escherichia coli for astragalin biosynthesis. 2016 doi: 10.1021/acs.jafc.6b03447. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Huang X. Z., Tan L. X., Gu K., Li C. Studies on chemical constituents from leaves of Acer truncatum. 2007;32(32):1544–1546. [PubMed] [Google Scholar]
- 22.Han J. T., Bang M. H., Chun O. K., Kim D. O., Lee C. Y., Baek N. I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. 2004;27(27):390–395. doi: 10.1007/bf02980079. [DOI] [PubMed] [Google Scholar]
- 23.Kato H., Li W., Koike M., Wang Y., Koike K. Phenolic glycosides from Agrimonia pilosa. 2010;71(71):1925–1929. doi: 10.1016/j.phytochem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Ivanova A., Mikhova B., Najdenski H., Tsvetkova I., Kostova I. Chemical composition and antimicrobial activity of wild garlic Allium ursinum of Bulgarian origin. 2009;4(4):1059–1062. [PubMed] [Google Scholar]
- 25.Lee K. T., Choi J. H., Kim D. H., et al. Constituents and the antitumor principle of Allium victorialis var. platyphyllum. 2001;24(24):44–50. doi: 10.1007/bf02976492. [DOI] [PubMed] [Google Scholar]
- 26.Chiang H. C., Lo Y. J., Lu F. J. Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (Hook) Tryon. 1994;8(8):61–71. doi: 10.3109/14756369409040777. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y., Liu C., Zhang Z., Wang J., Wu G., Li S. Comprehensive separation and identification of chemical constituents from Apocynum venetum leaves by high-performance counter-current chromatography and high performance liquid chromatography coupled with mass spectrometry. 2010;878(878):3149–3155. doi: 10.1016/j.jchromb.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Huong N. T., Cu N. K., Quy T. V., Zidorn C., Ganzera M., Stuppner H. A new phenylpropanoid glycoside from Jasminum subtriplinerve Blume. 2008;10(10):1035–1038. doi: 10.1080/10286020802320897. [DOI] [PubMed] [Google Scholar]
- 29.Krasteva I., Platikanov S., Nikolov S., Kaloga M. Flavonoids from Astragalus hamosus. 2007;21(21):392–395. doi: 10.1080/14786410701236871. [DOI] [PubMed] [Google Scholar]
- 30.Kiem P. V., Minh C. V., Huong H. T., Lee J. J., Kim Y. H. Caesaldecan, a cassane diterpenoid from the leaves of Caesalpinia decapetala. 2005;53(53):428–430. doi: 10.1248/cpb.53.428. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed H., Moawad A., Owis A., AbouZid S., Ahmed O. Flavonoids of Calligonum polygonoides and their cytotoxicity. 2016;54(54):2119–2126. doi: 10.3109/13880209.2016.1146778. [DOI] [PubMed] [Google Scholar]
- 32.Chung D. W., Lee S. B. Novel synthesis of leucoside by enzymatic hydrolysis of tea seed extract. 2013;93(93):362–367. doi: 10.1002/jsfa.5769. [DOI] [PubMed] [Google Scholar]
- 33.Lee H. B., Kim E. K., Park S. J., Bang S. G., Kim T. G., Chung D. W. Isolation and anti-inflammatory effect of astragalin synthesized by enzymatic hydrolysis of tea seed extract. 2011;91(91):2315–2321. doi: 10.1002/jsfa.4457. [DOI] [PubMed] [Google Scholar]
- 34.Lee H. B., Kim E. K., Park S. J., Bang S. G., Kim T. G., Chung D. W. Isolation and characterization of nicotiflorin obtained by enzymatic hydrolysis of two precursors in tea seed extract. 2010;58(58):4808–4813. doi: 10.1021/jf9045182. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z. M., Ling T. J., Li L. X., et al. A new norisoprenoid and other compounds from Fuzhuan brick tea. 2012;17(17):3539–3546. doi: 10.3390/molecules17033539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng W. S., Hao Z. Y., Zheng X. K., Kuang H. X. Chemical constituents from leaves of Celastrus gemmatus Loes. 2007;42(42):625–630. [PubMed] [Google Scholar]
