Abstract
Inefficient T-cell homing to tissues limits adoptive T-cell immunotherapy of solid tumors. αLβ2 and α4β1 integrins mediate trafficking of T cells into tissues via engagement of ICAM-1 and VCAM-1, respectively. Inhibiting Protein Kinase A(PKA)-mediated phosphorylation of α4 integrin in cells results in an increase in αLβ2-mediated migration on mixed ICAM-1-VCAM-1 substrates in vitro, a phenomenon termed “integrin trans-regulation.” Here we created an α4(S988A)-bearing mouse, which precludes PKA-mediated α4 phosphorylation, to examine the effect of integrin trans-regulation in vivo. The α4(S988A) mouse exhibited a dramatic and selective increase in migration of lymphocytes, but not myeloid cells, to sites of inflammation. Importantly, we found that the α4(S988A) mice exhibited a marked increase in T cell entry into and reduced growth of B16 melanomas, consistent with anti-tumor roles of infiltrating T cells and pro-growth functions of tumor-associated macrophages. Thus, increased α4 trans-regulation of αLβ2 integrin function biases leukocyte emigration towards lymphocytes relative to myeloid cells and enhances tumor immunity.
Keywords: α4 Integrin, Transregulation, Immunotherapy, Migration, T-cell
Introduction
Different classes of leukocytes have opposing effects on the growth of tumors. Lymphocytes, particularly T Cells, are critical components of the host defense that limit tumorigenesis (1). In sharp contrast, myeloid cells contribute cytokines that promote both tumor growth and angiogenesis (2, 3). α4 integrins play an important role in lymphocyte entry into sites of tissue injury (4, 5) in part because they markedly potentiate cell migration via signaling mediated by binding of paxillin to the α4 cytoplasmic domain (tail) (6). Protein Kinase A (PKA)-mediated phosphorylation of the α4 integrin tail at Ser988 inhibits paxillin binding in migrating cells (7); consequently the α4(S988A) mutation stabilizes the α4-paxillin interaction and increases α4 integrin signaling. The increased signaling downstream of α4(S988A) enhances integrin αLβ2(LFA-1)-mediated migration of cells, a phenomenon termed integrin trans-regulation (8).
Here we examined the biological consequences of blockade of α4 phosphorylation by generating α4(S988A) mutant mice and find that these mice manifest a dramatic increase in recruitment of lymphocytes, but not myeloid cells, to an inflammatory site. We found that the α4(S988A) mutation markedly increased the abundance of T cells but not myeloid cells in heterotopic B16 melanomas. Increased lymphocyte homing is needed for efforts to develop adoptive immuno-therapies for solid tumors (9–14). The potential therapeutic value of this form of integrin trans-regulation was established by the finding that subcutaneously implanted melanomas grew more slowly in α4(S988A) mice associated with markedly enhanced recruitment of T cells but not myeloid cells to the tumors. Thus increasing integrin trans-regulation by blocking α4 integrin phosphorylation selectively recruits lymphocytes, but not myeloid cells, thereby reducing tumor growth. This finding suggests that modulation of integrin trans-regulation may be useful in overcoming a limitation of adoptive cellular immunotherapy of solid tumors.
Methods
Mice
α4(S988A) mice were generated as described in previous supplementary material (16). These mice were bred to create homozygous germline knock-ins and backcrossed to BL6 for >8 generations for all experiments, unless otherwise noted. For assessment of T cell cytotoxic function, α4(S988A) mice were crossed with the OT-1 strain. Rag1−/− mice were used as recipients for in vivo competitive migration experiments. All mice were housed at the University of California San Diego animal facility, and all experiments were approved by the Institutional Animal Care and Use Committee (IACUC).
Hematological analysis
Blood from adult (10–20 wk-old) α4(S988A) and α4(wildtype) mice mice was collected into tubes containing EDTA. Cell counts were obtained using an MS9 automated cell counter by the University of California San Diego Animal Care Program Diagnostic Laboratory who also manually performed differential counts on DiffQuick–stained smears.
