Abstract
Background
The aim of the study was to confirm the hepatotoxicity induced by small-sized gold nanoparticles (GNPs) and evaluate the role of quercetin (Qur) and arginine (Arg) against hepatotoxicity caused by GNPs.
Methods
Twenty-five healthy male Wistar-Kyoto rats were used. GNPs were administered intraperitoneally to these rats at the dose of 50 μL for seven consecutive days. The role of Qur and Arg antioxidants against toxicity induced by GNPs was detected through the measurement of serum liver function and oxidative stress biomarkers in the liver tissues.
Results
Coadministration of Qur and Arg along with GNPs significantly induced dramatic alterations in the biochemical parameters. Levels of malondialdehyde, gamma-glutamyl transferase, alanine aminotransferase, alkaline phosphatase, and total protein increased significantly in the GNPs injected group than in the control group, while reduced glutathione was greatly reduced in the GNPs group than in the control group. It also significantly decreased liver enzymes and the oxidative stress, therefore improving the liver damage and hepatotoxicity induced by GNPs.
Conclusion
This study demonstrated that Qur and Arg antioxidants effectively improved the hepatic oxidative damage induced by GNPs. It also substantiates the application of Qur and Arg as protecting stand-in against GNPs’ hepatotoxicity.
Keywords: antioxidants, oxidative stress, gold nanoparticles, hepatotoxicity, quercetin, arginine, rats
Introduction
Nanoparticles (NPs) have novel properties, and thus, can offer great possibilities for the environment and human diseases. Toxicological studies indicate that small-sized gold NPs (GNPs) have harmful effects on the health of humans and living species in the environment.1–4 It has been reported that exposure to smaller sized GNPs produced more inflammatory and cytotoxic reactions when compared with exposure to larger sized GNPs of same mass concentration due to their highly reactive role, surface area, and number of NPs within the biological tissues.1–4
The exposure to small-sized GNPs induces inflammatory response, venous intimal disturbance, fatty change, and Kupffer–Browicz cells’ hypergenesis. The histological alterations induced in hepatocytes were mainly hydropic degeneration, cytoplasmic vacuolation, and necrosis.1–3 Moreover, cardiac tissue damage, pneumonia, fibrosis, and chronic inflammation depend on the size and duration of exposure.5,6
The toxicity induced by GNPs was due to the changes in the physicochemical properties such as length, form, chemical make-up, combination, high specific surface area, and solubility.7–9 The pathogenic mechanisms initiated by GNPs are dominated by inflammation in liver, kidney, heart, and lung tissues, oxidative stress, and DNA damage.
Quercetin (Qur) is a known antioxidant and anti- inflammatory/antiallergy agent, which inhibits the release of histamine and other inflammatory mediators in the body.10 In contrast, arginine (Arg) has various metabolic and immunologic effects,11,12 Arg can also act as a substance for polyamine production, which is reported to be taking part in protein production enrichment.13
It has been reported that treatment with either Qur or Arg as a protective agent may be effective against DNA, liver, and kidney damage caused by zinc oxide NPs.14 However, studies dealing with preventing the toxicity by inducing small-sized GNPs have not been recorded and acknowledged. Thus, the objective of this study is to confirm the hepatotoxicity induced by GNPs and to evaluate the function of Qur and Arg as defensive proxies against the hepatotoxicity in rats.
Methods
Animals
Twenty-five 12-week-old male Wistar-Kyoto rats weighing 220–240 g were obtained from the animal house (College of Pharmacy, KSU, Riyadh, Saudi Arabia). These rats were housed in cages under normal conditions (22°C±5°C temperature, 55%±5% humidity, 12-h light/dark cycle) with free access to water and chow diet. All procedures related to animal care and treatments strictly adhered to the ethical procedures and policies approved by King Saud University Local Animal Care and Use Committee.