- 37.Nhiem N. X., Tai B. H., Quang T. H., et al. A new ursane-type triterpenoid glycoside from Centella asiatica leaves modulates the production of nitric oxide and secretion of TNF-α in activated RAW 264.7 cells. 2011;21(21):1777–1781. doi: 10.1016/j.bmcl.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 38.Van Oanh H., Sinh P. X., An N. T., et al. A new rearranged abietane diterpene and other constituents from Clerodendrum philipinum. 2009;4(4):323–325. [PubMed] [Google Scholar]
- 39.Calzada F., Cedillo-Rivera R., Mata R. Antiprotozoal activity of the constituents of Conyza filaginoides. 2001;64(64):671–673. doi: 10.1021/np000442o. [DOI] [PubMed] [Google Scholar]
- 40.Yoshikawa M., Shimada H., Saka M., Yoshizumi S., Yamahara J., Matsuda H. Medicinal foodstuffs. V. Moroheiya. (1): absolute stereostructures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae) 1997;45(45):464–469. doi: 10.1248/cpb.45.464. [DOI] [PubMed] [Google Scholar]
- 41.Ye M., Yan Y., Guo D. A. Characterization of phenolic compounds in the Chinese herbal drug Tu-Si-Zi by liquid chromatography coupled to electrospray ionization mass spectrometry. 2005;19(19):1469–1484. doi: 10.1002/rcm.1944. [DOI] [PubMed] [Google Scholar]
- 42.Guo H., Li J. Flavonoids of Cuscuta australis R. Br. 1997;22(22):38–39. [PubMed] [Google Scholar]
- 43.He X., Yang W., Ye M., Wang Q., Guo D. Differentiation of Cuscuta chinensis and Cuscuta australis by HPLC-DAD-MS analysis and HPLC-UV quantitation. 2011;77(77):1950–1957. doi: 10.1055/s-0030-1271186. [DOI] [PubMed] [Google Scholar]
- 44.Lee J. H., Ku C. H., Baek N. I., Kim S. H., Park H. W., Kim D. K. Phytochemical constituents from Diodia teres. 2004;27(27):40–43. doi: 10.1007/bf02980043. [DOI] [PubMed] [Google Scholar]
- 45.Braunberger C., Zehl M., Conrad J., et al. LC-NMR, NMR, and LC-MS identification and LC-DAD quantification of flavonoids and ellagic acid derivatives in Drosera peltata. 2013;932(932):111–116. doi: 10.1016/j.jchromb.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Cordell G. A., Lyon R. L., Fong H. H., Benoit P. S., Farnsworth N. R. Biological and phytochemical investigations of Dianthus barbatus cv. “China Doll” (Caryophyllaceae) 1977;40(40):361–363. [PubMed] [Google Scholar]
- 47.Kim H. Y., Moon B. H., Lee H. J., Choi D. H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. 2004;93(93):227–230. doi: 10.1016/j.jep.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 48.Cheng J., Zhao Y. Y., Cui Y. X., Cheng T. M. Studies on flavonoids from leave of Eucommia ulmoides Oliv. 2000;25(25):284–286. [PubMed] [Google Scholar]
- 49.Li X., Tang Z., Fei D., Liu Y., Zhang M., Liu S. Evaluation of the sedative and hypnotic effects of astragalin isolated from Eucommia ulmoides leaves in mice. 2017;31(31):2072–2076. doi: 10.1080/14786419.2016.1272108. [DOI] [PubMed] [Google Scholar]
- 50.Elema E. T., Schripsema J., Malingre T. M. Flavones and flavonol glycosides from Eupatorium cannabinum L. 1989;11(11):161–164. doi: 10.1007/bf01959464. [DOI] [PubMed] [Google Scholar]
- 51.Qian S. H., Yang N. Y., Duan J. A., Yuan L. H., Tian L. J. Study on the flavonoids of Eupatorium lindleyanum. 2004;29(29):50–52. [PubMed] [Google Scholar]
- 52.Zhang J., Li X., Ren L., Fang C., Wang F. Chemical constituents from Exochorda racemosa. 2011;36(36):1198–1201. [PubMed] [Google Scholar]
- 53.Xie Q., Ding L., Wei Y., Ito Y. Determination of major components and fingerprint analysis of Flaveria bidentis (L.) Kuntze. 2014;52(52):252–257. doi: 10.1093/chromsci/bmt020. [DOI] [PubMed] [Google Scholar]