Peritonitis model
Adult (10–20 weeks old) α4(S988A) and control α4(wt) mice were injected intraperitoneally with 4% (wt/vol) thioglycollate (Sigma-Aldrich) and sacrificed at various time points for peritoneal lavage. Cells (1 × 105) were adhered to glass slides with a Cytospin4 instrument (ThermoShandon) and stained with DiffQuick to differentiate cell types by light microscopy. The percentages of T, B1, and B2 cells were assessed by flow cytometry using fluorochrome-conjugated anti-CD3, anti-B220, and anti-Mac-1 antibodies. For the competitive peritonitis assay, peritonitis was elicited as described above, but in Rag1−/− mice. 24h after thioglycollate injection, mice received an i.v. injection of a mixture of splenocytes from adult α4(S988A) or control Ly5.1 α4(wt). Splenocyte suspensions and peritoneal lavage were stained using antibodies against CD3, CD8, and Ly5.1. The ratio of α4(S988A) to Ly5.1+ α4(wt) splenocytes was compared between spleen and peritoneum as a measure of enrichment for α4(S988A) cells in the inflammatory site.
Lymphoid compartments
Bone marrow (BM) cell suspensions were prepared by flushing femur and tibia bones from adult α4(S988A) and α4(wildtype) mice. Single-cell suspensions from BM, spleen, and thymus were treated with ACK lysis buffer, counted, and stained with fluorochrome-conjugated antibodies (eBioscience) against mouse B220 (RA3-6B2), IgM (II/41), IgD (11-26c (11-26)), CD21/35 (7G6), CD23 (B3B4), CD3 (145-2C11), CD4(GK1.5), CD8 (53-6.7), at optimized concentrations. Cells were washed and analyzed by flow cytometry. For integrin expression analysis, blood was collected by tail bleed, treated with ACK lysis buffer and stained with anti-CD3, anti-α4, anti-αLβ2 integrin, or isotype control fluorochrome-conjugated antibodies before flow cytometry.
Humoral immune response
For antigen-specific antibody responses, α4(S988A) and α4(wildtype) mice (F5 backcross to BL6) were injected i.p. with 100 μg TNP-KLH (Biosearch) emulsified in 250 μl CFA (T cell-dependent antigen). Blood serum was collected by centrifugation of tail vein bleed (100–200 μl with 1–2 mM EDTA solution as an anticoagulant) before (pre-immune) and at 1, 2, and 3 wk after immunization. TNP-specific antibody concentrations in blood sera were assessed by direct ELISA with TNP-OVA as the coating antigen and AP-conjugated polyclonal anti-mouse IgG (Sigma), polyclonal anti-mouse IgM (Sigma), or anti-mouse IgG3 (Clone R40-82, BD Biosciences) as the detection antibody.
Migration assay
Resting B or T cells were purified from spleens of adult α4(S988A) and control α4(wt) mice by negative depletion. Macrophages were differentiated from α4(S988A) or control α4(wt) bone marrow by culture in 30% L292 supernatant for one week. In vitro migration was assessed following a modified Boyden Chamber assay (8). Briefly, Transwells (Costar) 3.0 μm polycarbonate membrane inserts were coated with VCAM-1 and/or ICAM-1 Fc fusion proteins in carbonate buffer, pH 8.0. Transwell membranes were blocked in PBS, 2% BSA 30 min at room temperature. Cells (2.0 × 105) were added to the top chamber in complete medium (10% FBS). Complete medium containing 15 ng/ml Stromal-derived factor-1α (SDF-1α; R&D Systems) added to the lower chamber. To observe macrophage migration, 20 ng/ml of both SDF-1 and MCP-1 was necessary. After a 4h incubation at 37°C (overnight for macrophages), cells in the lower chamber and underside of transwell were harvested and counted by hemacytometer.
B16 melanoma model
B16 (f1 subclone) cells were expanded in culture in complete medium (DMEM supplemented with 10% FBS, ℓ-glutamine, βME, and pen/strep antibiotics). B16 cells (3×105) were injected subcutaneously in the right hind flank of adult α4(S988A) or control α4(wt) mice. When tumors became visible, length and width were measured daily with calipers. Tumors were assumed to be ellipsoid and volume calculated using the formula: (length x width2)/2. Mice were sacrificed on day 15 and tumors excised and weighed. To analyze tumor-infiltrating leukocytes (TIL), tumors were digested with collagenase (Sigma) for 20–30 min. at 37C and further processed to a single-cell suspension using a 7ml tissue grinder (Kontes) and counted. Fluorochrome-conjugated antibodies were used to stain for tumor-infiltrating CD45+ leukocytes and identify CD4+ and CD8+ T cells (CD3+), as well as CD4+Foxp3+ Treg and NK1.1+ NK cells. Total subset numbers were calculated by multiplying the total cell number with %CD45+ and % of each subset. For lymphoid cell depletion, we injected anti-CD8 antibody (53-6.7, 100μg) or a combination of anti-CD4, anti-CD8, and anti-NK1.1 antibodies (150 μg each), compared with isotype control antibody(s) i.p. 2 days before and 5 days after B16 tumor cell inoculation. Splenocytes harvested on day 15 were stained with antibodies specific for CD3, CD4, and CD8 to determine efficiency of T cell depletion.
