Abstract
Rationale: Societal guideline recommendations vary with regard to the role of routine trans-thoracic echocardiography to screen for right ventricular strain in patients with hemodynamically stable acute pulmonary embolism.
Objective: To characterize national patterns in use of early trans-thoracic echocardiography for the evaluation of patients with hemodynamically stable acute pulmonary embolism and determine associations between trans-thoracic echocardiography use and patient outcomes.
Methods: Retrospective cohort study using Premier, Inc. database of approximately 20% of patients hospitalized in the United States with hemodynamically stable acute pulmonary embolism between 2008 and 2011. Multivariable, risk-adjusted hierarchical regression models were used to evaluate hospital variation in use of trans-thoracic echocardiography for pulmonary embolism and associations between hospital trans-thoracic echocardiography rates and patient outcomes. Patient-level trans-thoracic echocardiography exposure was used in sensitivity analyses.
Results: We identified 64,037 patients (mean age, 61.7 years; 54% women; 68% white) hospitalized at 363 U.S. hospitals. Trans-thoracic echocardiography rates for hemodynamically stable acute pulmonary embolism varied widely among hospitals (median trans-thoracic echocardiography rate, 41.4%; range, 0–89%; interquartile range, 32.7–51.7%). Hospital rates of trans-thoracic echocardiography were not associated with significant differences in risk-adjusted mortality (trans-thoracic echocardiography rate quartile 4 vs. quartile 1: odds ratio, 0.88; 95% confidence interval, 0.69–1.13) or use of thrombolytics (odds ratio, 1.28; 95% confidence interval, 0.84–1.96), but rates of intensive care unit admission (odds ratio, 1.57; 95% confidence interval, 1.18–2.07), hospital length of stay (relative risk, 1.08; 95% confidence interval, 1.03–1.15), and costs (relative risk, 1.15; 95% confidence interval, 1.07–1.23) were significantly higher at hospitals with high trans-thoracic echocardiography rates. Analyses of patient-level trans-thoracic echocardiography exposure produced similar results, except with higher rates of thrombolysis (odds ratio, 5.58; 95% confidence interval, 4.40–7.09) and bleeding (odds ratio, 1.37; 95% confidence interval, 1.24–1.51) among patients receiving trans-thoracic echocardiography.
Conclusions: Trans-thoracic echocardiography use in the evaluation of patients with hemodynamically stable acute pulmonary embolism varied widely between hospitals. Hospitals with high rates of pulmonary embolism–associated trans-thoracic echocardiography use did not achieve different patient mortality outcomes but had higher resource use and costs. Our findings support the 2016 American College of Chest Physicians guidelines for management of pulmonary embolism, which recommend selective, rather than routine, use of trans-thoracic echocardiography to risk stratify patients with hemodynamically stable pulmonary embolism.
Keywords: pulmonary embolism, echocardiogram, trans-thoracic echocardiography, intensive care unit, guideline
Pulmonary embolism (PE) accounts for more than 275,000 hospital admissions per year in the United States (1). In patients presenting with hemodynamically stable acute PE, treatment with systemic anticoagulants leads to case-fatality rates of approximately 2% (2–4). However, in the approximately 5% of patients with acute PE who develop hemodynamic instability, mortality is 20 to 50% (3, 5). Thus, research has focused on strategies to risk stratify patients with acute PE and guide use of potentially effective adjunctive therapies, such as thrombolytics.
Evidence of right ventricular (RV) strain measured from trans-thoracic echocardiography (TTE), RV characteristics on computed tomography (CT) scan, and cardiac biomarkers such as troponin and β-natriuretic peptide (BNP) enhance prediction of clinical deterioration during PE (2, 6–14). However, current guidelines do not clearly support use of adjunctive treatments (such as thrombolytics or catheter-based therapy) for patients with subclinical RV strain who may be at risk for hemodynamic decompensation after PE (4, 15, 16). For example, the American College of Chest Physicians guidelines for management of PE do not recommend routine use of adjunctive therapies (e.g., systemic lytic therapy or catheter-directed therapy) in hemodynamically stable patients with evidence of RV strain, reserving these interventions for “selected patients with acute PE who deteriorate after starting anticoagulant therapy but have yet to develop hypotension and who have a low bleeding risk” (4, 15). Because the presence of right heart strain does not have clear therapeutic implications, the most recent American College of Chest Physicians guidelines recommend against routine use of TTE or cardiac biomarkers to screen for RV strain or “intermediate-risk PE,” instead encouraging selected assessment of RV function in patients for whom there is clinical uncertainty about the need for adjunctive therapies or more intensive monitoring (4, 15). In contrast, the European Society of Cardiology guidelines for the management of PE recommend routine use of a validated clinical risk-assessment score such as a PE Severity Index in hemodynamically stable patients with acute PE and suggest that clinicians “consider further risk-stratification” with TTE, CT scan, or cardiac biomarkers for patients with intermediate or greater predicted risk (16). Similar to the American College of Chest Physicians guidelines, the European Society of Cardiology guidelines recommend against routine use of reperfusion therapies in patients with intermediate-risk PE, also reserving adjunctive therapy for patients with signs of decompensation despite systemic anticoagulation.
