For almost 70 years, intrapleural fibrinolytic therapy (IPFT) has been part of the therapeutic armamentarium to expedite pleural drainage in patients with empyema (EMP) and complicated parapneumonic pleural effusions (CPEs), as recently reviewed (1). Although we advocate the continued use of IPFT in adult patients with pleural infection, loculation, and failed drainage, a number of controversial points remain. These include which form of IPFT to use, the role of combinations of fibrinolysins with other enzymes, such as DNase, and the optimal dose and dosing interval of IPFT.
The previously published literature on “lone” IPFT (i.e., a fibrinolytic agent administered alone) suggests that it has utility in the pediatric population with pleural infection, but there is controversy concerning its use alone in adults (1, 2). It is likely that pediatric pleural infection is a different clinical entity than adult disease, with different diagnostic criteria, different comorbidities, and starkly different outcomes in adults and children. These considerations suggest that direct comparison of the efficacy of IPFT in adult versus pediatric populations may not be valid.
Early and small, but well-conducted, randomized, placebo-controlled trials of the fibrinolysins, urokinase and streptokinase, suggested benefit to surrogate outcomes in adults, such as chest tube fluid output and favorable radiological change, as recently reviewed (1). Conversely, the two largest and adequately powered randomized trials, MIST (Multicenter Intrapleural Sepsis Trial) 1 and MIST2, demonstrate clear lack of utility of the use of intrapleural fibrinolytic alone compared with placebo in adults (2, 3). The MIST2 study is the largest positive randomized trial to date using intrapleural agents, and demonstrated clear and clinically important benefits to the use of combined tissue plasminogen activator (tPA) and DNase intrapleurally. These benefits included improved radiological outcome, along with reductions in hospital length of stay and need for surgical referral (2).
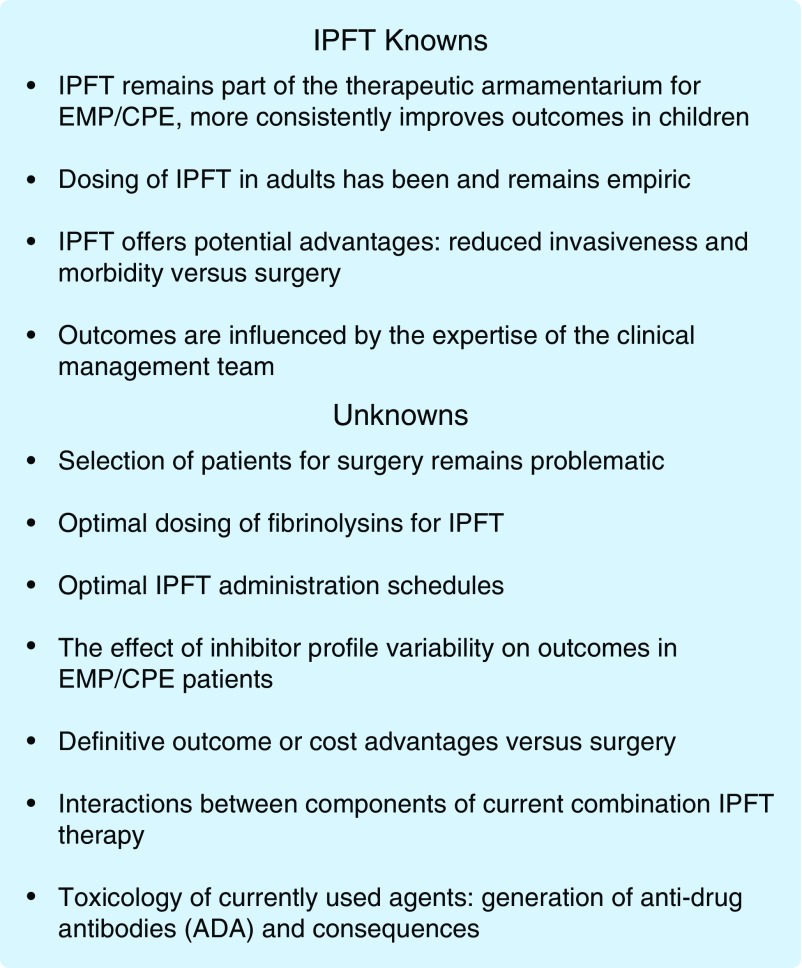
These two large studies offer a consistent message of IPFT alone being unhelpful in the management of adult pleural infection, but whether the results of these trials are generally applicable remains an open question. There remain several concerns about these landmark and well-designed studies that could have impacted the results. One important concern is that dosing of fibrinolysins in IPFT in adult patients has historically been entirely empiric (1), and was so in these studies (2, 3). This empiricism derives in large part from a paucity of evidence-based guidance and a heretofore poor understanding about how IPFT is processed within the pleural compartment. There has also been a rudimentary understanding of the variability of innate immune responses that affect the processing of IPFT and how such responses impact effective dosing and pleural drainage. As a result, administration of IPFT or decisions to use it instead of early surgery commonly defaults to local experience and the reported literature, as recently reviewed (1). Outcomes of IPFT in patients with EMP/CPE are also importantly influenced by a number of factors, including the expertise of the clinical team in the management of these patients (Figure 1). All of these concerns contribute to the ongoing uncertainty about the impact of IPFT on outcomes or costs of care versus surgery. Although surgery entails additional invasiveness and costs, these could be offset by potential reductions in hospital stay. Insufficient information about the impact of IPFT on hospital stay emerged from a recent meta-analysis (4), so the issue remains to be resolved.