Analysis of Clonal Expansion in vivo
To analyze clonal expansion on a polyclonal TCR background, α4(S988A) and wild type mice were immunized with a combination of 100 μg poly I:C (Invivogen), 50 μg anti-CD40 antibody (Biolegend), and 500 μg ovalbumin protein (Sigma) in PBS i.p. Six days later, mice were sacrificed and spleen cells stained using anti-CD8+ antibody and a H-2Kb-SIINFEKL(PE) tetramer (Coulter).
Assessment of CD8 T cell cytotoxic function
To generate functional CTL, splenocytes from α4(S988A) and wild type OT-1 mice were cultured with 1 μg/ml SIINFEKL and 100U/ml IL-2 (NCI) for 6 days. Degranulation as a measure of cytotoxicity was measured as exposure of CD107a (LAMP-1) on the outer cell membrane. On day 6 following SIINFEKL stimulation, CTL were harvested, counted, and cultured with SIINFEKL pulsed (1 μg/ml for 1–2h) or unpulsed splenocytes as targets in the presence of anti-LAMP-1-PE antibodies (eBioscience) for 2.5 h at 37C. Effector/target cultures were stained with anti-CD8 antibodies and analyzed by flow cytometry. For target lysis in vitro, CTL were generated as above and cultured with a ~50/50 mixture of peptide-pulsed (CFSElo) and unpulsed (CFSEhi) splenocyte targets overnight. Specific lysis is the %decrease in the percentage of the peptide-pulsed peak between CTL-containing and no-CTL control cultures.
Statistical Analysis
Two-tailed t-test was used for statistical comparison between groups in all experiments, except where otherwise noted. A value of p < 0.05 was considered statistically significant.
Results and Discussion
The α4(S988A) mutation selectively increases lymphocyte migration to an inflammatory site
We used homologous recombination to generate α4(S988A) mice (Supplemental, S1) and compared them to wild type C57BL/6 mice as controls. We observed no significant differences in formed elements of the blood (Supplemental, S2A) or in lymphocyte numbers (with the possible exception of increased Pro B cells in the bone marrow) in primary or secondary lymphoid tissue Supplemental, S2B) in α4(S988A) mice. Humoral immune responses to a T-dependent antigen were also similar between α4(S988A) and wild type mice (Supplemental, S2C).
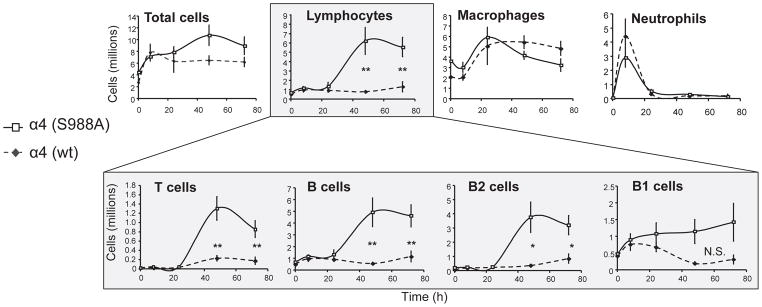
Because the α4(S988A) mutation inhibits migration on substrates containing purified α4 integrin ligands (7), we hypothesized that α4(S988A) mice might exhibit a similar defect in leukocyte migration in vivo. We therefore used a thioglycollate-induced peritonitis model to test the effect of this mutation on leukocyte to entry into an inflammatory site. To our great surprise, we observed a sharp increase in the number of lymphocytes infiltrating the peritoneum, (Fig. 1), whereas myeloid cell infiltration was unaffected. We stained the peritoneal lavage with antibodies to identify B1, B2, and T lymphocytes and found that both B2 and T cells exhibited significantly (>3–4-fold) greater numbers in the α4(S988A) mouse compared with controls (Fig. 1). In contrast, the numbers of B1 cells, a resident peritoneal population (15), showed no significant differences from controls, indicating that the α4(S988A) mutation specifically affects influx of lymphocytes. An in vivo competitive migration assay (Supplemental, S3) using a mix of wild-type (congenically marked) and α4(S988A) splenocytes confirmed that the increased migration of α4(S988A) T cells is intrinsic to leukocytes and not merely due to effects of α4 phosphorylation in endothelial cells (16) or other changes in the inflammatory environment (e.g. production of chemokines by resident cells) in these mice. Therefore, interfering with α4 integrin phosphorylation selectively increased homing of lymphocytes to a site of inflammation, but had no obvious effect on myeloid cells.
