Abstract
Rationale: In acute respiratory distress syndrome (ARDS), atelectatic solid-like lung tissue impairs transmission of negative swings in pleural pressure (Ppl) that result from diaphragmatic contraction. The localization of more negative Ppl proportionally increases dependent lung stretch by drawing gas either from other lung regions (e.g., nondependent lung [pendelluft]) or from the ventilator. Lowering the level of spontaneous effort and/or converting solid-like to fluid-like lung might render spontaneous effort noninjurious.
Objectives: To determine whether spontaneous effort increases dependent lung injury, and whether such injury would be reduced by recruiting atelectatic solid-like lung with positive end-expiratory pressure (PEEP).
Methods: Established models of severe ARDS (rabbit, pig) were used. Regional histology (rabbit), inflammation (positron emission tomography; pig), regional inspiratory Ppl (intrabronchial balloon manometry), and stretch (electrical impedance tomography; pig) were measured. Respiratory drive was evaluated in 11 patients with ARDS.
Measurements and Main Results: Although injury during muscle paralysis was predominantly in nondependent and middle lung regions at low (vs. high) PEEP, strong inspiratory effort increased injury (indicated by positron emission tomography and histology) in dependent lung. Stronger effort (vs. muscle paralysis) caused local overstretch and greater tidal recruitment in dependent lung, where more negative Ppl was localized and greater stretch was generated. In contrast, high PEEP minimized lung injury by more uniformly distributing negative Ppl, and lowering the magnitude of spontaneous effort (i.e., deflection in esophageal pressure observed in rabbits, pigs, and patients).
Conclusions: Strong effort increased dependent lung injury, where higher local lung stress and stretch was generated; effort-dependent lung injury was minimized by high PEEP in severe ARDS, which may offset need for paralysis.
Keywords: acute respiratory distress syndrome, spontaneous breathing, ventilator-induced lung injury, PEEP
At a Glance Commentary
Scientific Knowledge on the Subject
Transmission of negative swings in pleural pressure caused by diaphragmatic contraction is impaired in the injured (solid-like) lung; therefore, negative pleural pressure is localized at the dependent lung. The resulting higher local lung stress in the dependent lung is a key mechanism whereby spontaneous effort causes local overstretch and tidal recruitment in the dependent lung by drawing gas either from other lung regions (e.g., nondependent lung [pendelluft]) or from the ventilator. Thus, converting solid-like (injured, atelectatic) to fluid-like (recruited, normal) lung and/or lowering the level of spontaneous effort might lessen injury from spontaneous effort.
What This Study Adds to the Field
Lower levels of positive end-expiratory pressure (PEEP) resulted in stronger inspiratory effort, higher local lung stress in dependent lung, and greater inflammation (detected by positron emission tomography; pig) and histologic injury (rabbit) predominantly in dependent lung. Higher PEEP abrogated all of these effects. In patients with acute respiratory distress syndrome, higher PEEP reduced inspiratory effort and thus lowered Vt. These data identify the mechanism of dependent lung injury with spontaneous effort; the effort-dependent lung injury was minimized by high PEEP in severe acute respiratory distress syndrome, which could offset need for paralysis and protect against diaphragm disuse.
Spontaneous breathing (SB) is often permitted in patients with acute respiratory distress syndrome (ARDS) (1, 2), in part because oxygenation is better (1) and diaphragm disuse is avoided (3, 4). However, strong spontaneous effort injures lung and diaphragm (4–13) and in patients with severe ARDS, prevention of SB with neuromuscular blockade improves survival (14). The lung injury caused by spontaneous effort potentially seems to be proportional to the magnitude of the effort (9–13, 15, 16). This is consistent with the findings that high respiratory drive is independently associated with failure of noninvasive ventilation (17), and that such patients have a particularly poor prognosis (18).
In normal fluid-like lung, the inspiratory deflection (swing) in pleural pressure (Ppl) resulting from diaphragmatic contraction is rapidly dissipated across the whole pleural surface (19–21). In contrast, in the injured solid-like lung, the inspiratory Ppl swing is not dissipated, but is confined to the dependent regions where it is generated (11, 13). Thus, stronger spontaneous effort results in more negative swings in Ppl in the dependent lung. The resulting higher local lung stress causes injurious inflation patterns (i.e., local overstretch [11] and tidal recruitment in the dependent lung [12, 13]) by drawing gas either from other lung regions (e.g., nondependent lung [pendelluft]) (11) or from the ventilator. Therefore, it seems that stronger spontaneous effort would predominantly injure dependent lung; however, this has never been demonstrated.
Recruitment of injured lung (e.g., using higher positive end-expiratory pressure [PEEP]) is known to decrease tissue heterogeneity (22). This may help to convert solid-like (more atelectatic) to fluid-like (less atelectatic) lung; and if successful, PEEP may diminish the injurious inflation patterns associated with spontaneous effort (12).
We therefore hypothesized that in injured lungs, spontaneous effort would increase injury in dependent lung, and that such injury would be reduced by PEEP.
We investigated these two phenomena in established models of severe ARDS (rabbit and pig) and in patients with ARDS, and measured the impact of PEEP on regional injury associated with spontaneous effort (histology in the rabbit; positron emission tomography [PET] in the pig), regional inspiratory Ppl (intrabronchial balloon manometry in the pig) and lung stretch (electrical impedance tomography [EIT] in the pig), and the effect of PEEP on the intensity of inspiratory effort in patients with ARDS.
Methods
These studies were approved by the Laboratory Investigation Committee (No. 25041005, Osaka University Medical School; rabbit experiments), the Ethics Committee for Experimental Studies (No. 059/13, Faculdade de Medicina da Universidade de São Paulo; pig experiments), and the Ethics committee for Clinical Studies (No.17068/16298, Osaka University Medical School; human studies).
Experimental Protocol: Rabbit
Twenty-eight New Zealand white rabbits were anesthetized. An esophageal balloon (SmartCath, Bicore) was inserted to measure esophageal pressure (Pes). Severe ARDS was induced by lung lavage followed by injurious mechanical ventilation. Then, animals were randomly assigned to one of four groups: 1) high PEEP with SB, 2) high PEEP without SB, 3) low PEEP with SB, or 4) low PEEP without SB.
Animals were ventilated for 6 hours, using low Vt (6–6.5 ml/kg, regulated by adjusting inspiratory pressure) with pressure-controlled ventilation. The respiratory system compliance (Crs) was measured at each decremental PEEP step after lung recruitment. High and low PEEP were set as follows:
Dynamic Computed Tomography: Rabbit
Dynamic computed tomography (CT) scans were performed at 5–10 mm above the diaphragm at the start (0 h) and end (6 h) of the protocol. Tidal recruitment, distribution of aeration, and distribution of ventilation were estimated as previously described (13).
Regional Lung Histology: Rabbit
The right lung was fixed and stained with hematoxylin and eosin, and sliced at approximately the same transverse levels as the CT scans. Three noncoincident fields (lateral, center, medial) from each nondependent, middle, dependent lung region were assessed for 1) air space hemorrhage; 2) neutrophils (air space, vessel walls, and alveolar walls); and 3) thickness of the alveolar wall, interstitium, and hyaline membrane formation (23).