Figure 1.
The bullets indicate the current status of key issues related to intrapleural fibrinolytic therapy and are predicated upon the authors’ synthesis of the literature. ADA = anti-drug antibodies; CPE = complicated parapneumonic pleural effusions; EMP = empyema; IPFT = intrapleural fibrinolytic therapy.
To our knowledge, no form of IPFT to expedite pleural drainage in patients with EMP/CPE has been approved by the U.S. Food and Drug Administration or other regulatory agencies. Nor have any good laboratory practice formal toxicology or dose escalation safety trials been completed to inform the use of currently available regimens. The absence of information derived from such studies is largely responsible for the empiricism surrounding dosing of IPFT in adults. The recently reported (ADAPTAlteplase Dose Assessment for Pleural Infection Therapy) trial of de-escalating doses of tPA combined with DNase (5) represents a welcome attempt to address the issue of optimal IPFT dosing in adults. Such studies are imperative in lieu of information provided by the common drug development sequence of early dose escalation safety testing followed by efficacy trials. There is also information that saline irrigation flushes can be helpful in cases of EMP/CPE with failed drainage, suggesting that IPFT may not be required in all cases of EMP/CPE (6). Despite these limitations, the predicate for the use of IPFT remains strong, as alternative surgical interventions for EMP/CPE are invasive and entail morbidity with significant cost, and patient selection remains highly problematic (7). These considerations and the preponderance of available evidence (1, 2, 8) lead us to believe that the use of IPFT for adult patients with CPE/EMP and failed drainage should be continued and used as the preferred initial treatment over immediate surgical intervention.
Recently, a number of models, including a new rabbit Streptococcus pneumoniae EMP model, have been used to better understand the pathogenesis of pleural organization, identify new therapeutic targets, and understand the processing of IPFT (9, 10). These studies clearly show that different fibrinolysins undergo differential processing within the injured pleural space, and that high pleural fluid plasminogen activator inhibitor (PAI)-1 activity levels increase pleural organization and strongly influence the outcomes of IPFT (9, 10). It is now known that adult patients with EMP/CPE have a wide range of pleural fluid total PAI-1 antigen as well as its activity (9). This finding is especially important, as tPA is exquisitely sensitive to inhibition by PAI-1, raising the possibility that an effective dose of tPA-based IPFT in a given patient could be ineffective in another, with higher levels of pleural fluid PAI-1 activity (Figure 1). The same applies to all two-chain forms of urokinase plasminogen activator (uPA), commonly called urokinase in the literature. Although great variability of PAI-1 levels were found in patients from the MIST2 trial and another patient cohort from the University of Texas Health Science Center at Tyler (9), whether such interpatient variability affected IPFT outcomes in MIST2 or other trials remains unknown. This represents a potentially important gap in current knowledge that could be addressed by integration of inhibitor profile analyses into the design of future IPFT trials (Figure 1). The variability in the fibrinolysin inhibitor profile within the MIST2 trial population may offer an important avenue for improving current IPFT treatment, leaving a clear role for lone IPFT, albeit improved with an increase in precision and individualization of treatment.
Empiricism also currently extends to the dosing intervals currently for IPFT. For example, tPA or tPA/DNase regimens are commonly used in the United States, and each is commonly given twice daily. This approach assumes that sufficient pleural fluid plasminogen is available at the time of the next daily dose. Whether that dosing strategy is actually optimal in EMP/CPE patients is unknown. In rabbit models of EMP and tetracycline-induced pleural injury (9, 11), plasminogen appears to be restored by 24 hours after IPFT, but precisely when enough plasminogen is present within EMP/CPE pleural fluids to optimally support IPFT has not, to our knowledge, previously been studied. This gap represents yet another opportunity for further study. Theoretically, optimal IPFT dosing windows can be defined by serial analyses of pleural fluid drainage. These studies can be initiated in preclinical analyses and extended to clinical studies involving patients treated with IPFT for EMP/CPE with loculation and failed drainage. Identification of the best time for repeat IPFT dosing and how much interpatient variability exists in this regard may inform rational dosing schedules and advantage better outcomes.
