Abstract
Background
Limited information is available regarding medication use in COPD patients from Latin America. This study evaluated the type of medication used and the adherence to different inhaled treatments in stable COPD patients from the Latin American region.
Methods
This was an observational, cross-sectional, multinational, and multicenter study in COPD patients attended by specialist doctors from seven Latin American countries. Adherence to inhaled therapy was assessed using the Test of Adherence to Inhalers (TAI) questionnaire. The type of medication was assessed as: short-acting β-agonist (SABA) or short-acting muscarinic antagonist (SAMA) only, long-acting muscarinic antagonist (LAMA), long-acting β-agonist (LABA), LABA/LAMA, inhaled corticosteroid (ICS), ICS/LABA, ICS/LAMA/LABA, or other.
Results
In total, 795 patients were included (59.6% male), with a mean age of 69.5±8.7 years and post-bronchodilator FEV1 of 50.0%±18.6%. The ICS/LAMA/LABA (32.9%) and ICS/LABA (27.7%) combinations were the most common medications used, followed by LABA/LAMA (11.3%), SABA or SAMA (7.9%), LABA (6.4%), LAMA (5.8%), and ICS (4.3%). The types of medication most commonly used in each Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 category were ICS/LABA (A: 32.7%; B: 19.8%; C: 25.7%; D: 28.2%) and ICS/LAMA/LABA (A: 17.3%; B: 30.2%; C: 33%; D: 41.1%). The use of long-acting bronchodilators showed the highest adherence (good or high adherence >50%) according to the TAI questionnaire.
Conclusion
COPD management in specialist practice in Latin America does not follow the current guideline recommendations and there is an overuse of ICSs in patients with COPD from this region. Treatment regimens including the use of long-acting bronchodilators are associated with the highest adherence.
Keywords: adherence, inhaled therapy, COPD, Latin America
Introduction
Optimal pharmacological treatment for COPD patients reduces symptoms, reduces the frequency and severity of exacerbations, improves health status, and increases exercise tolerance.1 However, despite the frequent changes in COPD classification systems and the emergence of new inhaled therapies recommended in some new evidence-based guidelines,1–3 poor adherence to treatment and lack of accessibility to these drugs in many countries generate a considerable gap between optimal treatment and real-life prescribing patterns.4–6
In Latin America, the undertreatment and suboptimal treatment of COPD patients occur frequently, with wide variations between countries. The population-based prevalence study of COPD in Latin America (PLATINO)7 showed that only a quarter of COPD patients received any respiratory medication, 75% of patients with a previous medical diagnosis had received respiratory medication in the past year, and over half of treated subjects were on medication without a diagnosis of airway obstruction.8 In primary care settings in Latin America, long-acting bronchodilators (LA-BDs) are frequently underused as regular maintenance therapy for COPD, and the treatment received by patients does not strictly follow the COPD guidelines’ recommendations; short-acting bronchodilators (SA-BDs) were most commonly used as monotherapy in patients with COPD.9 Overtreatment with inhaled corticosteroids (ICSs) was also common in patients with a previous diagnosis of COPD.10
No information exists regarding the real-life types and patterns of inhaled medication use in COPD patients attended by specialist doctors in Latin America, nor is there any information on adherence to the different types of medication. Therefore, the aims of this non-interventional study were to describe the types of respiratory medication used in COPD patients attended by specialist doctors overall and according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2013 categories, and to determine the adherence to different inhaled therapies in stable COPD patients from seven Latin American countries.
Methods
The Latin American Study of 24-hour Symptoms in Chronic Obstructive Pulmonary Disease (LASSYC) was a prospective observational, multicenter, multinational, cross-sectional, non-interventional study in stable COPD patients from seven Latin American countries: Argentina, Chile, Colombia, Costa Rica, Guatemala, Mexico, and Uruguay.11,12 The study has been registered at ClinicalTrials.gov (identifier: NCT02789540). Here, we analyzed prescribed medication use and adherence to different inhaled therapies in this patient population.
In total, 795 patients were enrolled and distributed among convenient specialist doctors in the seven selected countries. Each country recruited 100–130 patients and each site around 10–15 patients. The recruitment was competitive in each country after the expected site recruitment time of 1 month, up to a total recruitment period of 3 months. The study team identified suitable ambulatory pulmonology sites (ie, those whose databases had information on diagnostic procedures, etc.) to ensure an adequate sample of both specialist doctors and patients.
The ethics committees for each site approved the study protocol and all patients provided written informed consent (Table S1).
Site staff collected, retrospectively, information from patients’ medical records to determine eligibility, and those patients with stable COPD were screened for inclusion.
The methodology of the study has been described previously. In brief, outpatients ≥40 years of age with a diagnosis of COPD for at least 1 year, at least one spirometry value with a COPD diagnosis using the post-bronchodilator FEV1/FVC <0.70 criterion in the previous 12 months, current or ex-smokers (≥10 pack-years), and stable disease (without treatment for an exacerbation or changes in current treatment in the previous 2 months) were included in the study.11 Patients with a diagnosis of sleep apnea or any other chronic respiratory disease, or any acute or chronic condition that would limit their ability to participate in the study were excluded.
The following information was collected for each patient: social demographics, health insurance system, lifestyle, smoking history, presence of comorbidities, level of dyspnea, disease severity, prescribed COPD treatments, exacerbation history, and health care resource utilization during the past 12 months.
COPD classification was performed according to the GOLD 2013 criteria for groups A–D based on post-bronchodilator FEV1, exacerbation risk, and level of symptoms.13
The type of medication used was assessed using the following classification: short-acting β-agonist (SABA) or short-acting muscarinic antagonist (SAMA) only, long-acting muscarinic antagonist (LAMA), long-acting β-agonist (LABA), LABA/LAMA, ICS, ICS/LABA, ICS/LAMA/LABA, and other (such as methylxanthine, mucolytics, and phosphodiesterase-4 inhibitors).