GNPs and dosing
A dose of 50 μL (0.25 mL/kg/day) of 10 nm GNPs (MKN-Au-010; M K Impex Corp, MKnano, Mississauga, ON, Canada) of spherical morphology shape determined by the scanning electron microscope (SEM) was administered intraperitoneally to the rats. Each dose of Qur and Arg was 200 mg/kg/day; the doses were determined according to the previous studies.14,15
Experimental design
Rats were allowed to acclimatize for a period of 1 week; before the commencement of dose administration, rats were fasted for 24 h and divided into four groups: group 1 (G1), normal healthy rats (n=10); group 2 (G2), GNPs intoxicated rats were left untreated (n=5), 50 μL of GNPs were administered intraperitoneally to the rats for seven consecutive days; group 3 (G3), GNPs intoxicated rats were treated with 200 mg/kg/day of Qur for seven consecutive days; and group 4 (G4), GNPs intoxicated rats were treated with 200 mg/kg/day of Arg for seven consecutive days.
Blood sampling and tissue preparation
After 24 h of the final dose injection, all rats were denied food for 12–14 h and thereafter euthanized, and blood samples from each rat were collected into sterilized tubes for serum separation. Centrifugation was carried out at 3,000 rpm for 10 min to separate the serum, and the serum was conserved at a temperature of −80°C for various biochemical assessments. The midline incision technique was used to harvest and collect liver tissues; they were rinsed with freezing isosmotic saline, blended, and frozen at a temperature of −80°C for biochemical tissue analyses (such as the estimations of reduced glutathione [GSH] and malondialdehyde [MDA] contents).
Serum liver function markers
A biochemical auto analyzer (model 7170; Hitachi Ltd., Tokyo, Japan) was used to determine alkaline phosphatase (ALP; Figure 1), alanine aminotransferase (ALT; Figure 2), gamma-glutamyl transferase (GGT; Figure 3), and total protein levels (Figure 4) in serum.
Figure 1.
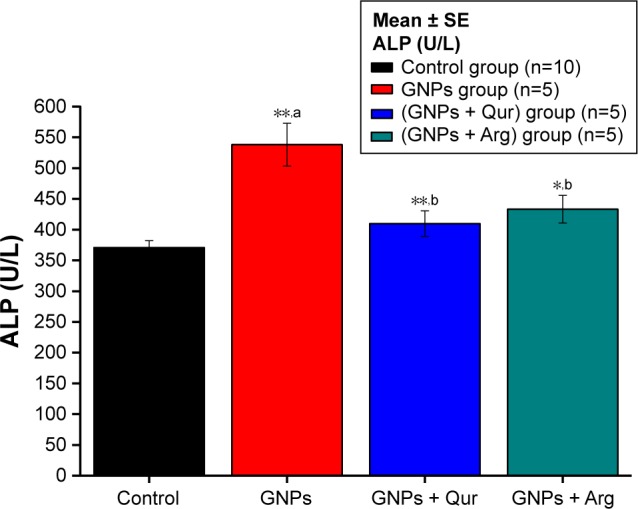
Effect of GNPs on ALP level in rats.
Notes: Image indicates a disturbance in the activity of liver ALP and a significant (P<0.05) elevation by 538.17±34.71 U/L in the GNPs group as compared with 370.67±11.17 U/L in the normal control group, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly reduced the ALP activity to 405.33±22.69 and 420±188.53 U/L, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. *P<0.05; **P<0.01.
Abbreviations: ALP, alkaline phosphatase; Arg, arginine; GNPs, gold nanoparticles; Qur, quercetin.
Figure 2.
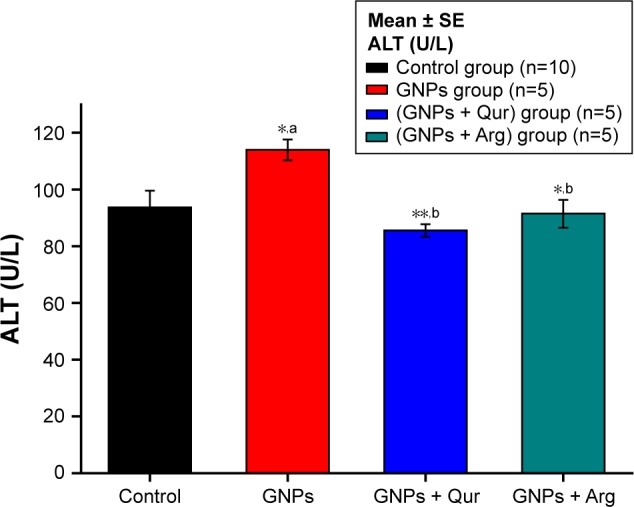
Effect of GNPs on ALT level in rats.