Estimation of Local Pleural Pressure: Pig
Local negative swing in Ppl (i.e., ΔPpl) was measured in nondependent and dependent regions (n = 5) by balloon catheter occlusion of subsegmental bronchi via a fiberoptic bronchoscope as follows: nondependent region, left B; dependent region, left lower lobe beyond D4. The pressure swings in the occluded subsegments were used as surrogates for ΔPpl, as described previously (24). Simultaneous pressure recording of ΔPpl and ΔPes were performed, while preserving spontaneous effort at high PEEP versus low PEEP.
Estimation of Local Lung Stretch: Pig
The local lung stretch imposed by spontaneous effort (vs. muscle paralysis) at high PEEP versus low PEEP was determined by secondary analysis of data from a previous experiment (12). EIT data were recorded using the Enlight impedance tomography monitor (Timpel). Local lung stretch (delta Z) was analyzed after division of the thorax into three zones (i.e., nondependent, middle, dependent regions).
Experimental Protocol: Pig
Thirteen Landrace pigs were anesthetized. An esophageal balloon (Copper Surgical) was inserted to measure Pes, and the electrical activity of the diaphragm (EAdi) was estimated by a specialized catheter (Maquet-Getinge). Severe ARDS was induced by lung lavage followed by injurious mechanical ventilation. Then, animals were randomly assigned to one of two groups: high PEEP plus SB, or low PEEP plus SB.
Animals were ventilated for 16 hours, using low Vt (6 ml/kg, titrated by adjusting inspiratory pressure) with pressure-controlled ventilation. High PEEP was defined as minimum PEEP required to maintaining lung collapse less than 1% using EIT during decremental PEEP steps after lung recruitment (25), and low PEEP was set according to the ARDSNet PEEP/FiO2 table (26). Sedatives were titrated in both groups to the same target level of ∆EAdi. The target levels of ∆EAdi were evaluated before randomization (i.e., effort titration phase) to maintain the magnitude of the swing in Pes (ΔPes) at −10 to −15 cm H2O, at a PEEP level of approximately 8 cm H2O.
PET: Pig
Regional lung inflammation was assessed with dynamic PET-CT of [18F] fluoro-2-deoxy-d-glucose net uptake rate at two phases: after lung injury (first PET scan) and at 16 hours of the protocol (second PET scan). Regional lung inflammation was estimated according to lung density (27).
Observational Data: Patients with ARDS
Eleven patients with ARDS were identified (four patients were included from a different ongoing clinical study as a secondary use of data, and seven patients were analyzed retrospectively). The change in Vt, ΔPL, and respiratory drive (by using ΔPes [Copper Surgical] and/or ΔEAdi [Maquet-Getinge]) was evaluated at two different levels of PEEP (5 and 15 cm H2O). All the other ventilator settings (except PEEP) remained unchanged during assisted pressure-controlled ventilation or pressure support ventilation. These measurements were collected 5–10 minutes after changing PEEP (either way, high to low or low to high).
Statistical Analysis
Two-way ANOVA for repeated measures evaluated the effects of time and group, followed by a Dunnett’s test (or paired t test) was used, and Tukey’s pairwise multiple comparison test was used to determine intergroup differences. Paired t test was used for patient data. Statistical significance was inferred where P was less than 0.05.
Results
Respiratory Variables: Rabbit and Pig
In rabbits, high-PEEP groups maintained higher oxygenation, with or without SB, than low-PEEP groups (Table 1). There was a transient increase in oxygenation in low PEEP with SB (vs. without SB) for the first 2 hours, and oxygenation was lower and similar thereafter with or without SB (Table 1). In pigs, high PEEP with SB maintained better oxygenation than low PEEP with SB (Table 2).
Table 1.
Respiratory Measurement: Rabbit
| PEEP | SB | Time after the Start of the Protocol |
||||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | 5 h | 6 h | ||
| PaO2/FiO2, mm Hg | ||||||||
| High | + | 454 ± 43*† | 451 ± 58*† | 454 ± 31*† | 446 ± 69*† | 465 ± 61*† | 454 ± 71*† | 457 ± 79*† |
| − | 446 ± 42*† | 463 ± 25*† | 469 ± 29*† | 469 ± 29*† | 466 ± 33*† | 446 ± 56*† | 437 ± 64*† | |
| Low | + | 68 ± 10 | 162 ± 75†‡ | 88 ± 21‡ | 79 ± 24 | 65 ± 15 | 69 ± 10 | 65 ± 16 |
| − | 67 ± 13 | 58 ± 9 | 56 ± 10 | 55 ± 17 | 75 ± 33 | 67 ± 19 | 64 ± 16 | |
| | ||||||||
| Plateau airway pressure, cm H2O | ||||||||
| High | + | 26.6 ± 1.7*† | 27.2 ± 0.8*† | 28.3 ± 1.1*‡ | 30.0 ± 0.9*‡ | 31.1 ± 1.0‡ | 32.2 ± 1.2‡ | 32.7 ± 1.5‡ |