The lack of consensus regarding evaluation and management of PE may have implications for practice variation. Currently, little is known about practice patterns in the evaluation of right heart strain during acute PE in the United States. Furthermore, associations between evaluation strategies for acute PE, use of adjunctive therapy, and resource use during acute PE have not been well described. Hospital-level variation in PE management may have substantial ramifications for healthcare delivery, costs, and patient outcomes (17); thus, we sought to characterize risk-stratification practices for acute PE in the United States. We hypothesized that testing for RV strain was common and associated with increased resource use without observed outcome benefits in the evaluation of patients hospitalized with hemodynamically stable acute PE.
The results of this article were presented previously as an abstract (18).
Methods
Cohort
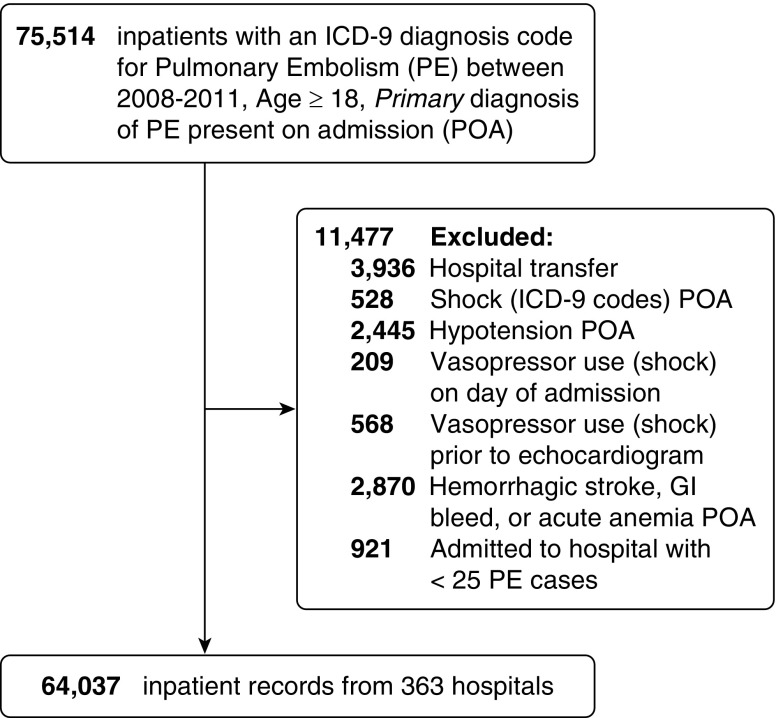
We performed a retrospective cohort study of patients hospitalized with acute, hemodynamically stable PE between 2008 and 2011 using the Premier Healthcare Database (Premier, Inc., Charlotte, NC) (19). We studied practice patterns before the 2012 publication of guideline recommendations regarding TTE in PE to attenuate potential unmeasured confounding by outcome improvements that may be associated with a hospital’s predilection to guideline adherence (20). Hospitals voluntarily submit data to Premier for quality improvement benchmarking, resulting in a nonrepresentative 20% sample of U.S. hospitalizations, with overrepresentation of southern states. Data undergo iterative quality check and include patient demographics, International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis and procedure codes, and a comprehensive log of laboratory, diagnostic studies, and pharmacy billing data (medication name, dates administered, and corresponding doses), as well as hospital characteristics. We identified adult patients (age ≥ 18 yr), hospitalized with a principal International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis of PE (see Table E1 in the online supplement), which was present on admission. To analyze practice patterns and outcomes among patients presenting with “hemodynamically stable” PE, we excluded patients who had International Classification of Diseases, Ninth Revision, Clinical Modification codes identifying shock or hypotension present on admission (Table E1) or who received vasopressors on the first hospital day or before echocardiography (i.e., we excluded “high-risk” PE). We also excluded patients with diagnoses present on admission that would be contraindications for use of thrombolytics, including intracranial hemorrhage, acute blood loss anemia, or gastrointestinal bleeding (Table E1). Finally, we excluded patients admitted as acute hospital–hospital transfer as well as patients admitted to hospitals with fewer than 25 PE hospitalizations over the study period, to increase the reliability of hospital-level analyses (Figure 1).
Figure 1.
Derivation of study cohort. GI = gastrointestinal; ICD-9 = International Classification of Diseases, Ninth Revision; PE = pulmonary embolism; POA = present on admission.
Exposures of Interest
We sought to characterize hospital-level variability in practice and outcomes associated with risk stratification strategies for acute PE. The primary analysis evaluated use of TTE after acute PE diagnosis. Although cardiac biomarkers (e.g., BNP, troponin) may provide evidence of RV strain and intermediate-risk PE, they are also often obtained on patient presentation as workup for undifferentiated chest pain and/or dyspnea before the diagnosis of acute PE, and also represent relatively low-cost interventions compared with TTE. To evaluate outcomes related to the use of TTE specifically obtained in the management of hemodynamically stable PE, we defined TTE use as any study conducted within the first 4 days of hospital admission, before use of any vasopressor medications. A cutoff of 4 days was chosen to capture patients who may have been admitted to hospitals in which nonurgent TTE is unavailable on weekends, including holiday weekends; TTEs obtained after 4 days were not included, to reduce potential bias from changing clinical status and/or indications.
Covariates and Data Abstraction
We abstracted hospital characteristics, patient demographics, medical comorbidities, and acute organ failures that were present on admission using previously validated International Classification of Diseases, Ninth Revision, Clinical Modification code algorithms (Table E1) (21, 22). We used procedure billing codes to identify patients who were admitted to an intensive care unit (ICU), had a TTE, or had cardiac troponin or BNP measured (Table E2). We used pharmacy charges to identify use of vasopressors or inotropic medications (Table E3) as well as use of thrombolytics (Table E4), excluding doses consistent with local use for central lines or chest tubes (e.g., alteplase 1 mg) (23). We identified clinically significant bleeding (not present on admission) on the basis of a previously validated International Classification of Diseases, Ninth Revision, Clinical Modification coding algorithm (Table E5) (24).