- 54.Wei Y., Xie Q., Fisher D., Sutherland I. A. Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. 2011;1218:6206–6211. doi: 10.1016/j.chroma.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 55.Yang Y., Zhao Y., Gu D., et al. Separation of the minor flavonols from Flos Gossypii by high-speed countercurrent chromatography. 2010;33(33):1502–1515. doi: 10.1080/10826076.2010.489000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tai Z. G., Yang X. Q., Cai L., Sun W. J., Ding Z. T., Yang Y. B. Studies on the chemical constituents from the aerial parts of Gladiolus gandavensis. 2010;33(33):1257–1259. [PubMed] [Google Scholar]
- 57.Biondi D. M., Rocco C., Ruberto G. Dihydrostilbene derivatives from Glycyrrhiza glabra leaves. 2005;68(68):1099–1102. doi: 10.1021/np050034q. [DOI] [PubMed] [Google Scholar]
- 58.Jia S. S., Ma C. M., Li Y. H., Hao J. H. Glycosides of phenolic acid and flavonoids from the leaves of Glycyrrhiza uralensis Ficsh. 1992;27(27):441–444. [PubMed] [Google Scholar]
- 59.Algariri K., Meng K. Y., Atangwho I. J., et al. Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. 2013;3(3):358–366. doi: 10.1016/s2221-1691(13)60077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trute A., Nahrstedt A. Identification and quantitative analysis of phenolic compounds from the dry extract of Hedera helix. 1997;63(63):177–179. doi: 10.1055/s-2006-957639. [DOI] [PubMed] [Google Scholar]
- 61.Calzada F., Alanis A. D. Additional antiprotozoal flavonol glycosides of the aerial parts of Helianthemum glomeratum. 2007;21(21):78–80. doi: 10.1002/ptr.2031. [DOI] [PubMed] [Google Scholar]
- 62.Ren Y. L., Yang J. S. Study on chemical constituents of Hemistepta lyrata Bunge. 2001;36(36):746–749. [PubMed] [Google Scholar]
- 63.Heinaaho M., Pusenius J., Julkunen-Tiitto R. Effects of different organic farming methods on the concentration of phenolic compounds in sea buckthorn leaves. 2006;54(54):7678–7685. doi: 10.1021/jf061018h. [DOI] [PubMed] [Google Scholar]
- 64.Luo J. G., Kong L. Y. Study on flavonoids from leaf of Ipomoea batatas. 2005;30(30):516–518. [PubMed] [Google Scholar]
- 65.Qu Q. H., Zhang L., Bao H., Zhang J. H., You X. J., Wang J. X. Chemical constituents of flavonoids from flowers of Koelreuteria paniculata. 2011;34(34):1716–1719. [PubMed] [Google Scholar]
- 66.Bernaert N., Wouters D., De Vuyst L., et al. Antioxidant changes of leek (Allium ampeloprasum var. porrum) during spontaneous fermentation of the white shaft and green leaves. 2013;93(93):2146–2153. doi: 10.1002/jsfa.6020. [DOI] [PubMed] [Google Scholar]
- 67.Ren D. C., Yang N. Y., Qian S. H., Xie N., Zhou X. M., Duan J. A. Chemical study on aerial parts of Ligusticum chuanxiong. 2007;32(32):1418–1420. [PubMed] [Google Scholar]
- 68.Xiao M., Cao N., Fan J. J., Shen Y., Xu Q. Studies on flavonoids from the leaves of Lindera aggregata. 2011;34(34):62–64. [PubMed] [Google Scholar]
- 69.Ye H., Yu J. The preliminary studies on antioxidation of three kinds of flavoniods from Litsea coreana. 2004;27(27):113–115. [PubMed] [Google Scholar]
- 70.Jung K. Y., Oh S. R., Park S. H., et al. Anti-complement activity of tiliroside from the flower buds of Magnolia fargesii. 1998;21(21):1077–1078. doi: 10.1248/bpb.21.1077. [DOI] [PubMed] [Google Scholar]
- 71.Vongsak B., Sithisarn P., Gritsanapan W. Simultaneous HPLC quantitative analysis of active compounds in leaves of Moringa oleifera Lam. 2014;52(52):641–645. doi: 10.1093/chromsci/bmt093. [DOI] [PubMed] [Google Scholar]
- 72.Doi K., Kojima T., Makino M., Kimura Y., Fujimoto Y. Studies on the constituents of the leaves of Morus alba L. 2001;49(49):151–153. doi: 10.1248/cpb.49.151. [DOI] [PubMed] [Google Scholar]
- 73.Sugiyama M., Katsube T., Koyama A., Itamura H. Varietal differences in the flavonol content of mulberry (Morus spp.) leaves and genetic analysis of quercetin 3-(6-malonylglucoside) for component breeding. 2013;61(61):9140–9147. doi: 10.1021/jf403136w. [DOI] [PubMed] [Google Scholar]