Assessment of medication adherence
Self-reported adherence to medication was measured using the Test of Adherence to Inhalers (TAI) questionnaire.14
The TAI questionnaire includes 10 items (patient domain), is self-administered, and is scored from 1 to 5 (where 1= worst possible score and 5= best possible score). The total score for the 10-item questionnaire ranges from 10 to 50. Adherence is rated as good (score =50), intermediate (score =46–49), or poor (score ≤45).14
The adherence profiles to inhaled therapies in general and the level of agreement between two patient self-report adherence questionnaires in stable COPD patients from seven Latin American countries have been published elsewhere.11
Statistical analysis
Descriptive statistics included the absolute and relative frequencies for categorical variables and the mean ± SD for numerical variables. To test for differences in some variables according to the type of medicine taken, a Student’s t-test was used for continuous variables as the medicines are all dichotomous variables. We considered a p-value less than 5% as statistically significant. All analyses were performed using Stata 13.1 (Stata Statistical Software: Release 13, 2013; StatCorp LP, College Station, TX, USA).
Results
In total, 795 patients were included between May and August 2016 (59.6% male), with a mean age of 69.5±8.7 years and a mean post-bronchodilator FEV1 of 50.0%±18.6% of predicted. The general characteristics of the overall patient population and by country are shown in Table 1. Among these patients, 787 (99%) completed the TAI questionnaire. The sample characteristics of the patient population according to the type of medication used are shown in Table 2.
Table 1.
Patients’ characteristics, overall and by country
| Variable | All | Argentina | Chile | Colombia | Costa Rica | Guatemala | Mexico | Uruguay |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 795 (100) | 193 (100) | 107 (100) | 150 (100) | 60 (100) | 32 (100) | 142 (100) | 111 (100) |
| Gender | ||||||||
| Male | 474 (59.6) | 106 (54.9) | 54 (50.5) | 90 (60.0) | 41 (68.3) | 26 (81.2) | 91 (64.1) | 66 (59.5) |
| Age (complete years) | ||||||||
| <60 | 114 (14.3) | 31 (16.1) | 14 (13.1) | 17 (11.3) | 10 (16.7) | 5 (15.6) | 18 (12.7) | 19 (17.1) |
| 60–69 | 280 (35.2) | 69 (35.8) | 35 (32.7) | 53 (35.3) | 16 (26.7) | 14 (43.8) | 50 (35.2) | 43 (38.7) |
| ≥70 | 401 (50.4) | 93 (48.1) | 58 (54.2) | 80 (53.4) | 34 (56.7) | 13 (40.6) | 74 (52.1) | 49 (44.1) |
| Highest level of schooling | ||||||||
| Less than primary school | 137 (17.2) | 4 (2.1) | 7 (6.5) | 69 (46.0) | 15 (25.0) | 2 (6.3) | 18 (12.7) | 22 (19.8) |
| Finished primary school | 200 (25.2) | 39 (20.2) | 23 (21.5) | 41 (27.3) | 23 (38.4) | 10 (31.2) | 21 (14.8) | 43 (38.7) |
| Finished secondary school | 252 (31.7) | 80 (41.5) | 51 (47.7) | 21 (14.0) | 14 (23.3) | 9 (28.1) | 41 (28.9) | 36 (32.4) |
| University/college degree | 206 (25.9) | 70 (36.3) | 26 (24.3) | 19 (12.7) | 8 (13.3) | 11 (34.4) | 62 (43.6) | 10 (9.0) |
| BMI (kg/m2) | ||||||||
| <18.5 | 235 (29.6) | 39 (20.2) | 18 (16.9) | 75 (50.0) | 20 (33.3) | 9 (28.1) | 51 (35.9) | 23 (20.7) |
| 18.5–24.9 | 425 (53.5) | 104 (53.9) | 62 (57.9) | 71 (47.3) | 33 (55.0) | 16 (50.0) | 73 (51.4) | 66 (59.5) |
| ≥25 | 135 (17.0) | 50 (25.9) | 27 (25.2) | 4 (2.7) | 7 (11.7) | 7 (28.1) | 18 (12.7) | 22 (19.8) |
| Physical activity level | ||||||||
| Low | 484 (60.9) | 72 (37.3) | 33 (30.8) | 71 (47.3) | 20 (33.3) | 5 (15.6) | 58 (40.9) | 40 (36.0) |