Notes: Image shows a disturbance in liver ALT and a significant (P<0.05) elevation by 114±3.71 U/L in the GNPs group as compared with 93.70±5.88 U/L in the normal control group, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly reduced the ALT activity to 85.57±2.20 and 91.5±4.91 U/L, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. *P<0.05; **P<0.01.
Abbreviations: ALT, alanine aminotransferase; Arg, arginine; GNPs, gold nanoparticles; Qur, quercetin.
Figure 3.
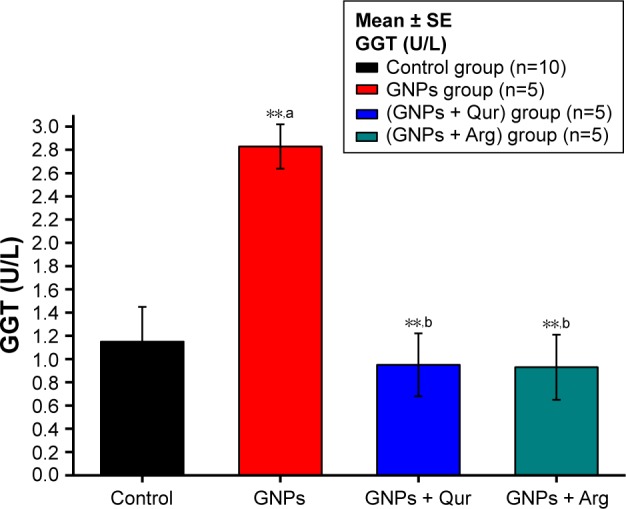
Effect of GNPs on GGT level in rats.
Notes: Image shows a disturbance in liver GGT and a significant (P<0.05) elevation by 2.83±0.18 U/L in the GNPs group as compared with 1.15±0.30 U/L in the normal control group, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly reduced the GGT activity to 0.92±0.27 and 86.5±4.91 U/L, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. **P<0.01.
Abbreviations: Arg, arginine; GGT, gamma-glutamyl transferase; GNPs, gold nanoparticles; Qur, quercetin.
Figure 4.
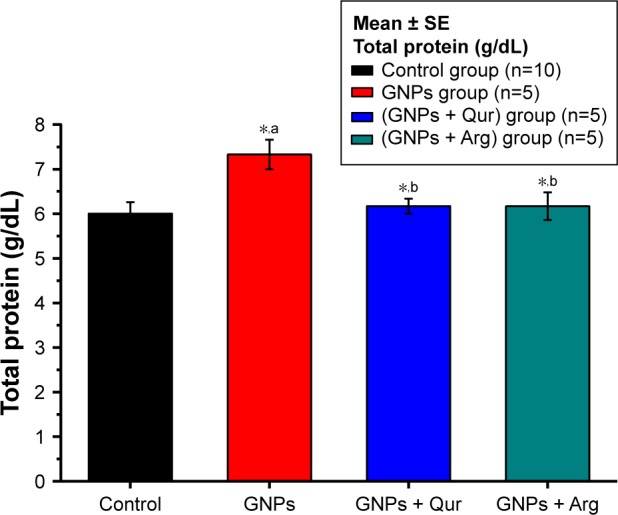
Effect of GNPs on total protein level in rats.
Notes: Image shows alterations in liver total protein, where a significant (P<0.05) elevation by 7.33±0.33 g/dL in the GNPs group as compared with 6.0±0.25 g/dL in the control group was observed, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly reduced the total protein activity to 6.15±0.27 and 6.17±4.91 g/dL, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. *P<0.05.
Abbreviations: Arg, arginine; GNPs, gold nanoparticles; Qur, quercetin.
Oxidative stress biomarkers
Determination of GSH level
The measurement of GSH level (μg/mL; Figure 5) in liver tissues is carried out enzymatically according to the modified procedure of the previous study.16
Figure 5.