| − | 26.8 ± 2.2*† | 26.5 ± 2.2* | 27.1 ± 2.1 | 28.9 ± 2.0‡ | 29.8 ± 1.8‡ | 30.8 ± 1.9‡ | 31.7 ± 2.0*‡ | |
| Low | + | 22.6 ± 1.3 | 23.8 ± 1.1 | 25.1 ± 1.1‡ | 26.6 ± 2.2†‡ | 28.9 ± 1.9†‡ | 30.4 ± 2.4†‡ | 33.0 ± 1.9‡ |
| − | 22.9 ± 1.0 | 24.8 ± 1.7 | 26.8 ± 1.5‡ | 29.4 ± 2.3‡ | 31.7 ± 0.9‡ | 33.0 ± 0.8‡ | 35.0 ± 1.1‡ | |
| | ||||||||
| Mean airway pressure, cm H2O | ||||||||
| High | + | 18.6 ± 0.5*† | 19.0 ± 0.7*† | 19.8 ± 0.7*†‡ | 19.9 ± 0.8*†‡ | 20.1 ± 1.1‡ | 20.6 ± 1.2‡ | 20.6 ± 1.3*‡ |
| − | 19.2 ± 1.7*† | 19.3 ± 1.8*† | 19.5 ± 1.9*† | 20.1 ± 1.7*†‡ | 20.5 ± 1.4†‡ | 20.8 ± 1.4‡ | 20.9 ± 1.6*‡ | |
| Low | + | 12.5 ± 0.7 | 13.1 ± 0.5 | 13.4 ± 0.8 | 14.2 ± 1.5‡ | 15.4 ± 1.8‡§ | 16.4 ± 2.0‡§ | 17.8 ± 1.9‡ |
| − | 12.9 ± 1.0 | 14.4 ± 2.0‡ | 15.5 ± 2.2‡ | 16.7 ± 2.6‡ | 18.2 ± 1.3‡ | 18.8 ± 1.3‡ | 19.5 ± 1.5‡ | |
| | ||||||||
| PEEP, cm H2O | ||||||||
| High | + | 13.4 ± 0.8*† | 13.4 ± 0.8*† | 13.4 ± 0.8*† | 13.4 ± 0.8*† | 13.4 ± 0.8*† | 13.4 ± 0.8*† | 13.4 ± 0.8*† |
| − | 13.3 ± 1.4*† | 13.3 ± 1.4*† | 13.3 ± 1.4*† | 13.3 ± 1.4*† | 13.3 ± 1.4*† | 13.3 ± 1.4*† | 13.3 ± 1.4*† | |
| Low | + | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.0 ± 0.0 | 5.3 ± 0.8†‡ | 6.0 ± 1.2†‡ | 6.9 ± 1.1‡ | 7.4 ± 1.1‡ |
| − | 5.0 ± 0.0 | 5.7 ± 1.3 | 6.4 ± 1.4‡ | 7.4 ± 1.1‡ | 7.9 ± 0.4‡ | 7.9 ± 0.4‡ | 7.9 ± 0.4‡ | |
| | ||||||||
| Peak transpulmonary pressure, cm H2O | ||||||||
| High | + | 20.7 ± 2.3 | 21.2 ± 2.0 | 21.9 ± 2.8 | 23.3 ± 2.6 | 24.5 ± 2.1‡ | 25.9 ± 1.6‡ | 26.2 ± 1.8‡ |
| − | 20.2 ± 2.0 | 20.1 ± 1.9 | 21.5 ± 1.8 | 22.5 ± 1.6*‡ | 23.2 ± 1.6‡ | 24.0 ± 1.7‡ | 24.9 ± 1.9‡ | |
| Low | + | 21.2 ± 1.9 | 22.0 ± 2.3† | 24.8 ± 3.8† | 26.5 ± 2.8†‡ | 28.4 ± 3.9‡§ | 29.6 ± 2.4‡§ | 33.2 ± 3.4‡§ |
| − | 16.2 ± 1.5§ | 18.3 ± 1.8 | 20.3 ± 1.3‡ | 22.3 ± 2.5‡ | 24.7 ± 1.2‡ | 26.1 ± 1.0‡ | 27.9 ± 1.5‡ | |
| | ||||||||
| Peak ∆ transpulmonary pressure, cm H2O | ||||||||
| High | + | 12.6 ± 1.0*† | 13.3 ± 0.4*† | 14.1 ± 1.1*†‡ | 15.3 ± 0.6*†‡ | 16.6 ± 0.7‡ | 17.4 ± 0.8‡ | 18.0 ± 0.5‡ |
| − | 12.1 ± 1.3*† | 12.2 ± 1.2*† | 13.5 ± 1.1*†‡ | 14.6 ± 1.0*†‡ | 15.6 ± 1.1‡ | 16.3 ± 1.3‡ | 17.2 ± 1.0‡ | |
| Low | + | 16.8 ± 0.9 | 18.1 ± 1.5 | 20.5 ± 2.5‡ | 22.2 ± 1.7‡ | 25.0 ± 2.8‡§ | 26.5 ± 1.6†‡ | 29.6 ± 2.7‡§ |
| − | 16.5 ± 0.7 | 17.2 ± 1.0‡ | 18.1 ± 0.9‡ | 20.1 ± 1.1‡ | 22.0 ± 1.1‡§ | 23.0 ± 0.8‡§ | 24.5 ± 1.4‡§ | |
| | ||||||||
| Dynamic compliance of respiratory system, ml/cm H2O | ||||||||
| High | + | 1.24 ± 0.1*† | 1.18 ± 0.1*† | 1.11 ± 0.1‡ | 1.02 ± 0.1*†‡ | 0.98 ± 0.1*†‡ | 0.91 ± 0.0‡ | 0.86 ± 0.0‡ |
| − | 1.27 ± 0.1*† | 1.25 ± 0.1*† | 1.13 ± 0.1‡ | 1.06 ± 0.1*†‡ | 1.01 ± 0.1*†‡ | 0.94 ± 0.1‡ | 0.88 ± 0.1‡ | |
| Low | + | 0.89 ± 0.1 | 0.82 ± 0.1‡ | 0.72 ± 0.1‡§ | 0.68 ± 0.0‡ | 0.59 ± 0.0‡ | 0.56 ± 0.1‡§ | 0.50 ± 0.0‡§ |
| − | 0.93 ± 0.1 | 0.89 ± 0.0‡ | 0.83 ± 0.1‡§ | 0.75 ± 0.0‡ | 0.71 ± 0.0‡ | 0.68 ± 0.0‡§ | 0.62 ± 0.0‡§ | |
| | ||||||||
| Vt, ml/kg | ||||||||
| High | + | 6.3 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.3 ± 0.2 | 6.2 ± 0.2 | 6.1 ± 0.1 |
| − | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.1 ± 0.1 | |
| Low | + | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.1 |
| − | 6.2 ± 0.2 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | 6.2 ± 0.1 | |
| | ||||||||
| Respiratory rate, breaths/min | ||||||||
| High | + | 105 ± 10*† | 107 ± 7*† | 113 ± 6*†‡ | 111 ± 9*† | 108 ± 7*† | 106 ± 12*† | 108 ± 9*† |
| − | 109 ± 7*† | 109 ± 7*† | 109 ± 7*† | 109 ± 7*† | 111 ± 5*† | 111 ± 5*† | 111 ± 5*† | |
| Low | + | 139 ± 8 | 140 ± 7 | 141 ± 5 | 141 ± 9 | 141 ± 6 | 141 ± 7 | 143 ± 6 |
| − | 135 ± 2 | 136 ± 2 | 136 ± 2 | 137 ± 2 | 137 ± 2 | 137 ± 2 | 137 ± 2 | |
| | ||||||||
| PaCO2, mm Hg | ||||||||
| High | + | 64 ± 9 | 70 ± 13 | 69 ± 15 | 75 ± 14*‡ | 74 ± 13‡ | 72 ± 11‡ | 74 ± 11‡ |
| − | 62 ± 13 | 65 ± 15 | 67 ± 17 | 72 ± 19‡ | 72 ± 19‡ | 73 ± 19‡ | 74 ± 18‡ | |
| Low | + | 60 ± 5 | 55 ± 5 | 57 ± 3 | 54 ± 3‡ | 58 ± 3 | 58 ± 4 | 63 ± 4 |
| − | 60 ± 9 | 59 ± 6 | 57 ± 8 | 57 ± 8 | 58 ± 9 | 58 ± 8 | 58 ± 6 | |
| | ||||||||
| pH | ||||||||
| High | + | 7.25 ± 0.05 | 7.24 ± 0.05* | 7.27 ± 0.06 | 7.30 ± 0.04‡ | 7.32 ± 0.03‡ | 7.32 ± 0.03‡ | 7.30 ± 0.04‡ |
| − | 7.25 ± 0.08 | 7.27 ± 0.07 | 7.28 ± 0.08 | 7.31 ± 0.08‡ | 7.35 ± 0.06‡ | 7.35 ± 0.05‡ | 7.34 ± 0.05‡ | |
| Low | + | 7.29 ± 0.06 | 7.31 ± 0.02 | 7.32 ± 0.02 | 7.36 ± 0.03‡ | 7.35 ± 0.03‡ | 7.34 ± 0.05 | 7.32 ± 0.06 |
| − | 7.26 ± 0.03 | 7.26 ± 0.05 | 7.33 ± 0.04‡ | 7.34 ± 0.04‡ | 7.35 ± 0.05‡ | 7.33 ± 0.03‡ | 7.34 ± 0.04‡ | |
Definition of abbreviations: PEEP = positive end-expiratory pressure; SB = spontaneous breathing.