| Moderate | 268 (33.7) | 38 (18.7) | 37 (34.6) | 37 (24.7) | 13 (21.7) | 8 (25.0) | 33 (23.2) | 18 (16.2) |
| High | 43 (5.4) | 83 (43.0) | 37 (34.6) | 42 (28.0) | 27 (45.0) | 19 (59.4) | 51 (35.9) | 53 (47.8) |
| Payment for health care | ||||||||
| Insurance | 200 (25.5) | 110 (58.9) | 26 (24.8) | 46 (30.7) | 1 (1.8) | 0 (0.0) | 15 (10.7) | 2 (1.8) |
| Out of pocket | 145 (18.5) | 48 (25.3) | 13 (12.4) | 8 (5.3) | 7 (12.2) | 27 (84.4) | 37 (26.4) | 5 (4.5) |
| Public sector | 440 (56.0) | 32 (16.8) | 66 (62.9) | 96 (64.0) | 49 (86.0) | 5 (15.6) | 88 (62.9) | 104 (93.7) |
| Exacerbation in past year | ||||||||
| Yes | 470 (59.1) | 99 (51.3) | 102 (95.3) | 87 (58.0) | 19 (31.7) | 27 (84.4) | 87 (61.3) | 49 (44.1) |
| Smoking history (pack-years) | 41.8±19.0 | 47.5±18.1 | 34.7±15.5 | 39.2±18.9 | 42.2±20.3 | 33.5±12.0 | 39.7±19.7 | 49.5±19.7 |
| Lung function parameters | ||||||||
| FEV1 (post-BD, % predicted) | 49.4±17.5 | 48.9±16.5 | 52.3±16.4 | 48.7±18.4 | 46.3±15.9 | 45.9±16.6 | 47.0±18.1 | 54.6±18.6 |
| FVC (post-BD, % predicted) | 73.2±18.9 | 71.4±17.4 | 78.6±16.8 | 72.1±20.3 | 68.8±18.0 | 64.9±17.8 | 71.0±18.9 | 80.2±19.6 |
| FEV1/FVC (%) | 49.3±11.4 | 49.7±10.3 | 49.1±12.4 | 49.6±12.8 | 49.4±11.8 | 52.0±9.7 | 47.8±11.0 | 49.6±10.8 |
| mMRC grade | 1.8±1.0 | 1.5±0.9 | 2.2±0.8 | 2.0±1.1 | 1.8±1.2 | 1.8±1.3 | 1.9±1.1 | 1.8±0.9 |
| CAT score | 15.2±8.2 | 11.8±6.8 | 22.2±8.1 | 16.1±7.8 | 15.8±8.7 | 15.5±8.0 | 14.2±7.0 | 14.1±7.7 |
| BODEx index | 2.9±1.9 | 2.5±1.8 | 3.1±1.8 | 3.5±2.0 | 3.1±1.8 | 2.9±2.0 | 3.0±1.8 | 2.4±1.7 |
Note: Data are shown as n (%) or mean ± SD.
Abbreviations: BMI, body mass index; BD, bronchodilator; mMRC, modified Medical Research Council Dyspnea Scale; CAT, COPD Assessment Test; BODEx, BMI, Obstruction, Dyspnea, and Exacerbations.
Table 2.
Clinical profile of patients according to type of medication used
| Treatment | Gender (male) | Age (years) | FEV1 (post-BD, % predicted) | FVC (post-BD, % predicted) | FEV1/FVC (%) | mMRC grade | CAT score | BODEx index | Daytime symptom score | Early morning symptom score | Night-time symptom score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No treatment | 12 (52.2) | 67.8±9.6 | 51.9±17.8 | 70.6±19.5 | 54.0±10.6 | 1.4±1.0 | 13.7±10.1 | 2.4±1.9 | 8.8±6.3 | 2.5±2.5 | 2.4±3.3 |
| SABA or SAMA (only) | 43 (68.3) | 69.5±9.4 | 50.7±17.8 | 69.7±19.5 | 53.3±11.8 | 1.6±1.0 | 15.1±7.8 | 2.9±1.6 | 10.7±6.9 | 3.7±4.0 | 3.1±4.7 |
| LABA | 29 (56.9) | 69.2±8.1 | 51.3±16.6 | 74.3±16.5 | 50.3±11.0 | 1.6±1.1 | 156.8±9.6 | 2.5±1.8 | 10.2±8.1 | 3.5±4.1 | 3.1±4.4 |
| LAMA | 26 (56.5) | 70.3±8.1 | 55.9±19.9 | 81.8±20.9 | 49.6±10.8 | 1.7±1.0 | 11.9±7.2 | 2.3±2.0 | 7.1±5.6 | 2.2±2.6 | 1.5±2.6 |
| LABA/LAMA | 53 (58.9) | 69.2±9.0 | 48.9±15.7 | 75.4±17.1 | 47.6±10.9 | 1.8±0.9 | 15.1±7.9 | 2.7±1.7 | 10.0±7.1 | 2.6±2.9 | 1.5±2.3 |
| ICS | 22 (64.7) | 69.6±9.4 | 53.4±19.3 | 72.3±19.8 | 53.7±10.0 | 1.8±1.2 | 15.6±7.0 | 2.8±2.0 | 9.5±6.0 | 3.3±2.5 | 2.6±3.3 |
| ICS/LABA | 124 (56.4) | 69.2±8.9 | 52.4±17.2 | 74.7±18.0 | 51.2±10.7 | 1.8±1.1 | 15.5±8.7 | 2.7±1.9 | 10.5±7.5 | 3.6±3.7 | 2.4±3.6 |
| ICS/LAMA/LABA | 161 (61.5) | 69.8±8.5 | 44.7±16.8 | 71.0±19.3 | 46.0±11.5 | 2.0±1.0 | 15.7±7.6 | 3.3±1.8 | 10.2±6.6 | 3.2±3.7 | 2.2±3.7 |
| Other | 4 (66.7) | 70.5±8.2 | 45.0±25.2 | 61.4±29.6 | 52.7±11.2 | 1.3±0.8 | 14.0±7.5 | 2.4±1.8 | 5.3±4.0 | 3.0±3.3 | 1.7±2.3 |
Notes: Data are shown as n (%) or mean ± SD. LAMA, LABA/LAMA, ICS, ICS/LABA, ICS/LAMA/LABA, ICS/LAMA could be used alone or in combination with SABA or SAMA.