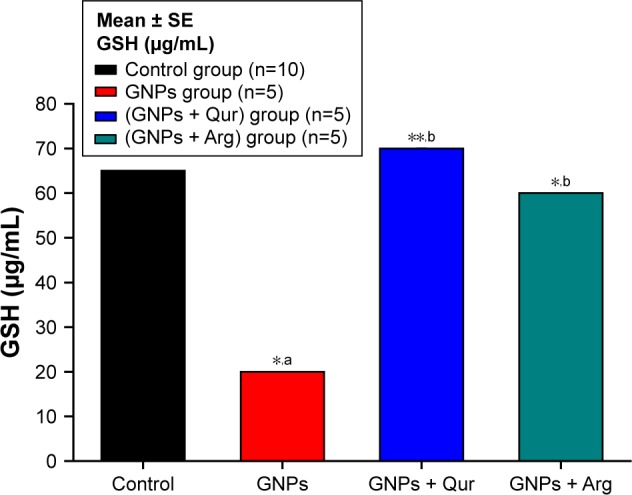
Effect of GNPs on liver GSH level in rats.
Notes: Image shows oxidative stress alterations in GSH liver homogenate level, where a significant (P<0.05) reduction by 20±0.01 μg/mL in the GNPs group as compared with 65±0.01 μg/mL in the normal control group was observed, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly elevated the GSH to 68.12±0.27 and 60.17±4.91 μg/mL, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. *P<0.05; **P<0.01.
Abbreviations: Arg, arginine; GNPs, gold nanoparticles; GSH, reduced glutathione; Qur, quercetin.
Determination of MDA
The index of fatty acid peroxidation and the level of MDA (μmol/mL; Figure 6) in hepatocytes of rats were determined using a spectrophotometer as defined in the previous study.17
Figure 6.
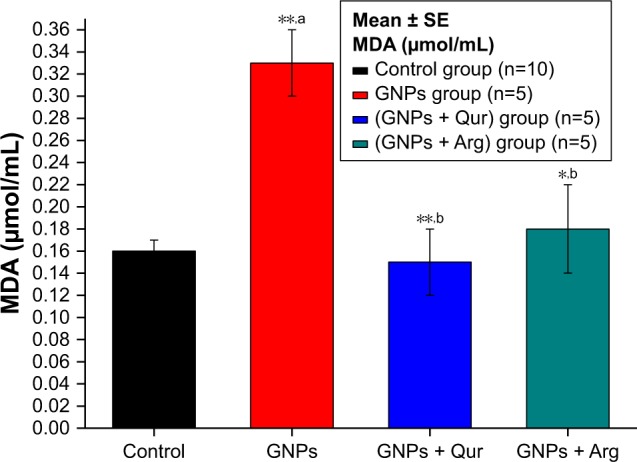
Effect of GNPs on liver MDA level in rats.
Notes: Image shows the level of MDA in rat liver. MDA level increased significantly (P<0.05) by 0.33±0.03 μmol/mL in the GNPs group as compared with 0.16±0.01 μmol/mL in the control group, while the coadministration of Qur (G3) and Arg (G4) with GNPs significantly reduced the lipid peroxidation MDA activity to 0.15±0.03 and 0.18±0.02 μmol/mL, respectively. The data of groups G3 and G4 were statistically significant compared with the data of group G2. aCompared with control group; bcompared with GNPs group. *P<0.05; **P<0.01.
Abbreviations: Arg, arginine; GNPs, gold nanoparticles; MDA, malondialdehyde; Qur, quercetin.
Statistical analysis
Results were expressed as mean ± standard error. Statistical analysis was carried out by means of one-way analysis of variance (ANOVA): 1) compared with control group and 2) compared with GNPs group (*P<0.05 and **P<0.01).
Results and discussion
The present study confirmed that GNPs trigger liver tissue destruction as demonstrated by the marked rise of serum liver markers in GNPs intoxicated rats as compared with normal rats. These findings indicate that liver is the target organ for GNPs’ toxicity and destruction of useful cohesion of hepatocyte membranes. Our finding was supported by the group of researchers18–23 as they stated that liver destruction could be caused by the overdose of zinc oxide NPs administration. Coadministration of Qur and Arg to GNPs rats significantly reduced all the serum liver markers (P<0.01) as compared with the GNPs intoxicated rats. This may indicate that the Qur and Arg antioxidants act as protective agents against the liver dysfunction caused by hepatotoxicity of GNPs.