Data presented are mean ± SD.
P < 0.05 compared with low PEEP + SB.
P < 0.05 compared with low PEEP − SB.
P < 0.05 compared with 0 (at the start of the protocol) within groups.
P < 0.05 compared with other groups.
Table 2.
Respiratory Measurement: Pig
| PEEP | SB | Time after the Start of the Protocol |
||
|---|---|---|---|---|
| 1 h | 8 h | 16 h | ||
| PaO2/FiO2, mm Hg | ||||
| High | + | 342 ± 105 | 409 ± 46* | 412 ± 53* |
| Low | + | 222 ± 118 | 221 ± 97 | 246 ± 100 |
| | ||||
| Plateau airway pressure, cm H2O | ||||
| High | + | 29 ± 4.2 | 26.2 ± 2.5 | 27 ± 2.2 |
| Low | + | 25.4 ± 2.7 | 25.7 ± 4.6 | 25 ± 3.5 |
| | ||||
| Mean airway pressure, cm H2O | ||||
| High | + | 21.1 ± 2.7* | 20 ± 2.1* | 20.3 ± 1.6* |
| Low | + | 14.8 ± 2.5 | 14.7 ± 2.5 | 14 ± 1.9 |
| | ||||
| PEEP, cm H2O | ||||
| High | + | 15 ± 2* | 15 ± 2* | 15 ± 2* |
| Low | + | 8 ± 3 | 7 ± 2 | 7 ± 2 |
| | ||||
| Peak transpulmonary pressure, cm H2O | ||||
| High | + | 23.3 ± 4.5 | 21.0 ± 2.6 | 21.4 ± 2.3 |
| Low | + | 26.4 ± 2.7 | 26.0 ± 4.1 | 26.2 ± 4.8 |
| | ||||
| Peak ∆ transpulmonary pressure, cm H2O | ||||
| High | + | 17.8 ± 2.0* | 15.6 ± 2.7* | 15.9 ± 3.4* |
| Low | + | 26.9 ± 2.7 | 26.0 ± 4.1 | 26.2 ± 4.8 |
| | ||||
| Dynamic compliance of respiratory system, ml/cm H2O | ||||
| High | + | 11.2 ± 2.0* | 12.2 ± 2.7* | 13.0 ± 2.9* |
| Low | + | 8.5 ± 1.6 | 8.9 ± 4.0 | 9.2 ± 3.5 |
| | ||||
| Vt, ml/kg | ||||
| High | + | 6.1 ± 0.6 | 5.7 ± 0.6 | 6.2 ± 0.8 |
| Low | + | 6.4 ± 0.7 | 6.0 ± 0.7 | 6.3 ± 1.0 |
| | ||||
| Respiratory rate, breaths/min | ||||
| High | + | 36 ± 5* | 36 ± 6* | 36 ± 8* |
| Low | + | 58 ± 4 | 54 ± 9 | 54 ± 11 |
| | ||||
| PaCO2, mm Hg | ||||
| High | + | 67 ± 15 | 61 ± 17 | 60 ± 11 |
| Low | + | 55 ± 9 | 61 ± 12 | 60 ± 16 |
| | ||||
| pH | ||||
| High | + | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 |
| Low | + | 7.3 ± 0.1 | 7.3 ± 0.1 | 7.3 ± 0.1 |
For definition of abbreviations, see Table 1.
Data presented are mean ± SD.
P < 0.05 compared with low PEEP + SB.
In rabbits and pigs, Vt was similar (∼6 ml/kg, low Vt) in all groups and PEEP was significantly greater in high- versus low-PEEP groups (Tables 1 and 2). In rabbits, peak ∆PL at the start was higher in low-PEEP than in high-PEEP groups, and was highest in low PEEP + SB at the end (Table 1). In pigs, peak ∆PL was higher in low PEEP + SB than in high PEEP + SB (Table 2).
Spontaneous Effort and PEEP: Rabbit, Pig, and Human
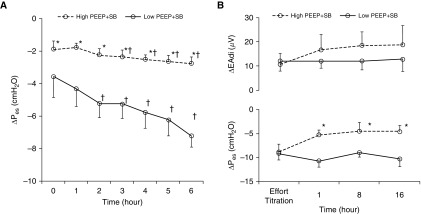
The intensity of spontaneous effort was estimated by ΔPes in rabbits and pigs, and in patients with ARDS by ΔPes (and/or ΔEAdi). In rabbits, ΔPes was lower in high PEEP than in low PEEP; it became significantly more negative in the low-PEEP group (Figure 1A), despite use of higher doses of sedatives (see Table E1 in the online supplement). The same relationship between ΔPes and PEEP was observed in pigs (Figure 1B), where ΔPes was more negative in the low-PEEP group than in the high-PEEP group, notwithstanding the higher sedative doses used (in low PEEP) to match the ∆EAdi levels in the two groups (see Table E1).
Figure 1.
Intensity of spontaneous effort in high versus low positive end-expiratory pressure (PEEP) in rabbit and pig. The intensity of inspiratory effort was evaluated as the magnitude of the negative swing in esophageal pressure (ΔPes). (A) In the rabbit, ΔPes was lower in high than in low PEEP throughout the protocol, despite higher doses of sedatives (titrated to prevent spontaneous limb movement). ΔPes became significantly more negative in low PEEP as lung injury progressed. (B) In the pig, ΔPes was lower in high than in low PEEP throughout the protocol, despite higher doses of sedatives (titrated to similar target levels of ∆EAdi in both groups). Data shown as mean ± SD. EAdi = electrical activity of the diaphragm; SB = spontaneous breathing. *P < 0.05 versus low PEEP + SB; †P < 0.05 versus start of the protocol within the group.
Because PaO2 was higher in high- than in low-PEEP groups, we performed additional experiments to maintain constant PaO2 (by adjusting FiO2) during SB at low and high PEEP, and ∆Pes was lower in the high-PEEP group (see Figure E1). But the behavior of ∆EAdi was not consistent with changing PEEP (∆EAdi was decreased, increased, or unchanged; see Figure E1) in pigs.
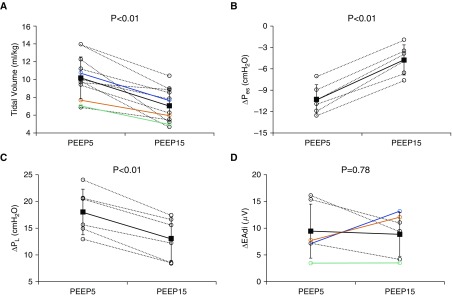
The clinical features of 11 patients with ARDS are described (Table 3). High PEEP of 15 cm H2O decreased ∆Pes and thus peak ∆PL, compared with PEEP of 5 cm H2O (∆Pes: −10.3 ± 2.0 to −4.8 ± 2.1 cm H2O; peak ∆PL: 18.1 ± 4.2 to 13.1 ± 4.0 cm H2O; n = 6; P < 0.01, respectively) (Figures 2 and 3). Vt was significantly lower in all patients with ARDS at high PEEP versus low PEEP (Vt: 10.2 ± 2.4 to 7.0 ± 1.9 ml/kg; n = 11; P < 0.01) (Figure 2). In accordance with the findings in pigs, however, the behavior of ∆EAdi was not consistent with changing PEEP (∆EAdi was decreased, increased, or unchanged) (Figure 2).