Abbreviations: BMI, body mass index; BD, bronchodilator; mMRC, modified Medical Research Council Dyspnea Scale; CAT, COPD Assessment Test; BODEx, BMI, Obstruction, Dyspnea, and Exacerbations; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
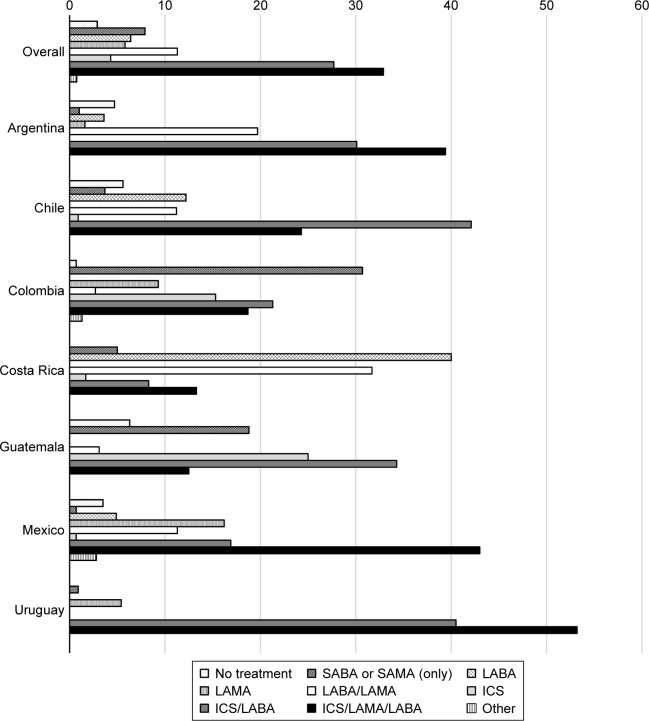
The type and frequency of medication used in patients with COPD, overall and by individual country, are shown in Figure 1. Approximately 3% of the overall study population were not receiving treatment. The ICS/LAMA/LABA and ICS/LABA combinations were the most frequently used treatments in most countries (Argentina, Chile, Guatemala, Mexico, and Uruguay), with more than one-third of patients using an ICS/LAMA/LABA combination. In Colombia, the most common medications used were SABA or SAMA, and in Costa Rica this was LABA monotherapy. A small proportion of patients were using LABA (6.4%) or LAMA (5.8%) monotherapy or dual bronchodilatation (LABA/LAMA [11.3%]) in the overall COPD population (Figure 1). The pattern of use of respiratory medication, either as-needed or as regular maintenance therapy, is shown in Figure S1. Of the patients who used SA-BD monotherapy (SABA or SAMA), more than 50% used it regularly as a maintenance therapy.
Figure 1.
Type and frequency of medication used, overall and by country.
Note: LABA, LAMA, LABA/LAMA, ICS, ICS/LABA, ICS/LAMA/LABA, and ICS/LAMA could be used alone or in combination with SABA or SAMA.
Abbreviations: LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist.
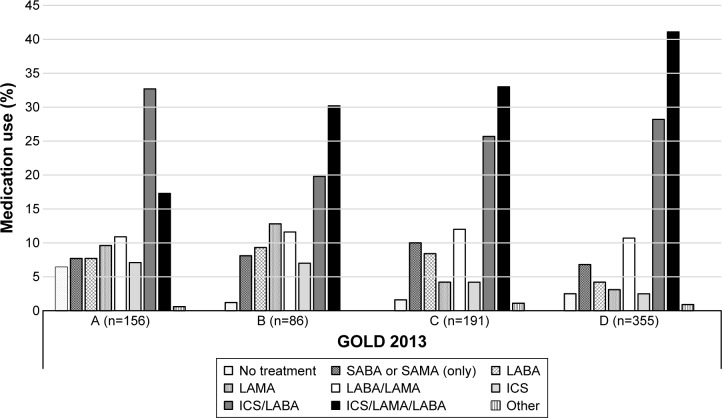
Figure 2 shows the types of medication used according to the GOLD 2013 COPD categories. The medication most frequently used in each category was ICS/LABA (A: 32.7%; B: 19.8%; C: 25.7%; D: 28.2%) and ICS/LAMA/LABA (A: 17.3%; B: 30.2%; C: 33%; D: 41.1%).
Figure 2.
Type of medication used according to the GOLD 2013 stage classification in the overall population.
Note: LABA, LAMA, LABA/LAMA, ICS, ICS/LABA, ICS/LAMA/LABA, and ICS/LAMA could be used alone or in combination with SABA or SAMA.
Abbreviations: GOLD, Global Initiative for Chronic Obstructive Lung Disease; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist.
Figures S2 and S3 show the type of medication used according to prior medical diagnosis of asthma and history of exacerbations (ambulatory or requiring hospitalization) in the past year, respectively. The majority of patients (≥90%) using any ICS combination did not have a prior medical diagnosis of asthma (Figure S2). In patients using any ICS combination, more than 35% and 75% of patients did not have an ambulatory exacerbation or an exacerbation requiring hospitalization, respectively.