Outcomes
We analyzed the association between use of TTE and inpatient mortality, rate of ICU admission, use of systemic thrombolytic therapy, episodes of major bleeding, hospital length of stay, and total hospital costs.
Statistical Methods
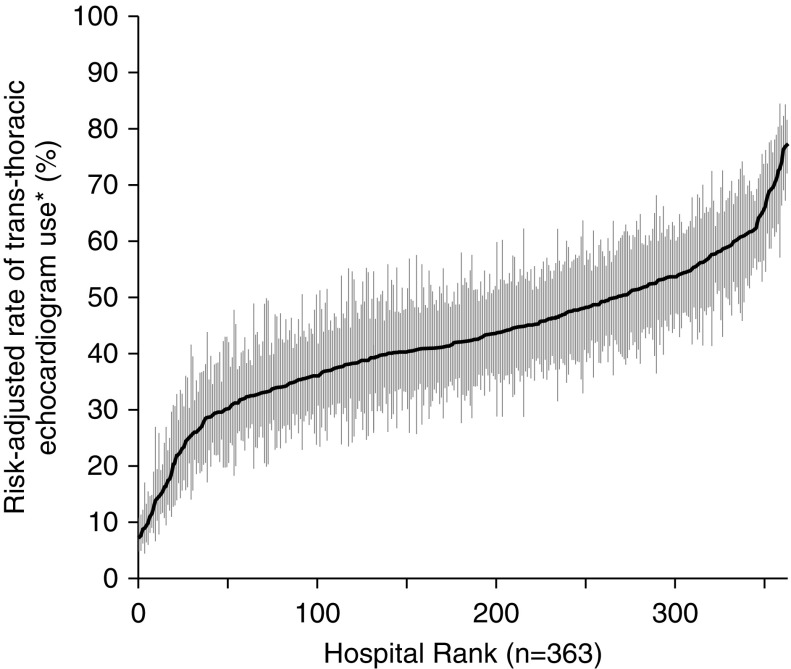
We used hierarchical logistic regression models with hospitals as random intercepts to obtain estimates for the associations of patient and hospital characteristics with the use of TTE during acute PE. Our model included 35 covariates capturing hospital characteristics, patient demographics and comorbidities, and acute organ failures (Table 1). We used the hospital-level random effects to calculate adjusted rates of TTE use for each hospital and generate a caterpillar plot to visually demonstrate hospital variation in evaluation of acute PE (Figure 2). We quantified the extent of interhospital variation in use of TTE for PE unexplained by measured patient and hospital characteristics using the median odds ratio derived from the hierarchical logistic regression model (25). The adjusted median odds ratio represents the median odds ratio of receiving a TTE as a result of practice pattern variation between identical patients at two randomly selected hospitals.
Table 1.
Patient and hospital characteristics of inpatients with hemodynamically stable acute pulmonary embolism, by trans-thoracic echocardiogram use (N = 64,037)
| No Trans-thoracic Echocardiography (n = 37,112) | Trans-thoracic Echocardiography (n = 26,925) | |
|---|---|---|
| Demographics | ||
| Age, yr | 61.1 ± 17.8 | 62.5 ± 17.0 |
| Race/ethnicity | ||
| White | 25,272 (68.1) | 1,8067 (67.1) |
| Black | 5,611 (15.1) | 4,158 (15.4) |
| Hispanic | 1,178 (3.2) | 959 (3.6) |
| Other | 5,051 (13.6) | 3,741 (13.9) |
| Female sex | 20,420 (55.0) | 14,358 (53.3) |
| Primary insurance payer | ||
| Medicare | 18,067 (48.7) | 13,499 (50.1) |
| Medicaid | 3,240 (8.7) | 2,055 (7.6) |
| Private insurance | 12,430 (33.5) | 9,187 (34.1) |
| Self-pay | 1,859 (5.0) | 1,101 (4.1) |
| Other | 1,516 (4.1) | 1,083 (4.0) |
| Hospital characteristics | ||
| No. of beds | 400.2 ± 227.8 | 407.9 ± 230.6 |
| Location | ||
| Rural | 4,204 (11.3) | 2,426 (9.0) |
| Urban | 32,908 (88.7) | 24,499 (91.0) |
| Teaching hospital | 13,670 (36.8) | 10,092 (37.5) |
| Geographic area | ||
| Northeast | 5,726 (15.4) | 6,110 (22.7) |
| Midwest | 7,707 (20.8) | 5,715 (21.2) |
| South | 16,292 (43.9) | 9,949 (37.0) |
| West | 7,387 (19.9) | 4,141 (19.1) |
| Comorbidities* | ||
| No. of comorbidities | 1.4 ± 1.2 | 1.5 ± 1.2 |
| Congestive heart failure | 1,460 (3.9) | 1,770 (6.6) |
| Atrial fibrillation | 3,080 (8.3) | 3,294 (12.2) |
| COPD | 1,913 (5.2) | 1,454 (5.4) |
| Chronic hypertension | 19,856 (53.5) | 15,936 (59.2) |
| Diabetes | 6,868 (18.5) | 5,886 (21.9) |
| Obesity | 5,660 (15.3) | 5,622 (20.9) |
| Coronary artery disease | 131 (0.4) | 88 (0.3) |
| Ischemic stroke | 244 (0.7) | 237 (0.9) |
| Solid tumor | 4,634 (12.5) | 2,290 (8.5) |
| Lymphoma | 291 (0.8) | 189 (0.7) |
| Metastatic cancer | 3,191 (8.6) | 1,501 (5.6) |
| Peripheral vascular disease | 1,004 (2.7) | 827 (3.1) |
| Rheumatologic disease | 1,031 (2.8) | 757 (2.8) |
| Dementia | 1,529 (4.1) | 1,207 (4.5) |
| Hemiplegia | 74 (0.2) | 53 (0.2) |
| Paralysis | 173 (0.5) | 98 (0.4) |
| Acute organ failures* | ||
| No. of acute organ failures | 0.3 ± 0.6 | 0.4 ± 0.7 |
| Respiratory failure | 2,135 (5.8) | 2,422 (9.0) |
| Renal failure | 1,462 (3.9) | 1,798 (6.7) |
| Neurological failure | 342 (0.9) | 316 (1.2) |
| Hematological failure | 1,401 (3.8) | 1,248 (4.6) |
| Hepatic failure | 49 (0.1) | 74 (0.3) |
| Metabolic failure | 278 (0.8) | 366 (1.4) |
| Electrolyte abnormalities | 5,452 (14.7) | 4,518 (16.8) |
| Acute myocardial infarction | 241 (0.7) | 407 (1.5) |
| Additional covariates | ||
| Attending specialty | ||
| Cardiology | 947 (2.6) | 1,187 (4.4) |
| Internal medicine | 24,523 (66.1) | 18,281 (67.9) |
| Medical oncology | 1,688 (4.6) | 544 (2.0) |
| Pulmonary–critical care | 2,276 (6.1) | 1,528 (5.7) |
| Surgery | 479 (1.3) | 243 (0.9) |