The combination of tPA with DNase has shown great promise as a treatment to expedite drainage in patients with EMP/CPE, with reported cure of over 90% of patients without requiring surgery (12). However, it should be noted that inherent case selection bias applies to the reporting of selective outcomes in expert centers. The administration of tPA/DNase is relatively labor intensive, as the combination is generally given twice daily, with administration of each component given separately via the chest tube about 2 hours apart (2, 12). Pain, commonly necessitating opiates for control, occurred in about 20% of patients and bleeding in about 2% (12). The premise for the addition of DNase to tPA for IPFT is built on in vitro evidence that EMP fluids are better liquefied with degradation of DNA and preclinical findings that outcomes of EMP are better in rabbits with Pasteurella-induced EMP (13, 14). No dose ranging studies were performed, and it is unknown whether the effects in vivo were related to changes in pleural fluid viscosity or proteolytic activity of IPFT or both. The superiority of tPA/DNase at an empirically selected 10-mg unit dose of tPA and 5 mg of DNase over tPA IPFT alone was subsequently confirmed in the MIST2 trial (2). However, whether tPA dosing was optimal or whether DNase is always required to expedite pleural drainage is not clear. Fibrinolysins can degrade cross-linked fibrin and decrease viscosity of purulent fluids, which uPA has been reported to efficiently do (15). Biofilms produced by infectious organisms incorporate fibrin as well as DNA, and expedited degradation by fibrinolysins supplementing antibiotics has also been reported (16). It is also plausible that activity of intrapleural tPA is affected when combined with DNase, because the size and quality of DNA fragments can alter tPA activity or that of uPA (17). Whether such effects occur in the pleural fluids of patients with EMP/CPE has not been studied. However, such interactions could be important, and they could conceivably have contributed to the bleeding complications reported in the MIST2 trial that were limited to the tPA/DNase IPFT group (2). At present, it is also unknown how the activity of DNase may be influenced by tPA or how their interaction could influence DNA fragmentation in human EMP/CPE fluids. Lastly, whether tPA-neutralizing anti-drug antibodies are generated by repetitive administration of tPA/DNase or tPA alone is also unknown, as are the consequences for subsequent administration of tPA for treatment of myocardial infarction or stroke. These gaps in current knowledge can be addressed in future clinical studies.
Although challenges remain, IPFT represents a useful approach in aiding pleural drainage of adults with EMP/CPE and pleural loculation. However, further understanding of the host, organism, and intrapleural specific factors associated with success is necessary. Optimization of dosing regimens and frequencies now require rigorous scientific evaluation. Lastly, integration of mechanistic studies into the design of future pleural infection/loculation trials will likely help optimize IPFT dosing/administration schedules, inform opportunities to develop personalized therapy or bedside dosing guidance (1, 9), and, thereby, improve prospects for better clinical outcomes for patients with EMP/CPE.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health (NIH) U.S. National Heart, Lung, and Blood Institute RO-1HL118401-01 (S.I., principal investigator [PI], Contact, multiple principal investigator [MPI]), R01HL130402-01A1 (S.I., MPI), 1 U54ES027698-01 (Site PI, subcontract), R01HL130133-01A1 (co-investigator [Co-I]), R01HL133067-01 (Co-I), R21ES025815-01A1 (Co-I), T. L. L. Temple Endowed Chair in Idiopathic Pulmonary Fibrosis, Texas Lung Injury Institute, NIH UO-1 HL 121841-01A1 (S.I., Contact PI, MPI), NIH Science Moving toward Research Translation and Therapy contract HHSN268201100014C (S.I., PI).