It has been reported that pathogenic mechanisms initiated by in vivo administration of GNPs are dominated by oxidative stress, apoptosis, and DNA damage.24,25 Thus, the protective mechanism reduces the generation of inflammatory mediators, ameliorates liver destruction, and improves organ failure. In our study, the injection of Qur or Arg immediately along with GNPs was beneficial in preventing the GNP-induced inflammatory liver injury. Our results were also consistent with those reported previously, in which Qur reduces the inflammation by inhibiting oxidative stress and cytokine production, indicating that Qur possesses anti-inflammatory effects in vivo.26,27 Moreover, Qur metabolites may act as antioxidants.27 In contrast, in other studies, it was found that Arg supplementation protects oxidative damage.18,19 The defense by Qur in contrast to the creation of oxidative DNA destruction caused by H2O2 was observed in vitro.20 Arg mitigates oxidative stress in the hepatocytes and brain tissues and has antioxidant activities, as well as reactive oxygen species (ROS)-scavenging properties.21 Coadministration with Arg was able to inhibit DNA fragmentation and increased wound DNA synthesis.22,23
It has been reported that 1.4 nm NPs react with DNA and caused strong toxicity near human tumors, since it is commonly reported that DNA double-strand disruptions may lead to cancer.1–3 NPs induced oxidative stress, and one of the likely means, which induce DNA destruction, may be fatty acid peroxidation and oxidative stress.26
ROS reacts with DNA particle leading to the destruction of purine and pyrimidine bases as well as DNA support.26 MDA is one of the main products of fatty acid peroxidation. It is a mutagenic and also a carcinogenic substance. It combines with DNA-forming compounds such as deoxyguanosine, deoxyadenosine, and deoxycytidine.27,28 DNA damage may trigger signal transduction pathways, giving rise to apoptosis or causing cell death.29 Coadministration of Qur and Arg to GNPs intoxicated rats effectively protected liver from lipid peroxidation and inflammatory damage. Our data were also consistent with the previously published study.29 Qur possesses anti-inflammatory, antioxidant, hepatoprotective, and anticarcinogenic activities.30
Conclusion
This study highlights the hepatotoxic effects induced by intraperitoneal administration of GNPs, which was confirmed by a significant increase in liver function markers, such as ALP, ALT, GGT, and total protein. In addition there was a significant elevation in the tissue lipid peroxidation biomarker MDA and a significant reduction in the oxidative stress biomarker GSH level. The treatment with either Qur or Arg successively alleviated ALP, ALT, GGT, total protein and MDA. While GSH increased, this in turn effectively ameliorated the lipid peroxidation and inflammatory liver damage induced in GNPs intoxicated rats. Our data demonstrated that Qur and Arg are highly potent antioxidants that have the ability to protect hepatocytes from damage, which is produced by oxidative stress and the associated vascular problems caused by the generation of GNPs, from the production of ROS. More research is required to assess the synergistic combination of Qur and Arg, which might be useful to completely inhibit the hepatotoxicity induced by GNPs.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project No RGP-285.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Abdelhalim MA, Jarrar BM. Histological alterations in the liver of rats induced by different gold nanoparticles size, dose and exposure duration. J Nanobiotechnology. 2012;10:5. doi: 10.1186/1477-3155-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelhalim MA, Jarrar BM. Renal tissue alterations were size-dependent with smaller ones induced more effects and related with time exposure of gold nanoparticles. Lipids Health Dis. 2011;10:163. doi: 10.1186/1476-511X-10-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelhalim MA, Jarrar BM. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011;10:166. doi: 10.1186/1476-511X-10-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nano level. Science. 2006;311(5761):622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 5.Abdelhalim MA. Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis. 2011;10:205. doi: 10.1186/1476-511X-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelhalim MA. Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids Health Dis. 2011;10:233. doi: 10.1186/1476-511X-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovern SB, Klaper R. Daphnia magna mortality when exposed to titanium dioxide and fullerene (C60) nanoparticles. Environ Toxicol Chem. 2006;25(4):1132–1137. doi: 10.1897/05-278r.1. [DOI] [PubMed] [Google Scholar]