- 74.He J., Feng Y., Ouyang H. Z., et al. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: application to a pharmacokinetic study of total flavonoids from mulberry leaves. 2013;84(84):189–195. doi: 10.1016/j.jpba.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 75.Choi J., Kang H. J., Kim S. Z., Kwon T. O., Jeong S. I., Jang S. I. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. 2013;36(36):912–917. doi: 10.1007/s12272-013-0090-x. [DOI] [PubMed] [Google Scholar]
- 76.Zou Y., Liao S., Shen W., et al. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. 2012;13(13):16544–16553. doi: 10.3390/ijms131216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao Y., Zhang Y., Cheng Y., Wang Y. Rapid screening and identification of alpha-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. 2013;27(27):148–155. doi: 10.1002/bmc.2761. [DOI] [PubMed] [Google Scholar]
- 78.Kim S. Y., Gao J. J., Lee W. C., Ryu K. S., Lee K. R., Kim Y. C. Antioxidative flavonoids from the leaves of Morus alba. 1999;22(22):81–85. doi: 10.1007/bf02976442. [DOI] [PubMed] [Google Scholar]
- 79.Ranarivelo Y., Skaltsounis A. L., Andriantsiferana M., Tillequin F. Glycosides from Mussaenda arcuata Lam. ex Poiret leaves. 1990;48(48):273–277. [PubMed] [Google Scholar]
- 80.Ohkoshi E., Miyazaki H., Shindo K., Watanabe H., Yoshida A., Yajima H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. 2007;73(73):1255–1259. doi: 10.1055/s-2007-990223. [DOI] [PubMed] [Google Scholar]
- 81.Tian N., Liu Z., Huang J., Luo G., Liu S., Liu X. Isolation and preparation of flavonoids from the leaves of Nelumbo nucifera Gaertn by preparative reversed-phase high performance liquid chromatography. 2007;25(25):88–92. [PubMed] [Google Scholar]
- 82.Zhao X. L., Wang Z. M., Ma X. J., Jing W. G., Liu A. Chemical constituents from leaves of Nelumbo nucifera. 2013;38(38):703–708. [PubMed] [Google Scholar]
- 83.Xu S., Sun Y., Jing F., Duan W., Du J., Wang X. Separation and purification of flavones from Nelumbo nucifera Gaertn. by silica gel chromatography and high-speed counter-current chromatography. 2011;29(29):1244–1248. [PubMed] [Google Scholar]
- 84.Deng S., Deng Z., Fan Y., et al. Isolation and purification of three flavonoid glycosides from the leaves of Nelumbo nucifera (Lotus) by high-speed counter-current chromatography. 2009;877(877):2487–2492. doi: 10.1016/j.jchromb.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Barakat H. H., El-Mousallamy A. M., Souleman A. M., Awadalla S. Flavonoids of Ochradenus baccatus. 1991;30(30):3777–3779. doi: 10.1016/0031-9422(91)80109-e. [DOI] [PubMed] [Google Scholar]
- 86.Je Ma C., Jung W. J., Lee K. Y., Kim Y. C., Sung S. H. Calpain inhibitory flavonoids isolated from Orostachys japonicus. 2009;24(24):676–679. doi: 10.1080/14756360802328075. [DOI] [PubMed] [Google Scholar]
- 87.Matsumoto M., Kotani M., Fujita A., et al. Oral administration of persimmon leaf extract ameliorates skin symptoms and transepidermal water loss in atopic dermatitis model mice, NC/Nga. 2002;146(146):221–227. doi: 10.1046/j.1365-2133.2002.04557.x. [DOI] [PubMed] [Google Scholar]
- 88.Xue Y. L., Miyakawa T., Hayashi Y., et al. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. 2011;59(59):6011–6017. doi: 10.1021/jf200940h. [DOI] [PubMed] [Google Scholar]
- 89.Kameda K., Takaku T., Okuda H., et al. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. 1987;50(50):680–683. doi: 10.1021/np50052a017. [DOI] [PubMed] [Google Scholar]