Figure 1.
Leukocyte migration in the α4(S988A) mouse. Adult α4(S988A) or wild type mice were injected with 1 ml thioglycollate medium i.p. Mice were sacrificed at various time points and leukocytes from peritoneal lavage were enumerated and identified by cytospin and DiffQuick staining. Peritoneal lavage was also stained with antibodies to identify T-cells (CD3+),Total B-cells (B220+), B1 cells (B220+CD11b+), and B2 cells (B220+CD11b−). Error bars are S.E.M. of n=5 for each group. * p < 0.03, ** p < 0.02; N.S. = not statistically significant.
Integrin trans-regulation explains increased migration of α4(S988A) lymphocytes
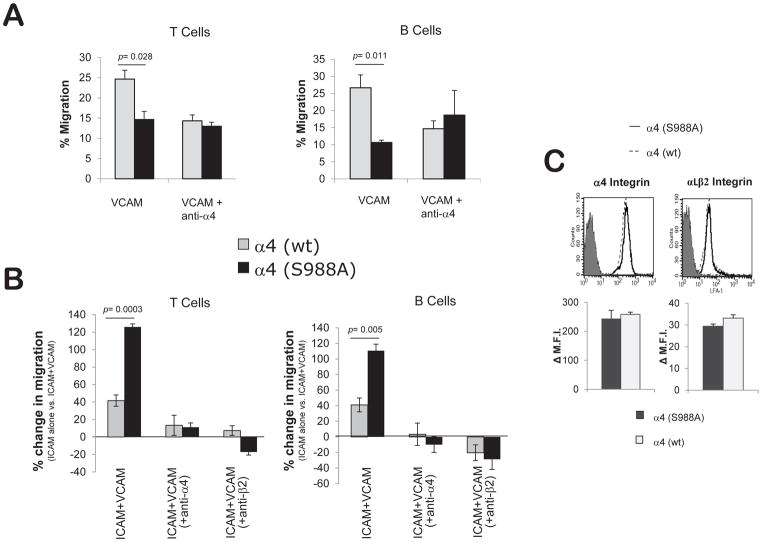
Our initial expectation that the α4(S988A) mutation would decrease homing was based on results from in vitro migration on purified α4 integrin ligands. In vivo migration to an inflammatory site is complex and involves several classes of integrins (17). We previously reported that the presence of small amounts of α4 integrin ligands (e.g. VCAM-1) increases the migration of Jurkat T cells on αLβ2 integrin substrates (e.g. ICAM-1) in vitro (8). Paxillin binding to α4 is required for this effect, and enforced paxillin-α4 association enhanced the trans-regulation of αLβ2 by α4 integrins through increasing the activation of FAK and Pyk2 kinases (8, 18). The α4(S988A) mutation that blocks Protein Kinase A phosphorylation of the α4 cytoplasmic tail could therefore enhance αLβ2-dependent migration. The in vivo peritonitis experiment requires migration on mixed substrates and is dependent on both α4 and β2 integrins (17), conditions in which the α4(S988A) mutation could increase migration of lymphocytes (8). To explore this possibility in a controlled setting, we purified B and T cells from α4(S988A) mice and measured their ability to migrate in vitro on purified α4 ligand (VCAM-1), purified β2 ligand (ICAM-1), or mixed substrates (VCAM-1+ICAM-1) in response to the chemokine SDF-1α. Using a modified Boyden chamber assay, we confirmed that α4(S988A) lymphocytes exhibited reduced migration on a purified α4 integrin ligand: VCAM-1 (Fig. 2A). In contrast, when plated on substrates containing predominantly ICAM-1 and small amounts of VCAM-1, both B and T cells from α4(S988A) mice displayed enhanced migration that was dependent on both α4 and β2 integrins (Fig. 2B, Supplemental, S4). This observation cannot be explained by differences in surface integrin expression levels, as T cells from α4(S988A) and α4(wt) mice show no difference in staining for α4 or αLβ2 integrins (Fig. 2C). These data indicate that the α4(S988A) mutation provides an increase in β2 integrin-dependent migration, i.e. integrin trans-regulation in primary lymphocytes.