Table 3.
Characteristics of 11 Patients with ARDS
| No. | Sex | Age (yr) | BMI (kg/m2) | Cause of ARDS | P/F Ratio at Baseline (mm Hg) | Ventilation Days | RASS | Sedation/Analgesia | P/F Ratio at Study (mm Hg) | Pes or EAdi |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 70 | 24 | Community-acquired pneumonia | 97 | 1 | −4 | Propofol fentanyl | 97 | Pes |
| 2 | F | 53 | 26 | Pneumocystis pneumonia | 199 | 9 | −3 | Midazolam dexmedetomidine | 164 | Pes and EAdi |
| 3 | M | 63 | 23 | Ventilator-associated pneumonia, cardiac surgery (MVP) | 138 | 11 | −1 | Propofol | 207 | Pes |
| 4 | M | 79 | 24 | Cardiac surgery (AVR + CABG) | 105 | 3 | −3 | Propofol | 185 | Pes |
| 5 | M | 72 | 25 | Aspiration pneumonia | 79 | 4 | −3 | Propofol fentanyl dexmedetomidine | 240 | Pes |
| 6 | M | 41 | 23 | Abdominal surgery (pelvic exenteration) | 248 | 3 | −2 | Propofol | 147 | EAdi |
| 7 | F | 44 | 25 | Abdominal surgery (simultaneous pancreas and kidney transplantation) | 262 | 5 | −2 | Propofol fentanyl | 338 | EAdi |
| 8 | M | 66 | 21 | Acute pancreatitis | 66 | 14 | −5 | Propofol midazolam morphine | 112 | EAdi |
| 9 | F | 61 | 17 | Abdominal surgery (simultaneous pancreas and kidney transplantation) | 265 | 3 | −4 | Propofol dexmedetomidine | 270 | EAdi |
| 10 | M | 43 | 21 | Cardiac surgery (LVAD) | 232 | 1 | 0 | Propofol fentanyl | 232 | EAdi |
| 11 | M | 68 | 34 | Ventilator-associated pneumonia, cardiac surgery (CABG) | 101 | 3 | −2 | Propofol fentanyl | 213 | Pes |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; AVR = arterial valve replacement; BMI = body mass index; CABG = coronary artery bypass grafting; EAdi = electrical activity of the diaphragm; LVAD = left ventricular assist device; MVP = mitral valve plasty; Pes = esophageal pressure; P/F = PaO2/FiO2; RASS = Richmond Agitation-Sedation Scale.
Figure 2.
Intensity of spontaneous effort in high versus low positive end-expiratory pressure (PEEP) in patients with acute respiratory distress syndrome. (A) Vt was significantly decreased in all patients with acute respiratory distress syndrome at high PEEP versus low PEEP. (B and C) High PEEP (PEEP of 15 cm H2O; PEEP15) decreased ∆Pes (B) and thus peak ∆PL (C), compared with low PEEP (PEEP of 5 cm H2O; PEEP5). (D) However, the response of ∆EAdi was variable after increased PEEP. The black solid line and the error bars indicate mean and SD of all data. The black dotted lines connect each variable at different PEEP levels measured in the same patient. The data shown in colored lines correspond to the same patients (A and D). EAdi = electrical activity of the diaphragm; Pes = esophageal pressure; Pl = transpulmonary pressure.
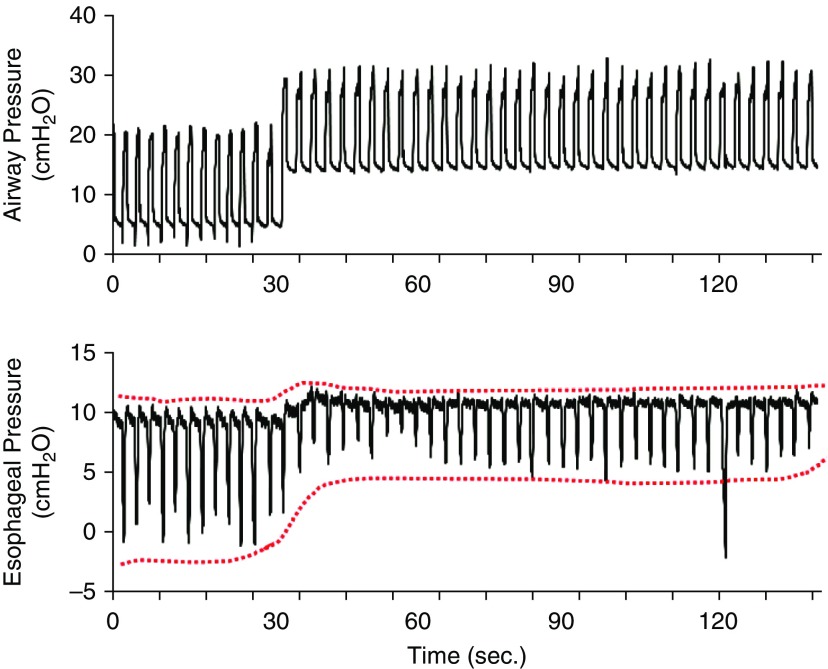
Figure 3.
Intensity of spontaneous effort in high versus low positive end-expiratory pressure in a patient with acute respiratory distress syndrome. Representative waveforms were obtained from patient 1. The magnitude of the negative swings of esophageal pressure was reduced by approximately 50% when positive end-expiratory pressure was increased from 5 to 15 cm H2O. Note that high positive end-expiratory pressure did not substantially reduce respiratory rate. The red dotted lines outline the esophageal pressures.
Regional Pleural Pressure and Lung Stretch: Pig
There was a large vertical gradient of inspiratory negative ΔPpl from nondependent to dependent regions at low PEEP. The magnitude of ∆Ppl in dependent lung regions was almost twofold greater than ∆Ppl in nondependent lung regions at low PEEP (−18.1 ± 4.0 vs. −9.8 ± 2.9 cm H2O; P < 0.01) (Table 4). In contrast, high PEEP significantly reduced a vertical gradient of ΔPpl from nondependent to dependent regions; ΔPpl in dependent lung regions was significantly reduced during spontaneous effort at high PEEP versus at low PEEP (Table 4).
Table 4.
Changes in Local Pleural Pressures during Spontaneous Effort at High PEEP versus Low PEEP: Pig
| PEEP | Lung Regions |
|
|---|---|---|
| Nondependent | Dependent | |
| Negative swing in local pleural pressures, cm H2O | ||
| High | −9.9 ± 2.8 | −13.3 ± 2.3* |
| Low | −9.8 ± 2.9 | −18.1 ± 4.0*† |
Definition of abbreviation: PEEP = positive end-expiratory pressure.