The views expressed in this article do not reflect the views of the authors’ institutions or the U.S. National Heart, Lung, and Blood Institute and are solely those of the authors. All human and animal studies described in this article were approved by the University of Texas Health Science Center at Tyler Human Subjects Institutional Review Board and Institutional Animal Care and Utilization Committees, respectively.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tucker T, Idell S. Plasminogen–plasmin system in the pathogenesis and treatment of lung and pleural injury. Semin Thromb Hemost. 2013;39:373–381. doi: 10.1055/s-0033-1334486. [DOI] [PubMed] [Google Scholar]
- 2.Rahman NM, Maskell NA, West A, Teoh R, Arnold A, Mackinlay C, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med. 2011;365:518–526. doi: 10.1056/NEJMoa1012740. [DOI] [PubMed] [Google Scholar]
- 3.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, et al. First Multicenter Intrapleural Sepsis Trial (MIST1) Group. U.K. controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865–874. doi: 10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 4.Redden MD, Chin TY, van Driel ML. Surgical versus non-surgical management for pleural empyema. Cochrane Database Syst Rev. 2017;3:CD010651. doi: 10.1002/14651858.CD010651.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Popowicz N, Bintcliffe O, De Fonseka D, Blyth KG, Smith NA, Piccolo F, et al. Dose de-escalation of intrapleural tissue plasminogen activator therapy for pleural infection: the Alteplase Dose Assessment for Pleural Infection Therapy Project. Ann Am Thorac Soc. 2017;14:929–936. doi: 10.1513/AnnalsATS.201609-673OC. [DOI] [PubMed] [Google Scholar]
- 6.Hooper CE, Edey AJ, Wallis A, Clive AO, Morley A, White P, et al. Pleural irrigation trial (PIT): a randomised controlled trial of pleural irrigation with normal saline versus standard care in patients with pleural infection. Eur Respir J. 2015;46:456–463. doi: 10.1183/09031936.00147214. [DOI] [PubMed] [Google Scholar]
- 7.Corcoran JP, Hallifax R, Rahman NM. New therapeutic approaches to pleural infection. Curr Opin Infect Dis. 2013;26:196–202. doi: 10.1097/QCO.0b013e32835d0b71. [DOI] [PubMed] [Google Scholar]
- 8.Diacon AH, Theron J, Schuurmans MM, Van de Wal BW, Bolliger CT. Intrapleural streptokinase for empyema and complicated parapneumonic effusions. Am J Respir Crit Care Med. 2004;170:49–53. doi: 10.1164/rccm.200312-1740OC. [DOI] [PubMed] [Google Scholar]
- 9.Komissarov AA, Florova G, Azghani AO, Buchanan A, Boren J, Allen T, et al. Dose dependency of outcomes of intrapleural fibrinolytic therapy in new rabbit empyema models. Am J Physiol Lung Cell Mol Physiol. 2016;311:L389–L399. doi: 10.1152/ajplung.00171.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karandashova S, Florova G, Azghani AO, Komissarov AA, Koenig K, Tucker TA, et al. Intrapleural adenoviral delivery of human plasminogen activator inhibitor-1 exacerbates tetracycline-induced pleural injury in rabbits. Am J Respir Cell Mol Biol. 2013;48:44–52. doi: 10.1165/rcmb.2012-0183OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komissarov AA, Florova G, Azghani AO, Buchanan A, Bradley WM, Schaefer C, et al. The time course of resolution of adhesions during fibrinolytic therapy in tetracycline-induced pleural injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2015;309:L562–L572. doi: 10.1152/ajplung.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccolo F, Pitman N, Bhatnagar R, Popowicz N, Smith NA, Brockway B, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease for pleural infection: an effective and safe alternative to surgery. Ann Am Thorac Soc. 2014;11:1419–1425. doi: 10.1513/AnnalsATS.201407-329OC. [DOI] [PubMed] [Google Scholar]
- 13.Light RW, Nguyen T, Mulligan ME, Sasse SA. The in vitro efficacy of varidase versus streptokinase or urokinase for liquefying thick purulent exudative material from loculated empyema. Hai. 2000;178:13–18. doi: 10.1007/s004080000002. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Hawthorne ML, Guo Y, Drake W, Bilaceroglu S, Misra HL, et al. Tissue plasminogen activator combined with human recombinant deoxyribonuclease is effective therapy for empyema in a rabbit model. Chest. 2006;129:1577–1583. doi: 10.1378/chest.129.6.1577. [DOI] [PubMed] [Google Scholar]
- 15.Park JK, Kraus FC, Haaga JR. Fluid flow during percutaneous drainage procedures: an in vitro study of the effects of fluid viscosity, catheter size, and adjunctive urokinase. AJR Am J Roentgenol. 1993;160:165–169. doi: 10.2214/ajr.160.1.8416618. [DOI] [PubMed] [Google Scholar]
- 16.Jørgensen NP, Zobek N, Dreier C, Haaber J, Ingmer H, Larsen OH, et al. Streptokinase treatment reverses biofilm-associated antibiotic resistance in Staphylococcus aureus. Microorganisms. 2016;4:pii: E36. doi: 10.3390/microorganisms4030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komissarov AA, Florova G, Idell S. Effects of extracellular DNA on plasminogen activation and fibrinolysis. J Biol Chem. 2011;286:41949–41962. doi: 10.1074/jbc.M111.301218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.