- 90.Ma Z., Piao T., Wang Y., Liu J. Astragalin inhibits IL-1β-induced inflammatory mediators production in human osteoarthritis chondrocyte by inhibiting NF-κB and MAPK activation. 2015;25(25):83–87. doi: 10.1016/j.intimp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Li F., Wang W., Cao Y., et al. Inhibitory effects of astragalin on lipopolysaccharide-induced inflammatory response in mouse mammary epithelial cells. 2014;192(192):573–581. doi: 10.1016/j.jss.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 92.Li F., Liang D., Yang Z., et al. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. 2013;17(17):478–482. doi: 10.1016/j.intimp.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Skalicka-Wozniak K., Szypowski J., Glowniak K. HPLC analysis of kaempherol and quercetin derivatives isolated by different extraction techniques from plant matrix. 2011;94(94):17–21. [PubMed] [Google Scholar]
- 94.Laparra J. M., Glahn R. P., Miller D. D. Assessing potential effects of inulin and probiotic bacteria on Fe availability from common beans (Phaseolus vulgaris L.) to Caco-2 cells. 2009;74(74):H40–H46. doi: 10.1111/j.1750-3841.2008.01027.x. [DOI] [PubMed] [Google Scholar]
- 95.Takeda Y., Isai N., Masuda T., et al. Phlomisflavosides A and B, new flavonol bisglycosides from Phlomis spinidens. 2001;49(49):1039–1041. doi: 10.1248/cpb.49.1039. [DOI] [PubMed] [Google Scholar]
- 96.Agyare C., Lechtenberg M., Deters A., Petereit F., Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: Geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. 2011;18(18):617–624. doi: 10.1016/j.phymed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 97.Klein-Junior L. C., Santin J. R., Lemos M., et al. Role of gastric mucus secretion, oxinitrergic system and sulfhydryl groups on the gastroprotection elicited by Polygala cyparissias (Polygalaceae) in mice. 2013;65(65):767–776. doi: 10.1111/jphp.12038. [DOI] [PubMed] [Google Scholar]
- 98.Calis I., Kuruuzum A., Demirezer L. O., Sticher O., Ganci W., Ruedi P. Phenylvaleric acid and flavonoid glycosides from Polygonum salicifolium. 1999;62(62):1101–1105. doi: 10.1021/np9900674. [DOI] [PubMed] [Google Scholar]
- 99.Olszewska M. A., Kwapisz A. Metabolite profiling and antioxidant activity of Prunus padus L. flowers and leaves. 2011;25(25):1115–1131. doi: 10.1080/14786410903230359. [DOI] [PubMed] [Google Scholar]
- 100.Olszewska M. High-performance liquid chromatographic identification of flavonoid monoglycosides from Prunus serotina ehrh. 2005;62(62):435–441. [PubMed] [Google Scholar]
- 101.Krauze-Baranowska M., Sowinski P., Kawiak A., Sparzak B. Flavonoids from Pseudotsuga menziesii. 2013;68(68):87–96. doi: 10.5560/znc.2013.68c0087. [DOI] [PubMed] [Google Scholar]
- 102.Kwon H. J., Park Y. D. Determination of astragalin and astragaloside content in Radix astragali using high-performance liquid chromatography coupled with pulsed amperometric detection. 2012;1232:212–217. doi: 10.1016/j.chroma.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 103.Jian J., Wu Z. Influences of traditional Chinese medicine on non-specific immunity of Jian Carp (Cyprinus carpio var. Jian) 2004;16(16):185–191. doi: 10.1016/s1050-4648(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 104.Li G., Gao T., Wen J., Yang R., Yu C., Zhang S. A research on the quality of radix Astragali. 1992;17(17):454–456. [PubMed] [Google Scholar]
- 105.Ding Y., Nguyen H. T., Choi E. M., Bae K., Kim Y. H. Rhusonoside A, a new megastigmane glycoside from Rhus sylvestris, increases the function of osteoblastic MC3T3-E1 cells. 2009;75(75):158–162. doi: 10.1055/s-0028-1088373. [DOI] [PubMed] [Google Scholar]