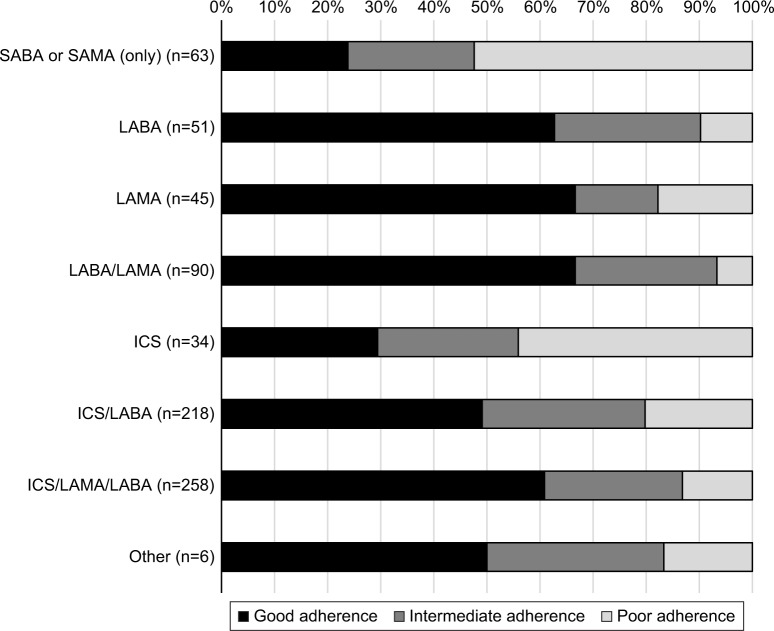
Adherence to treatment in the overall population according to the type of medication used, based on the TAI questionnaire, is shown in Figure 3. According to the TAI questionnaire, the use of SA-BDs and ICS monotherapy showed the lowest adherence (poor adherence in 52.4% and 44.0% of patients, respectively); treatment with LA-BDs had better adherence (good adherence in >50% of patients) (Figure 3).
Figure 3.
Adherence to treatment (TAI questionnaire) according to the type of medication used in the overall population.
Note: LABA, LAMA, LABA/LAMA, ICS, ICS/LABA, ICS/LAMA/LABA, and ICS/LAMA could be used alone or in combination with SABA or SAMA.
Abbreviations: TAI, Test of Adherence to Inhalers; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist.
Discussion
The main findings of this study on the treatment of stable COPD patients attended by specialist doctors in Latin America were: first, ICS/LAMA/LABA or ICS/LABA combinations were the most frequently used classes of medication in the overall population and also in each of the GOLD 2013 categories; second, a substantial proportion of patients were using an ICS combination without a prior medical diagnosis of asthma or exacerbation history; and third, the use of treatment regimens involving LA-BDs showed the highest adherence (good or high adherence >50%) according to the TAI questionnaire.
Pharmacological overtreatment, in particular the over-prescription of triple therapy in patients with COPD, many of whom have mild disease, has been frequently reported.4,9,10,15–21 In an analysis of a large UK primary care database, Price et al reported that ~50% of COPD patients received an ICS, in combination with either a LABA (26.7%) or a LABA/LAMA (23.2%).4 Similarly, here we found that ICS/LABA and triple therapy were the most frequently used treatments in patients categorized as GOLD A or B and only a small proportion of patients were using LABA or LAMA monotherapy (1.8% and 7.9%, respectively). In another large database study in Spain, up to 45.2% of patients newly diagnosed with COPD were treated with an ICS and up to 12% were receiving triple therapy.6 In a real-life study from France, ICS/LAMA/LABA and ICS/LABA combinations were used in 33% and 24% of the total COPD population, respectively.16 In addition, these combinations were commonly used in patients categorized as GOLD A (ICS/LAMA/LABA [20%] and ICS/LABA [16%]) or B (ICS/LAMA/LABA [32%] and ICS/LABA [14%]). A retrospective study from the USA indicates that 25.5% of COPD patients were treated with triple therapy, and the majority of the patients had mild/moderate disease.15
Limited information is available regarding the real-life types and frequencies of medication used in the treatment of COPD patients in Latin America. Data from patients with prior COPD diagnosis in a primary care setting indicate that 64.7% used any bronchodilator, 37.6% used a corticosteroid, and 25.6% used a bronchodilator plus corticosteroid.9,10 LA-BDs appeared to be underused as regular maintenance therapy (<30%), while 79% of patients were using a corticosteroid despite not having airway obstruction or exacerbation.9,10
The results of this study are consistent with those reported in other populations showing the underuse of LA-BD monotherapy (LAMA or LABA) and dual bronchodilatation (LAMA/LABA) as maintenance therapies in COPD patients, as well as the overuse of ICS/LAMA/LABA and ICS/LABA combinations in patients in GOLD groups A and B. Regarding the prescribing pattern of ICSs among the countries participating in the study, in six out of seven countries (Argentina, Chile, Guatemala, Mexico, Colombia, and Uruguay) ICSs were used in more than 55% of the patients; only Costa Rica had a low use of ICSs (28%). On the other hand, the countries with the highest use of SA-BDs were Colombia (31%) and Guatemala (19%), and those with the highest use of LA-BDs (without ICS) were Costa Rica (72%) followed by Argentina (25%) and Chile (23%). This suggests that a substantial proportion of patients are not treated with the most appropriate medication according to GOLD recommendations or local guidelines.1,2,22 These findings can be explained by a number of factors, one of which is the low implementation of or low physicians’ adherence to the current COPD guidelines around the world.18–21,23–25 Another factor that may help to explain the differences from the prescribing pattern previously reported in primary care physicians from Latin America,9,10 as well as the high prescription of triple therapy found in the present study, is the sites selected for the study, which were all COPD patients’ centers attended by specialist physicians, who have a greater tendency to prescribe this type of therapy. It is also possible that other factors, not assessed in the present study (such as variations in the characteristics of the local health care systems, medical coverage for respiratory medications, accessibility to medication, and reimbursement or regulatory policies), may contribute to the current COPD treatment practice and explain in part the differences observed among the countries.