Figure 2.
Increased integrin trans-regulation in α4(S988A) lymphocytes. B and T-cells were purified from spleens of α4(S988A) or control α4(wt) mice. Chemotactic migration towards SDF-1α (15ng/ml) was assessed using a modified Boyden Chamber assay in (A) wells coated with VCAM-1 alone (2 ug/ml), or (B) ICAM-1 (5 ug/ml) +/− VCAM-1 (0.02 ug/ml). For anti-integrin antibody blocking studies, cells were treated with 10μg/ml of either anti-α4 or anti-β2 integrin prior to the assay. Part (B) is the % increase in migration on ICAM-1+VCAM-1 compared to ICAM-1 alone 100*[(ICAM+VCAM migration – ICAM migration)/ICAM migration]. Error bars are S.E.M. of n=4 for each group. (C) Surface integrin expression levels on circulating α4(S988A) T-cells. Blood leukocytes from α4(S988A) and α4(wt) control adult C57BL/6 mice (n=3 per group) were stained with antibodies specific for T-cells (CD3), α4 integrins (CD49d), and αLβ2 integrin heterodimer and analyzed by flow cytometry. Representative histograms show α4 or αLβ2 staining (soild and dotted lines) compared with non-specific isotype control staining (filled peak). Bar graphs summarize staining from n= 3 mice per group; No significant differences were observed. Δ M.F.I = Change in Mean Fluorescence Intensity.
Increased integrin trans-regulation reduces tumor growth by increasing T cell homing
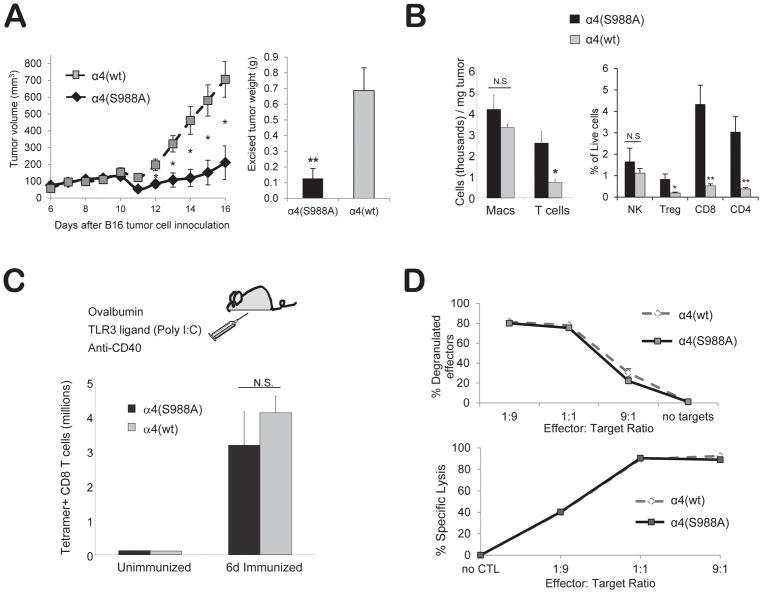
Specifically increasing lymphocyte entry into an inflammatory site might offer therapeutic benefit during an immune response to a tumor. T cell migration to solid tumors is important for tumor immunity (1), whereas tumor infiltration by macrophages may promote tumor growth through increased angiogenesis and suppressed immunity (2, 3). In adoptive immunotherapy (11–14, 19), naïve T cells are modified and activated in vitro; however T cell homing is a critical limiting variable in solid tumor adoptive immunotherapy (9, 10). Based on our results with the peritonitis model, we hypothesized that α4(S988A) mice may have increased ability to resist tumors due to selective migration of lymphocytes to the tumor site. We tested this idea using a melanoma model (20), in which B16 melanoma cells are injected subcutaneously and tumor size is measured by weighing excised tumors 15 days later. α4(S988A) mice had ~5-fold smaller tumors than wild type BL6 controls (Fig. 3A), indicating that blocking α4 integrin phosphorylation on Ser988 increased tumor protection. This observation did not appear to be unique to the B16 model, as α4(S988A) mice also displayed increased resistance to the growth of Lewis Lung Carcinoma (Supplemental, S5)
Figure 3.