- 106.Yang C., Li F., Zhang X., Wang L., Zhou Z., Wang M. Phenolic antioxidants from Rosa soulieana flowers. 2013;27(27):2055–2058. doi: 10.1080/14786419.2013.811660. [DOI] [PubMed] [Google Scholar]
- 107.Nguelefack T. B., Mbakam F. H., Tapondjou L. A., et al. A dimeric triterpenoid glycoside and flavonoid glycosides with free radical-scavenging activity isolated from Rubus rigidus var. camerunensis. 2011;34(34):543–550. doi: 10.1007/s12272-011-0404-9. [DOI] [PubMed] [Google Scholar]
- 108.Wang H. Q., Zhao C. Y., Chen R. Y. Studies on chemical constituents from leaves of Sapium sebiferum. 2007;32(32):1179–1181. [PubMed] [Google Scholar]
- 109.Kamel M. S., Ohtani K., Hasanain H. A., Mohamed M. H., Kasai R., Yamasaki K. Monoterpene and pregnane glucosides from Solenostemma argel. 2000;53(53):937–940. doi: 10.1016/s0031-9422(99)00447-1. [DOI] [PubMed] [Google Scholar]
- 110.Apati P., Houghton P. J., Kery A. HPLC investigation of antioxidant components in Solidago herba. 2004;74(74):223–231. [PubMed] [Google Scholar]
- 111.Olszewska M. A., Michel P. Activity-guided isolation and identification of free radical-scavenging components from various leaf extracts of Sorbus aria (L.) Crantz. 2012;26(26):243–254. doi: 10.1080/14786419.2010.537271. [DOI] [PubMed] [Google Scholar]
- 112.Xiang W., Li R. T., Mao Y. L., et al. Four new prenylated isoflavonoids in Tadehagi triquetrum. 2005;53(53):267–271. doi: 10.1021/jf0483117. [DOI] [PubMed] [Google Scholar]
- 113.Shen G., Oh S. R., Min B. S., et al. Phytochemical investigation of Tiarella polyphylla. 2008;31(31):10–16. doi: 10.1007/s12272-008-1113-x. [DOI] [PubMed] [Google Scholar]
- 114.Hosoi S., Shimizu E., Ohno K., et al. Structural studies of zoospore attractants from Trachelospermum jasminoides var. pubescens: taxifolin 3-O-glycosides. 2006;17(17):20–24. doi: 10.1002/pca.876. [DOI] [PubMed] [Google Scholar]
- 115.Aishan H., Baba M., Iwasaki N., Kuang H., Okuyama T. The constituents of Urtica cannabina used in Uighur medicine. 2010;48(48):577–583. doi: 10.3109/13880200903214215. [DOI] [PubMed] [Google Scholar]
- 116.Majinda R. R., Motswaledi M., Waigh R. D., Waterman P. G. Phenolic and antibacterial constituents of Vahlia capensis. 1997;63(63):268–270. doi: 10.1055/s-2006-957671. [DOI] [PubMed] [Google Scholar]
- 117.Singab A. N., Youssef D. T., Noaman E., Kotb S. Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata Desf. against CCl4-induced liver damage in rats. 2005;28(28):791–798. doi: 10.1007/bf02977344. [DOI] [PubMed] [Google Scholar]
- 118.Apers S., Huang Y., Van Miert S., et al. Characterisation of new oligoglycosidic compounds in two Chinese medicinal herbs. 2002;13(13):202–206. doi: 10.1002/pca.642. [DOI] [PubMed] [Google Scholar]
- 119.Weiss U. Inflammation. 2008;454(454):p. 427. doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- 120.Sarfraz I., Rasul A., Jabeen F., et al. Fraxinus: a plant with versatile pharmacological and biological activities. 2017;2017:12. doi: 10.1155/2017/4269868.4269868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walker J., Reichelt K. V., Obst K., et al. Identification of an anti-inflammatory potential of Eriodictyon angustifolium compounds in human gingival fibroblasts. 2016;7(7):3046–3055. doi: 10.1039/c6fo00482b. [DOI] [PubMed] [Google Scholar]
- 122.Soromou L. W., Chen N., Jiang L., et al. Astragalin attenuates lipopolysaccharide-induced inflammatory responses by down-regulating NF-κB signaling pathway. 2012;419(419):256–261. doi: 10.1016/j.bbrc.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 123.Choi E. J., Lee S., Chae J. R., Lee H. S., Jun C. D., Kim S. H. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. 2011;88(88):1121–1126. doi: 10.1016/j.lfs.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Zhang W., Lu X., Wang W., et al. Inhibitory effects of emodin, thymol, and astragalin on leptospira interrogans-induced inflammatory response in the uterine and endometrium epithelial cells of mice. 2017;40(40):666–675. doi: 10.1007/s10753-017-0513-9. [DOI] [PubMed] [Google Scholar]