All measurements recorded at Δ esophageal pressure approximately −10 cm H2O. Data presented are mean ± SD.
P < 0.01 compared with pleural pressures in nondependent lung regions.
P < 0.01 compared with pleural pressures in dependent lung regions at high PEEP.
Regional lung stretch estimated using EIT reflected the vertical gradient of ∆Ppl in the presence of low PEEP: stronger effort at low PEEP (ΔPes: −5.6 ± 1.3 cm H2O) shifted the ventilation into dependent lung regions, increasing dependent lung stretch almost fivefold greater than that of muscle paralysis at low PEEP, whereas mild effort at high PEEP (ΔPes: −2.0 ± 0.7 cm H2O) increased dependent lung stretch only by 1.6 times that of muscle paralysis at high PEEP (Table 5).
Table 5.
The Impact of Spontaneous Effort on Local Lung Stretch (Delta Z) at High versus Low PEEP: Pig
| High PEEP |
Low PEEP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nondependent |
Middle |
Dependent |
Nondependent |
Middle |
Dependent |
|||||||
| −SB | +SB | −SB | +SB | −SB | +SB | −SB | +SB | −SB | +SB | −SB | +SB | |
| Delta Z (lung stretch) | 4 ± 1.8*† | 3.7 ± 1.7*† | 9.9 ± 3.1 | 8.7 ± 2.9 | 6.6 ± 2.1 | 10.3 ± 5.3† | 8.9 ± 3.8 | 7.1 ± 2.5 | 14.1 ± 5.1‡ | 9.8 ± 3.8 | 2.9 ± 2.1 | 8.6 ± 6.0† |
| Fold vs. −SB | ×0.9 ± 0.1 | ×0.9 ± 0.2 | ×1.6 ± 0.4 | ×0.8 ± 0.2 | ×0.8 ± 0.5 | ×4.5 ± 4.5 | ||||||
For definition of abbreviations, see Table 1.
Data presented are mean ± SD.
P < 0.05 compared with low PEEP + SB.
P < 0.05 compared with low PEEP − SB.
P < 0.05 compared with other groups.
Dynamic CT: Rabbit
Representative CT images (progression of no-aeration) at end-expiration in all groups are shown in Figure E2. In accordance with the distribution of ventilation in EIT (Table 5), dynamic CT confirmed that SB in low PEEP shifted the ventilation into dependent, atelectatic lung regions (see Table E2), causing the greatest degree of tidal recruitment in dependent lung (14.1 ± 1.6%; P < 0.05 vs. other groups; see Table E3 and Figure E3). In contrast, muscle paralysis at low PEEP shifted the ventilation into nondependent lung (see Table E2). Thus, tidal recruitment was restricted in the upper parts of the atelectatic regions (i.e., mid-lung; see Figure E3).
Distribution of Histologic Injury: Rabbit
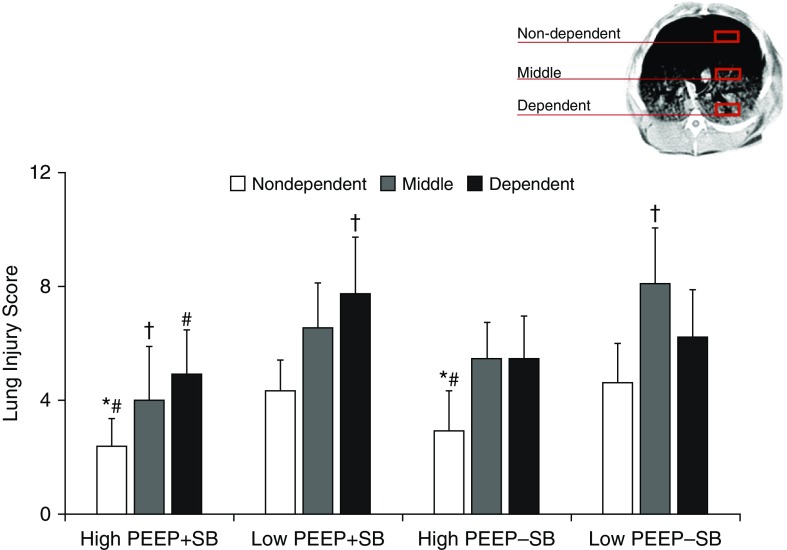
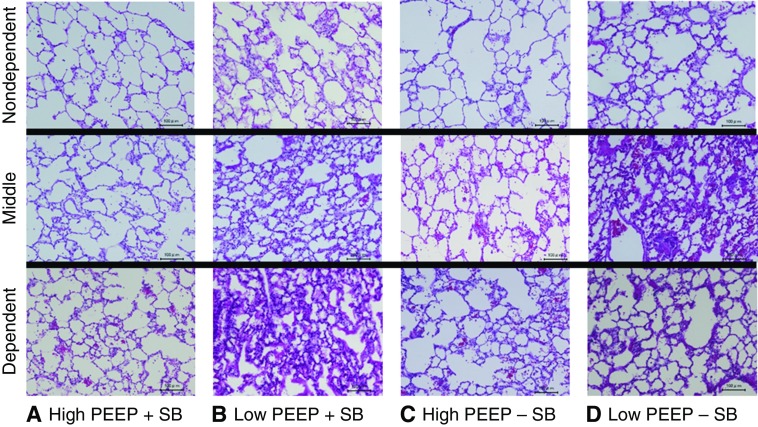
The distribution of histologic injury in each group is presented (Figure 4; see Table E4) with illustrative sections (Figure 5).
Figure 4.
Regional lung injury (quantitative). During spontaneous breathing (SB) low positive end-expiratory pressure (PEEP) increased injury in all lung regions, especially in dependent lung (severest among all groups); but high PEEP during SB reduced injury in all lung regions. In contrast, low PEEP during paralysis increased injury in nondependent and especially in middle lung regions (severest among all groups). High PEEP during paralysis reduced injury in nondependent and middle lung, but not in dependent lung. *P < 0.05 versus low PEEP + SB; #P < 0.05 versus low PEEP − SB; †P < 0.05 compared with other groups.
Figure 5.
Regional lung injury (illustrative). Representative images (hematoxylin and eosin; scale bars, 100 μm) are shown. (A) High positive end-expiratory pressure (PEEP) + spontaneous breathing (SB). (B) Low PEEP + SB. (C) High PEEP − SB. (D) Low PEEP − SB.
In nondependent lung, injury was greatest in the two low-PEEP groups, and least in the two high-PEEP groups (i.e., low PEEP − SB ≈ low PEEP + SB > high PEEP − SB ≈ high PEEP + SB). In mid lung regions, injury was greatest in low PEEP − SB and least in high PEEP + SB (i.e., low PEEP − SB > low PEEP + SB ≈ high PEEP − SB > high PEEP + SB). In dependent lung, injury was greatest in low PEEP + SB, and least in high PEEP + SB (i.e., low PEEP + SB > low PEEP − SB ≈ high PEEP − SB > high PEEP + SB).
Thus, during SB low PEEP increased injury in all lung regions, but especially in dependent lung (most severe lung injury among all groups); in contrast, high PEEP during SB reduced injury in all lung regions. In contrast, during paralysis low PEEP increased injury in nondependent and especially in mid lung regions (most severe lung injury among all groups). High PEEP during paralysis reduced injury in nondependent and mid lung, but not in dependent lung.