| Other | 7,199 (19.4) | 5,142 (19.1) |
| Year of hospitalization | ||
| 2008 | 8,413 (22.7) | 6,034 (22.4) |
| 2009 | 11,055 (29.8) | 7,747 (28.8) |
| 2010 | 11,589 (31.2) | 8,626 (32.0) |
| 2011 | 6,055 (16.3) | 4,518 (16.8) |
Definition of abbreviation: COPD = chronic obstructive pulmonary disease; SD = standard deviation.
Data presented as mean ± SD or n (%).
Present on hospital admission.
Figure 2.
Hospital variation in use of early trans-thoracic echocardiography (TTE) for the evaluation of hemodynamically stable acute pulmonary embolism. Early TTE was defined as occurring within 4 days of hospital admission. Point estimates represent the risk-adjusted rates of TTE use for individual hospitals, with error bars denoting 95% confidence intervals. *Risk-adjusted rate of early TTE use is based on patient demographic factors, comorbidities, and hospital characteristics.
Using similar hierarchical regression models, we also performed a mixed-level analysis of the associations between hospital rates of TTE use with patient-level outcomes (inpatient mortality, ICU admission, use of systemic thrombolytic therapy, occurrence of major bleeding, hospital length of stay, hospital costs) adjusting for patient- and hospital-level covariates. Using a hospital-level exposure variable in patient-level models reduces confounding by indication without being subject to the “ecological fallacy,” in which associations between exposures and outcomes occur at an ecological, but not individual, level (26, 27). We categorized hospitals into TTE-usage quartiles and compared risk-adjusted outcomes between quartiles to account for nonlinear associations between TTE rates and outcomes. We used hierarchical logistic regression for binary outcomes, negative binomial regression to model hospital length of stay, and linear regression with log transformation to model hospital costs. We chose to use hospital-level TTE rates as the ecological exposure variable in hierarchical regression models that produce average effect estimates (26, 27), rather than two-stage least squares regression approach often used for instrumental variables that produce marginal effect estimates, to maintain consistent effect measures across analyses of patient-level and hospital-level TTE exposure.
To evaluate consistency between hospital-level practices and patient-level exposures, we evaluated associations between patient-level exposure to TTE and patient outcomes. Because of the time-varying nature of TTE exposure and outcomes and risk of immortal person-time bias (28) from death before TTE, we used Cox proportional hazards models with TTE as a time-varying exposure to evaluate the association between patient-level use of TTE and mortality, ICU admission, and use of thrombolytic therapy. In patients who received TTE, ICU admission and use of a thrombolytic were defined as occurring on or after the day of TTE. Robust sandwich estimation was used to account for clustering by hospital. Similar to hospital-level TTE exposure models, we used a mixed-effects negative binomial regression model to evaluate hospital length of stay and mixed-effects linear regression to evaluate log-transformed hospital costs.
All study procedures were performed using SAS version 9.4 (SAS Institute). This study was deemed nonhuman subjects research by the Boston University Medical Center Institutional Review Board (#H-31821).
Sensitivity Analyses
To attenuate bias by potentially changing indication for TTE after admission for acute PE and to address the possibility that TTE may offer the most potential benefit when performed within 24 hours of admission, we performed a sensitivity analysis examining hospital-level and patient-level outcomes on the basis of use of TTE limited to hospital Day 0 or 1.
In a second sensitivity analysis, we evaluated both ICU and ICU step-down admissions as outcomes. In a third analysis designed to evaluate ICU admissions that occurred after TTE with greater certainty, we examined ICU admission outcomes occurring only on or after hospital Day 2, with hospital-level use of TTE on Day 0 or 1.