- 125.Inaba H., Tagashira M., Honma D., et al. Identification of hop polyphenolic components which inhibit prostaglandin E2 production by gingival epithelial cells stimulated with periodontal pathogen. 2008;31(31):527–530. doi: 10.1248/bpb.31.527. [DOI] [PubMed] [Google Scholar]
- 126.Liu J., Cheng Y., Zhang X., et al. Astragalin attenuates allergic inflammation in a murine asthma model. 2015;38(38):2007–2016. doi: 10.1007/s10753-015-0181-6. [DOI] [PubMed] [Google Scholar]
- 127.Kim M. S., Kim S. H. Inhibitory effect of astragalin on expression of lipopolysaccharide-induced inflammatory mediators through NF-κB in macrophages. 2011;34(34):2101–2107. doi: 10.1007/s12272-011-1213-x. [DOI] [PubMed] [Google Scholar]
- 128.Wang Z., Li Q., Xiang M., et al. Astragaloside alleviates hepatic fibrosis function via PAR2 signaling pathway in diabetic rats. 2017;41(41):1156–1166. doi: 10.1159/000464122. [DOI] [PubMed] [Google Scholar]
- 129.Cho I. H., Gong J. H., Kang M. K., et al. Astragalin inhibits airway eotaxin-1 induction and epithelial apoptosis through modulating oxidative stress-responsive MAPK signaling. 2014;14(14):p. 122. doi: 10.1186/1471-2466-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li H., Shi R., Ding F., et al. Astragalus polysaccharide suppresses 6-hydroxydopamine-induced neurotoxicity in Caenorhabditis elegans. 2016;2016:p. 4856761. doi: 10.1155/2016/4856761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yan L., Zhou Q. H. Study on neuroprotective effects of astragalan in rats with ischemic brain injury and its mechanisms. 2012;28(28):373–377. [PubMed] [Google Scholar]
- 132.Cho I. H., Choi Y. J., Gong J. H., Shin D., Kang M. K., Kang Y. H. Astragalin inhibits autophagy-associated airway epithelial fibrosis. 2015;16(16):p. 51. doi: 10.1186/s12931-015-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wasik A., Antkiewicz-Michaluk L. The mechanism of neuroprotective action of natural compounds. 2017;69(69):851–860. doi: 10.1016/j.pharep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 134.Habertheuer A., Kocher A., Laufer G., et al. Cardioprotection: a review of current practice in global ischemia and future translational perspective. 2014;2014:11. doi: 10.1155/2014/325725.325725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tanaka K., Kersten J. R., Riess M. L. Opioid-induced cardioprotection. 2014;20(20):5696–5705. doi: 10.2174/1381612820666140204120311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Testai L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. 2015;135(135):68–76. doi: 10.1016/j.lfs.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 137.Qu D., Han J., Ren H., et al. Cardioprotective effects of astragalin against myocardial ischemia/reperfusion injury in isolated rat heart. 2016;2016:11. doi: 10.1155/2016/8194690.8194690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang S., Moustaid-Moussa N., Chen L., et al. Novel insights of dietary polyphenols and obesity. 2014;25(25):1–18. doi: 10.1016/j.jnutbio.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alam U., Asghar O., Azmi S., Malik R. A. General aspects of diabetes mellitus. 2014;126(126):211–222. doi: 10.1016/b978-0-444-53480-4.00015-1. [DOI] [PubMed] [Google Scholar]