Distribution of Inflammation: PET-Pig
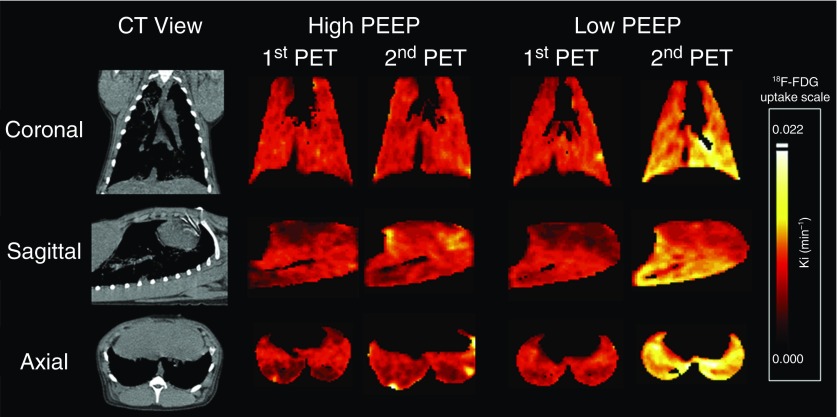
Global lung inflammation at the start of protocol was similar in high- and low-PEEP groups (whole-lung Ki 0.004 ± 0.001 vs. 0.004 ± 0.002 min−1). Stronger effort at low PEEP increased inflammation at normal, poorly aerated, and nonaerated lung regions, compared with high PEEP (see Figure E4). Stronger effort at low PEEP increased inflammation especially in dependent lung regions close to the diaphragm during 16-hour protocol (Figure 6); in addition, inflammation was progressively lower in lung regions more distant from the diaphragm (see Figure E5). Finally, lung inflammation predominantly occurred in dependent lung regions, the same regions where the magnitude of local dependent lung stretch was equivalent to that applied by Vt of 14 ml/kg during muscle paralysis (i.e., local volutrauma) (Figure 7; see Video E1). In contrast, high PEEP during SB reduced inflammation across all lung regions, especially in normal, and poorly aerated and nonaerated lung regions (Figure 6; see Figure E4).
Figure 6.
Distribution of inflammation in lung. Representative positron emission tomography (PET) scan images of [18F]fluoro-2-deoxy-d-glucose (18F-FDG) uptake after lung injury (first PET scan) and after 16 hours (second PET scan) in high versus low positive end-expiratory pressure (PEEP). Pixels are represented in the heat color scale, showing higher 18F-FDG uptake as lighter shades. Spontaneous effort with low PEEP increased lung inflammation, especially in dependent regions close to the diaphragm; in contrast, high PEEP during spontaneous effort resulted in less lung inflammation in these regions. CT = computed tomography.
Figure 7.
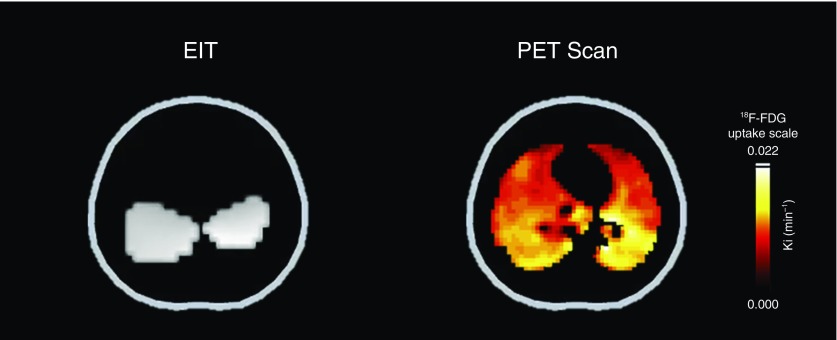
Local volutrauma and inflammation. Representative electrical impedance tomography (EIT) and positron emission tomography (PET) in low positive end-expiratory pressure images are presented. This EIT image shows lung regions where lung stretch was increased because of pendelluft (translocation of gas from nondependent to dependent lung regions during inspiration), as white. Vt was maintained at 7 ml/kg, but the magnitude of local dependent lung stretch (white regions), due to the localization of more negative ∆ pleural pressure (i.e., higher local lung stress), was equivalent to that applied by Vt of 14 ml/kg during muscle paralysis (i.e., local volutrauma). Correspondingly, PET imaging confirmed that lung inflammation predominantly occurred in the dependent regions, the same regions where local volutrauma occurred. 18F-FDG = [18F]fluoro-2-deoxy-d-glucose.
Discussion
The main findings in this study are that although ventilator-induced lung injury during muscle paralysis is predominantly in baby lung (i.e., the nondependent) and especially middle lung at low (vs. high) PEEP, strong spontaneous effort increased injury (indicated by PET and histology) especially in the dependent lung; in addition, higher levels of PEEP minimized this dependent lung injury but preserved spontaneous effort.
A key mechanism whereby strong effort increased dependent lung injury was a large vertical gradient of inspiratory Ppl swings, between the nondependent (less negative ΔPpl) and the dependent (more negative ΔPpl) lung. The resulting higher local lung stress in the dependent lung caused injurious inflation patterns (i.e., local overstretch and tidal recruitment) in the dependent lung by drawing gas either from other lung regions (e.g., nondependent lung [pendelluft]) (11) (see Video E1) or from the ventilator.
Higher levels of PEEP rendered spontaneous effort less injurious by two mechanisms: lowering the level of spontaneous effort (via neuromechanical uncoupling), and by converting solid-like (more atelectatic) lung to fluid-like (less atelectatic) lung, which in turn reduces the vertical gradient of inspiratory ΔPpl. Thus, both mechanisms work together to promote a more homogeneous lung expansion by avoiding a disproportionately strong diaphragmatic contraction in relation to the other respiratory muscles.
Positive Pressure Ventilation Injures Baby Lung
In accordance with previous findings (27–31), the current study confirmed that at low levels of PEEP (in which case atelectasis predominated in the dependent lung), positive pressure ventilation during muscle paralysis worsened lung injury in baby (nondependent and especially middle) lung regions (Figures 4 and 5).
Corroborating these findings, lung imaging confirmed that paralysis caused a shift of ventilation from dependent (during spontaneous effort) toward middle and nondependent lung (during paralysis) (Table 5; see Table E2), leading to tidal recruitment that occurred only in the upper parts of the atelectatic regions (i.e., middle lung; see Figure E3). Thus, lung injury predominantly occurred in baby lung where higher inspiratory stretch occurred during muscle paralysis at low PEEP, and tidal recruitment enhanced the progression of lung injury in middle lung (32–34).
Spontaneous Effort Injures Dependent Lung
The current data are the first to demonstrate that the bulk of effort-dependent lung injury occurred in the dependent lung, the same region where strong effort increased greater inspiratory stress (Table 4) and stretch (Table 5 and Figure 7). The findings are consistent using two techniques and two species: histology in rabbits (Figures 4 and 5) and PET scans in pigs (Figures 6 and 7).