Finally, to evaluate associations between general evaluation for RV strain and patient outcomes, we repeated our analyses incorporating a modified “combined RV strain workup” exposure variable, defined as any use of cardiac troponin, BNP, or TTE within 4 days of diagnosis.
Results
Description of Cohort
We identified 64,037 patients admitted to 363 hospitals with hemodynamically stable acute PE, of whom 26,925 (42.0%) had a TTE, 48,377 (75.6%) had a troponin, and 27,836 (43.5%) had a BNP measured. Characteristics of patients and hospitals are shown in Table 1; briefly, the mean (standard deviation [SD]) age of patients in the cohort was 61.7 (17.5) years, 54.3% were women, and 67.7% were white. Patients had a mean (SD) of 1.4 (1.2) chronic comorbidities and 0.3 (0.6) acute organ failures present on admission. Hospitals in the cohort were disproportionately in the southern United States (41.0%) and were primarily urban (89.6%) and nonteaching (62.9%), with a mean (SD) of 403 (229) beds.
Patient and Hospital Factors Associated with Trans-Thoracic Echocardiography Use
Hospital rates of TTE among patients with acute PE ranged from 0 to 89% (median, 42.0%; interquartile range [IQR], 32.7–51.7%; Figure 2), with a risk-adjusted median odds ratio (OR) of 1.82 (95% confidence interval [CI], 1.73–1.91) between hospitals; thus, if identical patients presented to two randomly selected hospitals, the odds of having a TTE would differ by a median factor of 1.82 as a result of hospital practice variability. Factors associated with increased use of TTE (Table E6) included increasing age, private insurance, a history of cardiac comorbidities, obesity, diabetes, ischemic stroke or dementia, acute organ failures, larger hospital size, and attending physician specializing in cardiology or pulmonary–critical care. Factors associated with decreased use of TTE included female sex, Hispanic race, history of cancer, geographic region in the southern United States, or attending physician specializing in medical oncology or surgery. We found no association between use of TTE and teaching hospital status and no significant interaction between hospital size and teaching status (P = 0.14).
Outcomes Associated with Hospital-Level and Patient-Level Echocardiogram Usage
Hospital rates of TTE were not associated with significant differences in risk-adjusted mortality, use of systemic thrombolytics, or episodes of major bleeding. However, admission to hospitals in the highest TTE use quartile was associated with significantly higher rates of ICU admission, hospital length of stay, and hospitalization costs (Table 2). Similarly, patient-level analysis showed that patients who received TTE did not have different hospital mortality but had increased risk for ICU admission and increased hospital length of stay and hospitalization costs (Table 3). Patient-level analyses also showed that patients who underwent TTE were more likely to subsequently receive systemic thrombolytics and experience a major bleeding episode (Table 3).
Table 2.
Outcomes associated with trans-thoracic echocardiogram use for inpatients with hemodynamically stable, acute pulmonary embolism: hospital-level exposure and patient-level outcomes (N = 64,037)
| Outcome | Hospital Echocardiogram Rate for Pulmonary Embolism |
P Value* | |||
|---|---|---|---|---|---|
| Quartile 1 0–33% | Quartile 2 33–42% | Quartile 3 42–52% | Quartile 4 52–89% | ||
| Mortality, OR (95% CI) | Ref | 1.05 (0.84–1.32) | 1.09 (0.87–1.37) | 0.88 (0.69–1.13) | 0.27 |
| ICU admission, OR (95% CI) | Ref | 0.86 (0.66–1.11) | 1.19 (0.91–1.55) | 1.57 (1.18–2.07) | < 0.01 |
| Thrombolytic use, OR (95% CI) | Ref | 1.09 (0.72–1.63) | 1.22 (0.81–1.84) | 1.28 (0.84–1.96) | 0.65 |
| Major bleeding, OR (95% CI) | Ref | 1.10 (0.88–1.39) | 0.98 (0.77–1.24) | 0.91 (0.70–1.17) | 0.42 |
| Hospital LOS, RR (95% CI) | Ref | 1.04 (0.98–1.09) | 1.03 (0.98–1.09) | 1.08 (1.03–1.15) | 0.04 |
| Hospital costs, RR (95% CI) | Ref | 1.07 (1.01–1.15) | 1.09 (1.02–1.16) | 1.15 (1.07–1.23) | < 0.01 |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; LOS = length of stay; OR = odds ratio; Ref = reference; RR = relative risk.
From hierarchical regression models accounting for demographics, comorbidities, acute organ failures, and hospital characteristics.
Table 3.
Outcomes associated with trans-thoracic echocardiogram use for inpatients with hemodynamically stable, acute pulmonary embolism: patient-level exposure and patient-level outcomes (N = 64,037)
| Outcome | Cohort Mean | Adjusted Effect Estimate (95% CI)* | P Value |
|---|---|---|---|
| Mortality | 2.0% | 1.02 (0.89–1.16) | 0.83† |
| ICU admission | 19.3% | 2.07 (1.93–2.22) | < 0.01† |
| Thrombolytic use | 1.3% | 5.58 (4.40–7.09) | < 0.01† |
| Major bleeding | 2.9% | 1.37 (1.24–1.51) | < 0.01‡ |
| Hospital LOS, d | 5.4 | 1.15 (1.14–1.16) | < 0.01§ |
| Hospitalization cost, USD | $ 9,587 | 1.31 (1.30–1.32) | < 0.01ǁ |
Definition of abbreviations: CI = confidence interval; ICU = intensive care unit; LOS = length of stay; USD = U.S. dollars.