- 140.Jung M., Park M., Lee H. C., Kang Y. H., Kang E. S., Kim S. K. Antidiabetic agents from medicinal plants. 2006;13(13):1203–1218. doi: 10.2174/092986706776360860. [DOI] [PubMed] [Google Scholar]
- 141.Ke M., Hu X. Q., Ouyang J., Dai B., Xu Y. The effect of astragalin on the VEGF production of cultured Muller cells under high glucose conditions. 2012;22(22):113–119. doi: 10.3233/BME-2012-0696. [DOI] [PubMed] [Google Scholar]
- 142.Kim G. E., Kang H. K., Seo E. S., et al. Glucosylation of the flavonoid, astragalin by Leuconostoc mesenteroides B-512FMCM dextransucrase acceptor reactions and characterization of the products. 2012;50(50):50–56. doi: 10.1016/j.enzmictec.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 143.Jung S. Y., Jung W. S., Jung H. K., et al. The mixture of different parts of Nelumbo nucifera and two bioactive components inhibited tyrosinase activity and melanogenesis. 2014;65(65):377–388. [PubMed] [Google Scholar]
- 144.Svobodova A., Psotova J., Walterova D. Natural phenolics in the prevention of UV-induced skin damage. A review. 2003;147(147):137–145. doi: 10.5507/bp.2003.019. [DOI] [PubMed] [Google Scholar]
- 145.Chen M., Cai F., Zha D., et al. Astragalin-induced cell death is caspase-dependent and enhances the susceptibility of lung cancer cells to tumor necrosis factor by inhibiting the NF-κB pathway. 2017;8(8):26941–26958. doi: 10.18632/oncotarget.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rasool M., Malik A., Manan A., et al. Roles of natural compounds from medicinal plants in cancer treatment: structure and mode of action at molecular level. 2015;11(11):618–628. doi: 10.2174/1573406411666150430120038. [DOI] [PubMed] [Google Scholar]
- 147.Neergheen V. S., Bahorun T., Taylor E. W., Jen L. S., Aruoma O. I. Targeting specific cell signaling transduction pathways by dietary and medicinal phytochemicals in cancer chemoprevention. 2010;278(278):229–241. doi: 10.1016/j.tox.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 148.Jafari S., Saeidnia S., Abdollahi M. Role of natural phenolic compounds in cancer chemoprevention via regulation of the cell cycle. 2014;15(15):409–421. doi: 10.2174/1389201015666140813124832. [DOI] [PubMed] [Google Scholar]
- 149.Sun J., Li F., Zhao Y., et al. LZ-207, a newly synthesized flavonoid, induces apoptosis and suppresses inflammation-related colon cancer by inhibiting the NF-κB signaling pathway. 2015;10(10) doi: 10.1371/journal.pone.0127282.e0127282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li W., Hao J., Zhang L., Cheng Z., Deng X., Shu G. Astragalin reduces hexokinase 2 through increasing miR-125b to inhibit the proliferation of hepatocellular carcinoma cells in vitro and in vivo. 2017;65(65):5961–5972. doi: 10.1021/acs.jafc.7b02120. [DOI] [PubMed] [Google Scholar]
- 151.You O. H., Shin E. A., Lee H., et al. Apoptotic effect of astragalin in melanoma skin cancers via activation of caspases and inhibition of Sry-related HMg-box gene 10. 2017;31(31):1614–1620. doi: 10.1002/ptr.5895. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J., Li N., Zhang K., et al. Astragalin attenuates UVB radiation-induced actinic keratosis formation. 2017 doi: 10.2174/1871520618666171229190835. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chiang Y. Y., Wang S. L., Yang C. L., et al. Extracts of Koelreuteria henryi Dummer induce apoptosis and autophagy by inhibiting dihydrodiol dehydrogenase, thus enhancing anticancer effects. 2013;32(32):577–584. doi: 10.3892/ijmm.2013.1441. [DOI] [PubMed] [Google Scholar]
- 154.Ammar O. In silico pharmacodynamics, toxicity profile and biological activities of the Saharan medicinal plant Limoniastrum feei. 2017;53(53):1–10. doi: 10.1590/s2175-97902017000300061. [DOI] [Google Scholar]