The main indication for the use of ICS combination therapy in COPD patients is two or more exacerbations or one hospitalization due to an exacerbation in the past year (GOLD 2013 groups C and D). In addition, the use of ICS has been recommended for patients with the overlap phenotype (asthma plus COPD).26
Analysis from a UK primary care setting showed that 53.7% of the COPD patients without a concomitant diagnosis of asthma were receiving an ICS, as well as 49% and 64% of the patients who had experienced no exacerbations or one exacerbation in the previous year, respectively.4 Similarly, a study involving a primary care population from Latin America reported that 70.4% of patients with a correct prior diagnosis of COPD were receiving a corticosteroid despite not having experienced any exacerbations in the past year.10 In the present study, we also found that more than 90% of the COPD patients using any ICS combination did not have a prior medical diagnosis of asthma, and more than 35% and 75% did not have ambulatory exacerbation or exacerbations requiring hospitalization, respectively. These data allow us to conclude that there is an overuse of ICSs in patients with COPD in the Latin American region. Difficulties in distinguishing between asthma and COPD in adults with airways disease, or in establishing when these two conditions coexist (asthma and COPD overlap), could play an important role in the ICS prescribing pattern.27
Several studies have reported frequent suboptimal adherence to inhaled medication in COPD patients.28–30 Limited information exists regarding adherence according to the different types of medication in these patients. However, a study using pharmacy records found that adherence to medications was poor, with 19.8% of patients adherent to ICS, 30.6% adherent to LABA, and 25.6% adherent to ipratropium.30 A separate study analyzing pharmacy records showed that 54% of patients prescribed a LABA were adherent to therapy while only 40% were adherent to an ICS.28 Data from the Copenhagen General Population Study found that adherence varied from 29% to 56% across COPD GOLD stages 1–4 for ICS/LABA therapy, from 51% to 68% for LAMA monotherapy, and from 25% to 62% for LABA monotherapy.29
A study using a PHARMO database showed that the persistence rates with initial therapy for COPD at 1, 2, and 3 years were 25%, 14%, and 8%, respectively, for LAMA therapy; 21%, 10%, and 6% for LABA therapy; and 27%, 14%, and 8% for ICS/LABA fixed-dose combination therapy.31 Another study reported that ~37% of new users of tiotropium continued treatment for 1 year compared with only 14% for ipratropium, 13% for LABA, and 17% for ICS/LABA.32 In contrast, a large database study from Spain found higher levels of adherence: around 50% of patients newly initiated with LAMA therapy were persisting with their treatment after 9 months.33
To our knowledge, no previous study has evaluated adherence to different inhaled medications using patients’ self-report methods in a selected COPD population. The results of the present study are in line with previous studies showing improved adherence with the use of LA-BDs compared with SA-BD or ICS monotherapy regimens. In addition, improved adherence to LAMA, LABA, or LABA/LAMA treatment regimens compared with ICS/LABA was observed. The well-documented superiority of LA-BDs for improving lung function and quality of life, and reducing exacerbations and hospital admissions compared with SA-BDs, together with the potential advantages of newer, easier-to-use devices, could help to explain the greater adherence to LA-BDs regimens.34,35 That said, the adherence to ICS therapy could be lower than that with LA-BDs because of the lack of a direct symptom-relieving effect of the corticosteroids.
Limitations
This study has some limitations that should be mentioned. Medication adherence was only assessed using self-reported measurements, and self-reported use frequently leads to an overestimation of medication use. Although the study included a large number of COPD patients from seven countries, it cannot be concluded that the sample is representative of the entire COPD population attended by specialist doctors in Latin America; however, the sample included patients with different degrees of severity and may provide a valid estimation of patients’ characteristics from this region. Finally, since this was a cross-sectional study, it was not possible to analyze prospectively the type of medication used and adherence according to the different types of medication over time.
Conclusion
Our results indicate that the management of COPD patients attended by specialist doctors in Latin America does not usually follow GOLD or local recommendations, particularly regarding the use of LA-BDs, ICS/LABA, and triple therapy in patients with low exacerbation risk or without a prior diagnosis of asthma. Treatment regimens that include LA-BDs are associated with the highest adherence. Further efforts are needed to improve our knowledge on COPD management in specialist practice in the Latin American region and to ensure that COPD patients have access to the most appropriate medication regimens.
Supplementary materials
Pattern of use of respiratory medication, either as-needed or as regular maintenance therapy.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Type of medication used according to prior medical diagnosis of asthma.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
History of exacerbations (ambulatory or requiring hospitalization) in the past year.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Table S1.
Approval committees in the various countries involved in the LASSYC study
| Country | Ethics committee |
|---|---|
| Argentina | IRB: Iniciativa y Reflexión Bioética |
| Mexico | LEC: Comité de Etica en Investigacion del Hospital Dr Angel Leaño, Comité de Etica |
| del Hospital General del Estado de Sonora, Comité de Ética del Hospital Clínico de la Fuerza Aérea de Chile | |
| IRB: Comite de Investigacion del Hospital Dr Angel Leaño | |
| Comité de Ética en Investigación de México Centre for Clinical Research SA de CV | |
| Comité Etica del Hospital General del Estado de Sonora | |
| Institudo Jalisciense de Investigacion Clinica SA | |
| Winsett Rethman SA de CV | |
| Comite de Etica en Investigacion de Mexico, Centre for Clinical Research, SA de CV | |
| Instituto Jaliscience de Investigacion Clinica SA de CV (IJICSA) | |
| Comité de Etica en Investigacion | |
| Comité de Investigacion del Hospital Angeles de Las Lomas | |
| Chile | Comité de Etica del Servicio de Salud Metropolitano Sur Oriente |
| Comité Etico Científico del Servicio de Salud Metropolitano Oriente (RC) | |
| Comité de ética del Hospital Clínico de la Fuerza Aérea de Chile | |
| Colombia | Comité de Etica en la Investigacion CAIMED |
| Comité de Etica en Investigación Fundación Neumológica Colombiana (CEI-FNC) | |
| Comité Corporativo de Etica (CCEI) | |
| Costa Rica | Comité ético Científico UCIMED |
| Guatemala | Comité de Ética Independiente Zugueme |
Abbreviations: LASSYC, Latin American Study of 24-hour Symptoms in Chronic Obstructive Pulmonary Disease; IRB, institutional review board; LEC, local ethics committee.