Adjusted effect estimates: hazard ratio (mortality, ICU admission, thrombolytic use), odds ratio (major bleeding), relative proportion (hospital LOS, hospital costs) associated with use of trans-thoracic echocardiogram.
From Cox proportional hazard models with robust sandwich estimators.
From mixed effects logistic regression models.
From mixed effects negative binomial regression model.
ǁFrom mixed effects linear regression model of log costs.
Sensitivity Analyses
Hospital-level analysis of the combined outcome of ICU and/or step-down admission yielded similar results to the ICU-only outcome of our primary analysis; admission to either an ICU or step-down unit was increased in hospitals with high TTE usage (Table E7). Analysis of outcomes on the basis of hospital-level use of combined RV-strain workup (TTE, BNP, or troponin) showed that a high proportion of hospitals used at least one of these tests, with relatively little variation in use (median, 82.2%; IQR, 78.0–86.2%). Admission to hospitals in the highest combined RV-strain workup use quartile was associated with higher rates of ICU admission; otherwise, there were no significant associations between hospital-level use of combined RV-strain workup and other outcomes (Table E8).
Sensitivity analyses examining use of TTE on hospital Day 0 or 1 showed similar results to primary analysis. For example, hospital rates of TTE on Day 0 or 1 (range, 0–44%; median, 11.8%; IQR, 7.4–17.8%) were not associated with significant differences in risk-adjusted mortality, use of systemic thrombolytics, or episodes of major bleeding; however, admission to hospitals in the highest TTE use (Day 0–1) quartile was associated with significantly higher hospitalization costs (quartile 4 vs. quartile 1: relative risk, 1.12; 95% CI, 1.05–1.19) and ICU use (quartile 4 vs. quartile 1: OR, 1.43; 95% CI, 1.09–1.87). In contrast to the primary analysis, higher hospital rate of TTE use on Day 0 or 1 was not associated with hospital length of stay (Table E9). Patient-level analysis showed that patients who received TTE on Day 0 or 1 did not have different hospital mortality but had increased risk for ICU admission, use of thrombolytics, episodes of major bleeding and increased hospital length of stay and hospitalization costs, similar to our primary analysis (Table E10). An additional sensitivity analysis evaluating only ICU admissions occurring after hospital Day 1 (i.e., excluding 83% of ICU admission outcomes) did not identify an association between hospital rate of TTE use on hospital Day 0 or 1 and ICU admission after hospital Day 1 (Table E11).
Discussion
We evaluated practice pattern and outcome variation in the United States associated with the use of trans-thoracic echocardiography among patients admitted with hemodynamically stable acute pulmonary embolism. Trans-thoracic echocardiography was performed for approximately 40% of patients with hemodynamically stable acute pulmonary embolism, with wide interhospital variation. Use of trans-thoracic echocardiography was associated with higher resource use (intensive care unit admissions, hospital costs, hospital length of stay) without associated differences in patient outcomes, including mortality. Our findings support the 2012 and 2016 American College of Chest Physicians guidelines for management of venous thromboembolism, which recommend selective, rather than routine, use of trans-thoracic echocardiography to risk stratify patients with hemodynamically stable acute pulmonary embolism (4, 15).
In an era of value-based care, there are growing initiatives to identify and reduce unnecessary medical testing (29, 30). Our study suggests that routine use of trans-thoracic echocardiography in patients with hemodynamically stable pulmonary embolism may have multiple downstream effects on resource use. Positive findings of right ventricular strain may lead clinicians to pursue escalations of care, such as intensive care unit admission for closer monitoring, specialist consultations, or use of thrombolytic therapies, which in the vast majority of cases would not be guideline indicated (15, 16) in the absence of hemodynamic instability. These escalations in care potentially increase medical costs, patient anxiety (31, 32), and exposure to adverse complications. Both hospital-level and patient-level analyses suggested an association between trans-thoracic echocardiography use and higher intensive care unit admission, which offered a potential explanation for the higher hospital costs observed in both analyses. Patient-level analyses also observed an association between use of trans-thoracic echocardiography and higher subsequent use of thrombolytics; however, these findings were not replicated in hospital-level analyses, which are less subject to confounding by indication of trans-thoracic echocardiography (26, 27).
In the context of prior studies, our findings provide additional insights into healthcare use associated with variation in practice during acute pulmonary embolism. Although practice pattern variation in use of trans-thoracic echocardiography during evaluation of pulmonary embolism has not been previously described, other studies have shown wide variation in intensive care unit use practices for patients with pulmonary embolism. Recent studies by Chang and Shapiro (33) and Admon and colleagues (17) have shown that hospitals with higher intensive care unit use rates in patients admitted with pulmonary embolism were more likely to perform invasive procedures and incur higher medical costs, with no identifiable associated improvements in patient mortality. Our findings provide evidence that routine use of trans-thoracic echocardiography among hemodynamically stable patients with pulmonary embolism may be an additional factor associated with resource use during hospitalization. Similar associations between hospital trans-thoracic echocardiography rates and resource use have also been reported among patients with suspected cardiac ischemia (34).