Blocking α4 integrin phosphorylation increases tumor-infiltrating T-cells and reduces tumor growth in mice. (A) Reduced tumor growth in α4 (S988A) mice. B16 melanoma cells (3×105) were injected into α4(S988A) or control α4(wt) mice. Tumor area was measured daily and converted to an ellipsoid volume: (length x width2)/2. Fifteen days later, tumors were excised and weighed. (B) α4(S988A) T-cells migrate more efficiently to a tumor site. B16 tumors were grown as in (A). On day 15, mice were sacrificed and excised tumors were weighed, digested with collagenase, and stained for (CD45+) CD11b+ macrophages, CD3+ T cells, CD4+ T-cells, CD8+ T-cells, Foxp3+(CD4+) Treg, and NK1.1+ NK cells. Error bars are S.E.M. of n 10 mice per group. * p < 0.015, ** p < 0.002. (C) T-cell clonal expansion. α4(S988A) and α4(wt) control littermate mice were immunized with ovalbumin protein, anti-CD40, and Poly I:C, i.p. in PBS. Six days later, splenocytes were stained with anti-CD8 antibody and an H-2Kb-SIINFEKL tetramer. (D) T-cell cytotoxic function. Splenocytes of 8–12 wk-old OT-I+ α4(S988A) or OT-1+ α4(wt) control littermate mice were differentiated to CTL in vitro with SIINFEKL (1μ/ml) and IL-2 for 5 days and cultured for 2 h at the indicated effector to target ratios (E:T) in the presence of anti-LAMP-1 antibody, followed by staining for CD8 and flow cytometric analysis. For target lysis, CTL were cultured with SIINFEKL-pulsed or unpulsed targets that had been differentially labeled with CFSE. Specific killing was detected by flow cytometry as the decrease in the percentage of specific targets. No significant differences were noted, with the exception of the 9:1 E:T ratio at which the α4(S988A) specific killing was 3.7% lower than α4(wt) CTL (p<.005). n = 3 mice per group. Experiment was performed twice.
We next asked whether α4(S988A) resistance tumors was associated with increased T cell homing. Indeed, B16 tumors in α4(S988A) mice had greater concentrations of T cells than those grown in wild type mice (Fig. 3B, left panel). This was in striking contrast to a similar number of macrophages found in tumors from mice of both genotypes, supporting the idea that the decreased tumor growth is a result of selective homing of lymphocytes vs. macrophages in the α4(S988A) mice. Among lymphoid cells, tumors in α4(S988A) mice had significantly greater numbers of CD4+, CD8+, and regulatory T cells; NK cell abundance showed a modest, but statistically insignificant, increase compared to controls (Fig. 3B, right panel). The increased density of tumor-infiltrating T cells seen in α4(S988A) mice could be due to greater clonal expansion of tumor-specific T cells; however, expansion of antigen-specific T cells (SIINFEKL-tetramer+) was similar between α4(S988A) and α4(wt) mice in response to immunization with ovalbumin (Fig. 3C). Furthermore, the cytotoxic function of α4(S988A) CD8+ T cells was nearly identical to that of wild type (Fig. 3D) cells as measured by degranulation of α4(S988A) OT-1 CD8+ T cells and specific lysis of SIINFEKL-pulsed target cells. Thus, we conclude that the reduction in tumor growth observed in α4(S988A) mice is largely ascribable to increased lymphoid cell homing. Intriguingly, we also observed smaller tumors in α4(S988A) mice partially depleted of CD8 T cells; however, reduced tumor growth in α4(S988A) mice could not be observed when the majority of CD4 T, CD8 T, and NK cells were depleted (Supplemental, S6). This finding suggests that increased migration of multiple lymphoid cell subsets can contribute to the tumor resistance in α4(S988A) mice.