Acknowledgments
We would like to acknowledge all members of the LASSYC team of Argentina, Brazil, Colombia, Mexico, Uruguay, Chile, Costa Rica, Venezuela, and Spain: Alejandro Casas (Colombia), Maria Montes de Oca (Venezuela), Ana Menezes and Fernando C Wehrmeister (Brazil), Maria Victorina Lopez Varela (Uruguay), Luis Ugalde (Costa Rica), Alejandra Ramirez-Venegas (México), Laura Mendoza (Chile), Ana López (Argentina), Larissa Ramirez (Costa Rica), and Marc Miravitlles (Spain).
This observational study was funded by AstraZeneca Latin America. Editorial support was provided by Dr Ian Wright, Wright Medical Communications Ltd, and funded by AstraZeneca. AstraZeneca had no input into the study design, analysis, or interpretation of the results.
Footnotes
Author contributions
For all data, all authors confirm that they have personally reviewed the data, understand the statistical methods employed for the analyses, and have an understanding of these analyses, that the methods are clearly described, and that they are a fair way to report the results. AC, MMO, AM, FCW, MVLV, LM, LR, and MM contributed substantially to the study design, data collection, interpretation, and the writing of the manuscript. AM and FCW performed the data analysis while all authors were involved with data interpretation. All authors approved the final version of the manuscript and agreed to its submission to the International Journal of Chronic Obstructive Pulmonary Disease.
Disclosure
AM has been paid for her work as a statistician for the LASSYC study. LR is an employee of AstraZeneca. The other authors report no conflicts of interest in this work.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Montes de Oca M, López Varela MV, Laucho-Contreras ME, et al. Classification of patients with chronic obstructive pulmonary disease according to the Latin American Thoracic Association (ALAT) staging systems and the global initiative for chronic obstructive pulmonary disease (GOLD) Arch Bronconeumol. 2017;53(3):98–106. doi: 10.1016/j.arbres.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Miravitlles M, Soler-Cataluña JJ, Calle M, et al. Spanish COPD guidelines (GesEPOC) 2017 Pharmacological treatment of stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2017;53(6):324–335. doi: 10.1016/j.arbres.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Price D, West D, Brusselle G, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–904. doi: 10.2147/COPD.S62750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Make B, Dutro MP, Paulose-Ram R, Marton JP, Mapel DW. Under-treatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. doi: 10.2147/COPD.S27032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrecheguren M, Monteagudo M, Ferrer J, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi: 10.1016/j.rmed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Menezes AM, Perez-Padilla R, Jardim JR, et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet. 2005;366(9500):1875–1881. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 8.Lopez Varela MV, Muiño A, Pérez Padilla R, et al. Treatment of chronic obstructive pulmonary disease in 5 Latin American cities: the PLATINO study. Arch Bronconeumol. 2008;44(2):58–64. doi: 10.1016/s1579-2129(08)60016-6. [DOI] [PubMed] [Google Scholar]
- 9.Montes de Oca M, Lopez Varela MV, Jardim J, Stirvulov R, Surmont F. Bronchodilator treatment for COPD in primary care of four Latin America countries: the multinational, cross-sectional, non-interventional PUMA study. Pulmon Pharmacol Ther. 2016;38:10–16. doi: 10.1016/j.pupt.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Jardim JR, Stirbulov R, Moreno D, Zabert G, Lopez-Varela MV, Montes de Oca M. Respiratory medication use in primary care among COPD subjects in four Latin American countries. Int J Tuberc Lung Dis. 2017;21(4):458–465. doi: 10.5588/ijtld.16.0633. [DOI] [PubMed] [Google Scholar]
- 11.Montes de Oca M, Menezes A, Wehrmeister FC, et al. Adherence to inhaled therapies of COPD patients from seven Latin American countries: the LASSYC study. PLoS One. 2017;12:e0186777. doi: 10.1371/journal.pone.0186777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miravitlles M, Menezes A, López Varela MV, et al. Prevalence and impact of respiratory symptoms in a population of patients with COPD in Latin America: the LASSYC observational study. Respir Med. 2018;134(1):62–69. doi: 10.1016/j.rmed.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 14.Plaza V, Fernandez-Rodriguez C, Melero C, et al. Validation of the “Test of the Adherence to Inhalers” (TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi: 10.1089/jamp.2015.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2016;12:73–83. doi: 10.2147/COPD.S122013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgel PR, Deslée G, Jebrak G, et al. Real-life use of inhaled corticosteroids in COPD patients versus the GOLD proposals: a paradigm shift in GOLD 2011? Eur Respir J. 2014;43(4):1201–1203. doi: 10.1183/09031936.00162313. [DOI] [PubMed] [Google Scholar]
- 17.Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi: 10.2147/COPD.S91694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White P, Thornton H, Pinnock H, Georgopoulou S, Booth HP. Overtreatment of COPD with inhaled corticosteroids – implications for safety and costs: cross-sectional observational study. PLoS One. 2013;8(10):e75221. doi: 10.1371/journal.pone.0075221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franssen FM, Spruit MA, Wouters EF. Determinants of polypharmacy and compliance with GOLD guidelines in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:493–501. doi: 10.2147/COPD.S24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sen E, Guclu SZ, Kibar I, et al. Adherence to GOLD guideline treatment recommendations among pulmonologists in Turkey. Int J Chron Obstruct Pulmon Dis. 2015;10:2657–2663. doi: 10.2147/COPD.S85324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turan O, Emre JC, Deniz S, Baysak A, Turan PA, Mirici A. Adherence to current COPD guidelines in Turkey. Expert Opin Pharmacother. 2016;17(2):153–158. doi: 10.1517/14656566.2016.1115482. [DOI] [PubMed] [Google Scholar]