Our results must be interpreted within the context of guidelines in place during the study years 2008 to 2011. Although current professional society guidelines give conditional recommendations (4, 15, 16) for use of trans-thoracic echocardiography in patients with pulmonary embolism, guidelines before 2012 provided no recommendations regarding the use of trans-thoracic echocardiography in the management of hemodynamically stable acute pulmonary embolism (35–37). The absence of guidelines for use of trans-thoracic echocardiography in pulmonary embolism during the time period of this study provided an opportunity to study clinical outcomes associated with institutional practice variation, without further confounding by factors associated with guideline uptake and adherence. For example, studies have shown that hospitals more adept at following guidelines also have better patient outcomes, likely independent of the guideline-based care processes (20). Subsequently, the presence of a new set of guidelines during the study period would have had the potential to confound the measured association between use of trans-thoracic echocardiography and patient outcomes, independent of trans-thoracic echocardiography–specific practices. Further studies in the period after 2012 are necessary to evaluate for changes in practice since publication of updated guidelines.
There are several limitations to this study that should be considered in interpreting its results. First, definitions of medical conditions were based on claims data and therefore may be subject to misclassification bias. We attempted to optimize specificity by including only patients with International Classification of Diseases, Ninth Revision, Clinical Modification primary discharge diagnosis of pulmonary embolism and specified that pulmonary embolism be present on admission (38). Second, because direct hemodynamic information was not available from claims data, we relied on International Classification of Diseases, Ninth Revision, Clinical Modification coding of shock and pharmacy administration of vasopressor medications; subsequently, there is the potential for unmeasured confounding due to cases of hemodynamic instability that were not captured by these methods. Third, the indication for trans-thoracic echocardiography also could not be determined from claims data; for the purposes of this study, we assumed that a trans-thoracic echocardiogram obtained within 4 days of pulmonary embolism diagnosis was obtained to evaluate right ventricular strain in pulmonary embolism. Although this assumption introduces the possibility of bias by indication, sensitivity analysis in which trans-thoracic echocardiography was restricted to hospital Day 0 or 1 corroborated the results of the primary analysis. Fourth, more than 80% of intensive care unit admissions occurred during the first day of hospital admission, limiting the ability to discern temporality between trans-thoracic echocardiography and intensive care unit admission occurring during the same day. Reverse and/or bidirectional causality may exist between trans-thoracic echocardiography use and intensive care unit admission, and subsequently the increased intensive care unit use associated with trans-thoracic echocardiography use observed in our study may in part reflect an increased propensity of patients admitted to an intensive care unit to receive trans-thoracic echocardiography, rather than the opposite. Similarly, the increased resource use (hospital length of stay, cost) associated with trans-thoracic echocardiography observed in our study may in part reflect an increased propensity of patients admitted to an intensive care unit to receive more diagnostic/therapeutic interventions (including trans-thoracic echocardiography) as a result of a higher severity of illness unaccounted for in risk adjustment.
Last, comparisons of outcomes on the basis of observational, patient-level use of trans-thoracic echocardiography may be subject to unmeasured, residual confounders related to the indication for trans-thoracic echocardiography. Although analyses using hospital-level rates of trans-thoracic echocardiography use attenuate confounding by indication on a patient level (26, 27), the use of hospital-level rates of trans-thoracic echocardiography may also be subject to bias resulting from “endogeneity,” or nonrandom practice pattern variation between hospitals. However, results were consistent across individual patient- and hospital-level exposure-based analyses. Alternative instruments for trans-thoracic echocardiography exposure (e.g., differential distance to high trans-thoracic echocardiography rate hospitals [39]) were unavailable from the data source used in the present study.
Conclusions
Trans-thoracic echocardiography was used frequently in the management of hemodynamically stable patients with acute pulmonary embolism. Hospitals varied widely in rates of pulmonary embolism–associated trans-thoracic echocardiography use. Although hospital trans-thoracic echocardiography use was not associated with inpatient mortality, high trans-thoracic echocardiography–use hospitals used more resources and had higher costs. Our results provide evidence in support of the 2012 and 2016 American College of Chest Physicians guidelines for management of venous thromboembolism, which recommend selective, rather than routine, use of trans-thoracic echocardiography to risk stratify patients with hemodynamically stable acute pulmonary embolism. Further studies are needed to determine if practice patterns have changed in response to the current American College of Chest Physicians guidelines.