- 8.Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol. 2007;4124(24):8484–8490. doi: 10.1021/es071445r. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhou J, Fan J, et al. Effect of rapamycin alone and in combination with sorafenib in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2008;14(16):5124–5130. doi: 10.1158/1078-0432.CCR-07-4774. [DOI] [PubMed] [Google Scholar]
- 10.Jung WJ, Sung MK. Effects of major dietary antioxidant on inflammatory markers of RAW 264.7 macrophages. Biofactors. 2004;21(1–4):113–117. doi: 10.1002/biof.552210122. [DOI] [PubMed] [Google Scholar]
- 11.Huang CC, Tsai SC, Lin WT. Potential ergogenic effects of Arg against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp Gerontol. 2008;43(6):571–577. doi: 10.1016/j.exger.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Ivanov V, Cha J, Ivanova S, et al. Essential nutrients suppress inflammation by modulating key inflammatory gene expression. Int J Mol Med. 2008;22(6):731–741. [PubMed] [Google Scholar]
- 13.Moinard C, Cynober L, de Bandt JP. Polyamines: metabolism and implications in human diseases. Clin Nutr. 2005;24(2):184–197. doi: 10.1016/j.clnu.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Faddah LM, Baky NA, Al-Rasheed NM, Al-Rasheed NM, Fatani AJ, Atteya M. Role of quercetin and arginine in ameliorating nano zinc oxide-induced nephrotoxicity in rats. BMC Complement Altern Med. 2012;12(1):1062. doi: 10.1186/1472-6882-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baky NA, Faddah LM, Al-Rasheed NM, Al-Rasheed NM, Shebali W. Role of quercetin and L-arginine in alleviating zinc oxide nanoparticle hepatotoxicity in rats. Chiang Mai J Sci. 2013;40(4):577–592. [Google Scholar]
- 16.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 17.Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118(1):29–32. [Google Scholar]
- 18.Gurr JR, Wang AS, Chen CH, Jan KY. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology. 2005;213(1–2):66–73. doi: 10.1016/j.tox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Collins AR, Dusinska M, Gedik CM, Stĕtina R. Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect. 1996;104(suppl 3):465. doi: 10.1289/ehp.96104s3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilms LC, Hollman PC, Boots AW, Kleinjans JC. Protection by qur and Qur-rich fruit juice against induction of oxidative DNA damage and formation of BPDE-DNA adducts in human lymphocytes. Mutat Res. 2005;582:155–162. doi: 10.1016/j.mrgentox.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Lass A, Suessenbacher A, Wolkart G, Mayer B, Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by Arg. Mol Pharmacol. 2002;61:1081–1088. doi: 10.1124/mol.61.5.1081. [DOI] [PubMed] [Google Scholar]
- 22.Piacenza L, Peluffo G, Radi R. Arg-dependent suppression of apoptosis in trypanosoma cruzi: contribution of the nitric oxide and polyamine path ways. Proc Natl Acad Sci U S A. 2001;98(13):7301–7306. doi: 10.1073/pnas.121520398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XJ, Chinkes DL, Wu Z, Herndon DN. Enternal Arg supplementation stimulates DNA synthesis in skin donor wound. Clin Nutr. 2011;30(3):391–396. doi: 10.1016/j.clnu.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S, Duffin R, Poland C, Daly P, Murphy F, Drost E. Efficacy of simple short-term in vitro assays for predicting the potential of metal oxide nanoparticles to cause pulmonary inflammation. Environ Health Perspect. 2009;117(2):241–247. doi: 10.1289/ehp.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong D, Fang T, Yu L, Sima X, Zhu W. Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage. Sci Total Environ. 2011;409(8):1444–1452. doi: 10.1016/j.scitotenv.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Marnett LJ. Oxy radicals, lipid peroxidation and DNA damage. Toxicology. 2002;181–182:219–222. doi: 10.1016/s0300-483x(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 28.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 29.Sharma V, Shukla RK, Saxena N, Parmar D, Das M, Dhawan A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett. 2009;185(3):211–218. doi: 10.1016/j.toxlet.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]