The differential requirement of β2 integrins for lymphocytes or myeloid cells in vivo can account for the remarkable leukocyte specificity of this form of trans-regulation. Whereas αLβ2 plays a major role in the migration of T-cells to inflammatory sites (17, 21–23), macrophage migration to inflamed peritoneum is not dependent on β2 integrins, and is reported to be solely dependent on α4β1 (24, 25). Thus trans-regulation of migration would be absent in mononuclear leukocytes since β2 integrins are not required. Indeed, we did not observe increased migration of monocytes/macrophages from α4(S988A) or α4(wt) mice (Supplemental, S7) on mixed ICAM-1-VCAM-1 substrates.
The finding that α4β1 trans-regulation of αLβ2 integrin-mediated migration promotes tumor immunity has important therapeutic implications. Inhibiting Focal Adhesion Kinase (FAK) can suppress such trans-regulation (8), a finding that sounds a cautionary note in the use of FAK inhibitors in tumor therapy (26–28) and suggests that the effect of these agents on lymphocyte trafficking to tumors should be evaluated. Homing of infused lymphocytes is currently a rate-limiting step in applying T cell immunotherapy to solid tumor cancers (9). Since lymphocytes are modified ex vivo before adoptive transfer for immunotherapy (11–14), the opportunity exists to simultaneously optimize their homing capacity by increasing integrin trans-regulation. α4 phosphorylation is Type I-Protein Kinase A-dependent (29), thus increased trans-regulation could be induced by introduction of a dominant α4(S988A) integrin subunit, a cell permeating Type I-specific A-kinase anchor protein (AKAP) peptide, or genetically-encoded Type I-specific Protein Kinase A inhibitor (29, 30). Increased integrin trans-regulation might also have utilities beyond tumor immunotherapy. Migration and survival of long-lived plasma cells in the bone marrow appears to be dependent on α4 and αLβ2 integrins (31–35) and could involve integrin trans-regulation. Thus, enhanced integrin trans-regulation offers a new approach to selectively potentiate β2 integrin-mediated homing of lymphocytes and plasma cells.
Supplementary Material
Acknowledgments
This work was funded in part by NIH R01 HL 31950 and HL 117807. J.M.C is funded by NIH NIDDK grant K01-DK090416 and MS Society grant RG4981A1/T.
References
- 1.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373–400. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 2.Porta C, Subhra Kumar B, Larghi P, Rubino L, Mancino A, Sica A. Tumor promotion by tumor-associated macrophages. Adv Exp Med Biol. 2007;604:67–86. doi: 10.1007/978-0-387-69116-9_5. [DOI] [PubMed] [Google Scholar]
- 3.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose DM, Han J, Ginsberg MH. Alpha4 integrins and the immune response. Immunol Rev. 2002;186:118–124. doi: 10.1034/j.1600-065x.2002.18611.x. [DOI] [PubMed] [Google Scholar]
- 5.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 6.Liu S, Thomas SM, Woodside DG, Rose DM, Kiosses WB, Pfaff M, Ginsberg MH. Binding of paxillin to alpha4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 7.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of alpha4 integrin phosphorylation regulates lamellipodial stability and alpha4beta1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose DM, Liu S, Woodside DG, Han J, Schlaepfer DD, Ginsberg MH. Paxillin binding to the alpha 4 integrin subunit stimulates LFA-1 (integrin alpha L beta 2)-dependent T cell migration by augmenting the activation of focal adhesion kinase/proline-rich tyrosine kinase-2. J Immunol. 2003;170:5912–5918. doi: 10.4049/jimmunol.170.12.5912. [DOI] [PubMed] [Google Scholar]
- 9.Ngo MC, Rooney CM, Howard JM, Heslop HE. Ex vivo gene transfer for improved adoptive immunotherapy of cancer. Hum Mol Genet. 2011;20:R93–99. doi: 10.1093/hmg/ddr102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parente-Pereira AC, Burnet J, Ellison D, Foster J, Davies DM, van der Stegen S, Burbridge S, Chiapero-Stanke L, Wilkie S, Mather S, et al. Trafficking of CAR-engineered human T cells following regional or systemic adoptive transfer in SCID beige mice. J Clin Immunol. 2011;31:710–718. doi: 10.1007/s10875-011-9532-8. [DOI] [PubMed] [Google Scholar]