Supplementary Material
Footnotes
Supported by U.S. National Heart, Lung, and Blood Institute grants K01HL116768 (A.J.W.) and K24HL132008 (P.K.L.). The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Contributions: A.J.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. D.M.C. and A.J.W. contributed to the study design, analysis and interpretation of data, writing the initial draft and revising the manuscript, and approval of the final manuscript; M.W. contributed to the analysis and interpretation of data, writing and revising the manuscript for important intellectual content, and approval of the final manuscript; and P.K.L. contributed to the acquisition and interpretation of data, revision of the manuscript for important intellectual content, and approval of the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention (CDC) Venous thromboembolism in adult hospitalizations - United States, 2007-2009. MMWR Morb Mortal Wkly Rep. 2012;61:401–404. [PubMed] [Google Scholar]
- 2.ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med. 2004;164:1685–1689. doi: 10.1001/archinte.164.15.1685. [DOI] [PubMed] [Google Scholar]
- 3.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–462. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 4.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation. 2006;113:577–582. doi: 10.1161/CIRCULATIONAHA.105.592592. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353:1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 7.Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G, et al. Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction. Circulation. 2000;101:2817–2822. doi: 10.1161/01.cir.101.24.2817. [DOI] [PubMed] [Google Scholar]
- 8.Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation. 2007;116:427–433. doi: 10.1161/CIRCULATIONAHA.106.680421. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez D, Uresandi F, Otero R, Lobo JL, Monreal M, Martí D, et al. Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest. 2009;136:974–982. doi: 10.1378/chest.09-0608. [DOI] [PubMed] [Google Scholar]
- 10.Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med. 2008;178:425–430. doi: 10.1164/rccm.200803-459OC. [DOI] [PubMed] [Google Scholar]
- 11.Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation. 2003;108:2191–2194. doi: 10.1161/01.CIR.0000100687.99687.CE. [DOI] [PubMed] [Google Scholar]
- 12.Lankeit M, Friesen D, Aschoff J, Dellas C, Hasenfuss G, Katus H, et al. Highly sensitive troponin T assay in normotensive patients with acute pulmonary embolism. Eur Heart J. 2010;31:1836–1844. doi: 10.1093/eurheartj/ehq234. [DOI] [PubMed] [Google Scholar]
- 13.Lankeit M, Jiménez D, Kostrubiec M, Dellas C, Kuhnert K, Hasenfuß G, et al. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratification of pulmonary embolism. Eur Respir J. 2014;43:1669–1677. doi: 10.1183/09031936.00211613. [DOI] [PubMed] [Google Scholar]
- 14.Becattini C, Agnelli G, Germini F, Vedovati MC. Computed tomography to assess risk of death in acute pulmonary embolism: a meta-analysis. Eur Respir J. 2014;43:1678–1690. doi: 10.1183/09031936.00147813. [DOI] [PubMed] [Google Scholar]
- 15.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033–3069. doi: 10.1093/eurheartj/ehu283. 3069a-3069k. [DOI] [PubMed] [Google Scholar]
- 17.Admon AJ, Seymour CW, Gershengorn HB, Wunsch H, Cooke CR. Hospital-level variation in ICU admission and critical care procedures for patients hospitalized for pulmonary embolism. Chest. 2014;146:1452–1461. doi: 10.1378/chest.14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen DM, Winter M, Walkey AJ. Use of echocardiogram in the management of hemodynamically stable acute pulmonary embolism: national practice patterns and clinical outcomes [abstract] Am J Respir Crit Care Med. 2017;195:A1093. [Google Scholar]
- 19.Premier Premier Applied Sciences supports health care transformation through generation of real-world evidence. Charlotte, NC: Premier, Inc. [Accessed 2017 May 5]. Available from: https://www.premierinc.com/transforming-healthcare/healthcare-performance-improvement/premier-applied-sciences/
- 20.Fawzy A, Walkey AJ. Association between hospital case volume of sepsis, adherence to evidence-based processes of care and patient outcomes. Crit Care Med. 2017;45:980–988. doi: 10.1097/CCM.0000000000002409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Healthcare Cost and Utilization Project (HCUP) Elixhauser comorbidity software version 3.7. Rockville, MD: Agency for Healthcare Research and Quality; June 2017 [accessed 2016 Sep 1]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
- 22.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Baskin JL, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC, Pui CH, et al. Thrombolytic therapy for central venous catheter occlusion. Haematologica. 2012;97:641–650. doi: 10.3324/haematol.2011.050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118:253–262. doi: 10.1016/j.thromres.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, et al. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. doi: 10.1136/jech.2004.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston SC. Combining ecological and individual variables to reduce confounding by indication: case study--subarachnoid hemorrhage treatment. J Clin Epidemiol. 2000;53:1236–1241. doi: 10.1016/s0895-4356(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 27.Johnston SC, Henneman T, McCulloch CE, van der Laan M. Modeling treatment effects on binary outcomes with grouped-treatment variables and individual covariates. Am J Epidemiol. 2002;156:753–760. doi: 10.1093/aje/kwf095. [DOI] [PubMed] [Google Scholar]
- 28.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time-varying Cox regression for observational cohort studies. Crit Care Med. 2009;37:2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberger SE. Providing high-value, cost-conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155:386–388. doi: 10.7326/0003-4819-155-6-201109200-00007. [DOI] [PubMed] [Google Scholar]
- 30.ABIM Foundation. ABIM Choosing Wisely campaign. Philadelphia, PA: ABIM Foundation. 2017 [accessed 2017 Jun 1]. Available from: http://www.choosingwisely.org/
- 31.Granja C, Lopes A, Moreira S, Dias C, Costa-Pereira A, Carneiro A JMIP Study Group. Patients’ recollections of experiences in the intensive care unit may affect their quality of life. Crit Care. 2005;9:R96–R109. doi: 10.1186/cc3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novaes MA, Knobel E, Bork AM, Pavão OF, Nogueira-Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25:1421–1426. doi: 10.1007/s001340051091. [DOI] [PubMed] [Google Scholar]
- 33.Chang DW, Shapiro MF. Association between intensive care unit utilization during hospitalization and costs, use of invasive procedures, and mortality. JAMA Intern Med. 2016;176:1492–1499. doi: 10.1001/jamainternmed.2016.4298. [DOI] [PubMed] [Google Scholar]
- 34.Safavi KC, Li SX, Dharmarajan K, Venkatesh AK, Strait KM, Lin H, et al. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174:546–553. doi: 10.1001/jamainternmed.2013.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S–428S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]
- 36.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 37.Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, et al. ESC Committee for Practice Guidelines (CPG) Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 38.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in administrative data. Health Serv Res. 2011;46:1946–1962. doi: 10.1111/j.1475-6773.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valley TS, Sjoding MW, Ryan AM, Iwashyna TJ, Cooke CR. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA. 2015;314:1272–1279. doi: 10.1001/jama.2015.11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.