- 22.Montes de Oca M, López Varela MV, Acuña A, et al. ALAT-2014 Chronic Obstructive Pulmonary Disease (COPD) Clinical Practice Guidelines: questions and answers. Arch Bronconeumol. 2015;51(8):403–416. doi: 10.1016/j.arbres.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Asche CV, Leader S, Plauschinat C, et al. Adherence to current guidelines for chronic obstructive pulmonary disease (COPD) among patients treated with combination of long-acting bronchodilators or inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis. 2012;7:201–209. doi: 10.2147/COPD.S25805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–19. doi: 10.1155/2008/173904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrado A, Rossi A. How far is real life from COPD therapy guidelines? An Italian observational study. Respir Med. 2012;106(7):989–997. doi: 10.1016/j.rmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Sin DD, Miravitlles M, Mannino DM, et al. What is asthma–COPD overlap syndrome (ACOS)? Towards a consensus definition from a roundtable discussion. Eur Respir J. 2016;48(3):664–673. doi: 10.1183/13993003.00436-2016. [DOI] [PubMed] [Google Scholar]
- 27.Plaza V, Álvarez F, Calle M, et al. Consensus on the Asthma-COPD Overlap Syndrome (ACOS) Between the Spanish COPD Guidelines (GesEPOC) and the Spanish Guidelines on the Management of Asthma (GEMA) Arch Bronconeumol. 2017;53(8):443–449. doi: 10.1016/j.arbres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Cecere LM, Slatore CG, Uman JE, et al. Adherence to long-acting inhaled therapies among patients with chronic obstructive pulmonary disease (COPD) COPD. 2012;9(3):251–258. doi: 10.3109/15412555.2011.650241. [DOI] [PubMed] [Google Scholar]
- 29.Ingebrigtsen TS, Marott JL, Nordestgaard BG, et al. Low use and adherence to maintenance medication in chronic obstructive pulmonary disease in the general population. J Gen Intern Med. 2015;30(1):51–59. doi: 10.1007/s11606-014-3029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huetsch JC, Uman JE, Udris EM, Au DH. Predictors of adherence to inhaled medications among veterans with COPD. J Gen Intern Med. 2012;27(11):1506–1512. doi: 10.1007/s11606-012-2130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Penning-van Beest F, van Herk-Sukel M, Gale R, Lammers JW, Herings R. Three-year dispensing patterns with long-acting inhaled drugs in COPD: a database analysis. Respir Med. 2011;105(2):259–265. doi: 10.1016/j.rmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Breekveldt-Postma NS, Koerselman J, Erkens JA, Lammers JW, Herings RM. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med. 2007;101(7):1398–1405. doi: 10.1016/j.rmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Monteagudo M, Roset M, Rodriguez-Blanco T, Muñoz L, Miravitlles M. Characteristics of COPD patients initiating treatment with aclidinium or tiotropium in primary care in Catalonia: a population-based study. Int J Chron Obstruct Pulmon Dis. 2017;12:1145–1152. doi: 10.2147/COPD.S131016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheyne L, Irvin-Sellers MJ, White J. Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;9:CD009552. doi: 10.1002/14651858.CD009552.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Appleton S, Poole P, Smith B, Veale A, Lasserson TJ, Chan MM. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;3:CD001104. doi: 10.1002/14651858.CD001104.pub2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pattern of use of respiratory medication, either as-needed or as regular maintenance therapy.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Type of medication used according to prior medical diagnosis of asthma.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
History of exacerbations (ambulatory or requiring hospitalization) in the past year.
Abbreviations: SABA, short-acting β-agonist; SAMA, short-acting muscarinic antagonist; LABA, long-acting β-agonist; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid.
Table S1.
Approval committees in the various countries involved in the LASSYC study
| Country | Ethics committee |
|---|---|
| Argentina | IRB: Iniciativa y Reflexión Bioética |
| Mexico | LEC: Comité de Etica en Investigacion del Hospital Dr Angel Leaño, Comité de Etica |
| del Hospital General del Estado de Sonora, Comité de Ética del Hospital Clínico de la Fuerza Aérea de Chile | |
| IRB: Comite de Investigacion del Hospital Dr Angel Leaño | |
| Comité de Ética en Investigación de México Centre for Clinical Research SA de CV | |
| Comité Etica del Hospital General del Estado de Sonora | |
| Institudo Jalisciense de Investigacion Clinica SA | |
| Winsett Rethman SA de CV | |
| Comite de Etica en Investigacion de Mexico, Centre for Clinical Research, SA de CV | |
| Instituto Jaliscience de Investigacion Clinica SA de CV (IJICSA) | |
| Comité de Etica en Investigacion | |
| Comité de Investigacion del Hospital Angeles de Las Lomas | |
| Chile | Comité de Etica del Servicio de Salud Metropolitano Sur Oriente |
| Comité Etico Científico del Servicio de Salud Metropolitano Oriente (RC) | |
| Comité de ética del Hospital Clínico de la Fuerza Aérea de Chile | |
| Colombia | Comité de Etica en la Investigacion CAIMED |
| Comité de Etica en Investigación Fundación Neumológica Colombiana (CEI-FNC) | |
| Comité Corporativo de Etica (CCEI) | |
| Costa Rica | Comité ético Científico UCIMED |
| Guatemala | Comité de Ética Independiente Zugueme |
Abbreviations: LASSYC, Latin American Study of 24-hour Symptoms in Chronic Obstructive Pulmonary Disease; IRB, institutional review board; LEC, local ethics committee.