- 11.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagarasan S, Watanabe N, Honjo T. Generation, expansion, migration and activation of mouse B1 cells. Immunol Rev. 2000;176:205–215. doi: 10.1034/j.1600-065x.2000.00604.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldfinger LE, Tzima E, Stockton R, Kiosses WB, Kinbara K, Tkachenko E, Gutierrez E, Groisman A, Nguyen P, Chien S, et al. Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ Res. 2008;103:177–185. doi: 10.1161/CIRCRESAHA.108.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulyanova T, Priestley GV, Banerjee ER, Papayannopoulou T. Unique and redundant roles of alpha4 and beta2 integrins in kinetics of recruitment of lymphoid vs myeloid cell subsets to the inflamed peritoneum revealed by studies of genetically deficient mice. Exp Hematol. 2007;35:1256–1265. doi: 10.1016/j.exphem.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Rose DM, Woodside DG, Goldfinger LE, Ginsberg MH. Integrin alpha 4 beta 1-dependent T cell migration requires both phosphorylation and dephosphorylation of the alpha 4 cytoplasmic domain to regulate the reversible binding of paxillin. J Biol Chem. 2003;278:34845–34853. doi: 10.1074/jbc.M304691200. [DOI] [PubMed] [Google Scholar]
- 19.Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials. Leuk Lymphoma. 2013;54:255–260. doi: 10.3109/10428194.2012.715350. [DOI] [PubMed] [Google Scholar]
- 20.Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol. 2001;Chapter 20(Unit 20):21. doi: 10.1002/0471142735.im2001s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelhardt B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J Neural Transm. 2006;113:477–485. doi: 10.1007/s00702-005-0409-y. [DOI] [PubMed] [Google Scholar]
- 22.Koboziev I, Karlsson F, Ostanin DV, Gray L, Davidson M, Zhang S, Grisham MB. Role of LFA-1 in the activation and trafficking of T cells: implications in the induction of chronic colitis. Inflamm Bowel Dis. 2012;18:2360–2370. doi: 10.1002/ibd.22947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeichi T, Mocevicius P, Deduchovas O, Salnikova O, Castro-Santa E, Buchler MW, Schmidt J, Ryschich E. alphaL beta2 integrin is indispensable for CD8+ T-cell recruitment in experimental pancreatic and hepatocellular cancer. Int J Cancer. 2012;130:2067–2076. doi: 10.1002/ijc.26223. [DOI] [PubMed] [Google Scholar]
- 24.Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- 25.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 26.Beierle EA, Ma X, Stewart J, Nyberg C, Trujillo A, Cance WG, Golubovskaya VM. Inhibition of focal adhesion kinase decreases tumor growth in human neuroblastoma. Cell Cycle. 2010;9:1005–1015. doi: 10.4161/cc.9.5.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Golubovskaya VM, Nyberg C, Zheng M, Kweh F, Magis A, Ostrov D, Cance WG. A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth. J Med Chem. 2008;51:7405–7416. doi: 10.1021/jm800483v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hochwald SN, Nyberg C, Zheng M, Zheng D, Wood C, Massoll NA, Magis A, Ostrov D, Cance WG, Golubovskaya VM. A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer. Cell Cycle. 2009;8:2435–2443. doi: 10.4161/cc.8.15.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim CJ, Han J, Yousefi N, Ma Y, Amieux PS, McKnight GS, Taylor SS, Ginsberg MH. Alpha4 integrins are type I cAMP-dependent protein kinase-anchoring proteins. Nat Cell Biol. 2007;9:415–421. doi: 10.1038/ncb1561. [DOI] [PubMed] [Google Scholar]
- 30.Burns-Hamuro LL, Ma Y, Kammerer S, Reineke U, Self C, Cook C, Olson GL, Cantor CR, Braun A, Taylor SS. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc Natl Acad Sci U S A. 2003;100:4072–4077. doi: 10.1073/pnas.2628038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, Hogg N. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med. 1999;189:1467–1478. doi: 10.1084/jem.189.9.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cyster JG. Homing of antibody secreting cells. Immunol Rev. 2003;194:48–60. doi: 10.1034/j.1600-065x.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 33.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med. 2001;193:741–754. doi: 10.1084/jem.193.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med. 2001;193:755–768. doi: 10.1084/jem.193.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minges Wols HA, Underhill GH, Kansas GS, Witte PL. The role of bone marrow-derived stromal cells in the maintenance of plasma cell longevity. J Immunol. 2002;169:4213–4221. doi: 10.4049/jimmunol.169.8.4213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.