In ARDS, atelectatic lung tissue behaves in a solid-like manner, therefore deflections of Ppl caused by diaphragmatic contraction are poorly transmitted beyond the dependent, atelectatic lung to the reminder of the lung surface (11, 13); this caused a large vertical gradient of ΔPpl from nondependent (less negative) to dependent (more negative) lung (Table 4). The resulting higher local lung stress in dependent lung caused substantial inspiratory stretch (approximately fivefold compared with muscle paralysis), by drawing gas either from other lung regions (e.g., nondependent lung [pendelluft]) (see Video E1) or from the ventilator without changing overall lung volume (i.e., Vt) (11–13). Thus, strong effort caused injurious inflation patterns including local volutrauma (Figure 7) and tidal recruitment (see Table E3 and Figure E3), and dependent lung injury (Figures 4–7).
Although injurious inflation patterns resulting from strong effort cannot be detected using standard airway monitoring (11, 13, 15), it is clear from the current data that effort-dependent lung injury cannot be prevented by limiting Vt or plateau pressure.
Minimizing Lung Injury from Spontaneous Effort
Higher levels of PEEP minimized effort-dependent lung injury while preserved spontaneous effort during mechanical ventilation (Figures 4–7), and there are two potential mechanisms of this effect: conversion of solid-like (more atelectatic) to fluid-like (less atelectatic) lung, and lowering the intensity of spontaneous effort.
First, high (vs. low) PEEP significantly reduced the amount of atelectatic solid-like lung and maximized fluid-like lung (see Table E3 and Figure E2), and this resulted in a smaller vertical gradient of negative Ppl deflections between dependent and nondependent regions (Table 4). High PEEP achieved more homogeneous distribution of ΔPpl over the whole lung surface, after diaphragmatic contraction. The even distribution of inspiratory stress and stretch could diminish (focal points of) injurious inflation associated with spontaneous effort.
Second, higher levels of PEEP also decreased spontaneous effort (reflected by ∆Pes, but not by ∆EAdi) (Figure 2; see Figure E1); this resulted in lower peak ∆PL (i.e., dynamic lung stress) and lower Vt in patients with ARDS (Figure 2). Also, we confirmed that PEEP was a modifier of respiratory drive because ∆Pes was lower at higher PEEP despite maintaining constant the other key parameters that influence respiratory drive (i.e., PaO2, PaCO2, and pH; see Figure E1).
Among several potential mechanisms whereby high PEEP may decrease spontaneous effort (reflected by ∆Ppl) (35–40), the impact on the force–length relationship and curvature of the diaphragm may be important. High PEEP may change the force–length relationship of the diaphragm and reduce curvature of the diaphragm, leading to electromechanical uncoupling (35–37, 40). ∆Pes (or Ppl) after phrenic nerve stimulation is known to be lessened as the end-expiratory lung volume is increased (36, 37, 40). Indeed, we demonstrate that a given level of ∆EAdi, ∆Pes, was kept less negative for 16 hours at high PEEP (vs. low PEEP) in pigs (Figure 1). Also, in pigs and patients with ARDS, high PEEP reduced ∆Pes, but not ∆EAdi (Figure 2; see Figure E1). This observation (i.e., neuromechanical uncoupling) explains, in part, why high PEEP reduced spontaneous effort (and why low PEEP increased it). Irrespective of the mechanism, higher levels of PEEP reduced the strength of spontaneous effort proportional to the greater magnitude of dependent inspiratory stretch, which in turn minimized effort-dependent lung injury.
Study Limitations
There are several limitations to the current work. First, in this study (rabbits and pigs), we established a model of severe ARDS by the combination of repeated surfactant depletion and injurious mechanical ventilation. Then, rabbits were ventilated for 6 hours (and pigs for 16 h). In addition, this was a recruitable model; by contrast, human ARDS has many etiologies (e.g., pneumonia, sepsis, trauma) and the time-course is usually days (rather than hours, as in experimental models). In addition, the lung in human ARDS often has a heterogeneous distribution of aeration and, in contrast to the experimental setting, it may be difficult to recruit.
Although this model has been successfully used to illustrate key principles underlying ventilator-induced lung injury, caution is necessary in extrapolating the current data to the clinical context. The effects of high PEEP on the intensity of spontaneous effort (and lung injury) in nonrecruitable lungs are unknown. Second, in this study, we did not separate inspiratory effort component and expiratory effort component from total spontaneous effort. This is an important issue in future work because expiratory effort may potentially worsen lung injury, in part by decreasing expiratory transpulmonary pressure (41).
Clinical Implications
These are the first data to report the localization of effort-dependent lung injury (dependent lung); taken together with earlier studies reporting that injury from mechanical breaths predominate in baby lung (27–31), the emerging picture is that in ventilator-induced lung injury, the injury occurs in the lung regions receiving the most stretch (or ventilation). This could certainly impact on future definitions of injury (to include region), and future developments in regional lung monitoring and management.
The study also has potentially important management implications. Effort-dependent lung injury was not preventable using global parameters, such as limitation of Vt or plateau pressure; instead, direct management of the strength of spontaneous effort and/or minimizing the proportion of solid-like (atelectatic) lung decreased effort-dependent lung injury without the use of muscle paralysis. The current study demonstrated that increasing PEEP can accomplish both of these aims, but in other situations, sedation or correction of acidosis may be used to reduce the inspiratory effort. This is important because even short-term blockade might cause serious ICU-acquired weakness; indeed, concerns about this may explain its infrequent use (<40%) in patients with severe ARDS, despite the strong evidence for survival benefit (14).
Conclusions
Strong effort increases injury in dependent lung, the regions in which higher local lung stress and stretch was generated. Such injury was lessened with high PEEP, and this acted by converting solid-like into fluid-like lung and by lowering the intensity of spontaneous effort. Therefore, higher PEEP may facilitate noninjurious spontaneous effort in severe ARDS, and this may offset the need of muscle paralysis.
Acknowledgments
Acknowledgement
The authors thank Jose Claudio Meneghetti, M.D., Ph.D. (Director of the Department of Nuclear Medicine and Molecular Imaging, Heart Institute of the University of São Paulo Medical School, São Paulo, Brazil), and Marco Antonio de Oliveira and Luis Tonello Gonçalves (Nuclear Medicine Technologists, Department of Nuclear Medicine and Molecular Imaging, Heart Institute of the University of São Paulo Medical School, São Paulo, Brazil) for image acquisition and reconstruction.
Footnotes
This work was supported by Grant-in-Aid for Young Scientists #26861230, the Ministry of Education, Culture, Sports, Science and Technology, Japan; Marumo Research Foundation for Acute Care Medicine, Japan; São Paulo Research Foundation (FAPESP) (#2014/02030-7), São Paulo, Brazil; National Council for Scientific and Technological Development, Brazil; and Coordination for the Improvement of Higher Level Personnel, Brazil. This study was performed at Osaka University Graduate School of Medicine, Japan (rabbits and patients) and Faculdade de Medicina da Universidade de São Paulo, Brazil (pigs). M.F.V.M. was funded by NIH–NHLBI (R01 HL121228).
Author Contributions: C.C.A.M. designed and conducted the study (pigs–positron emission tomography [PET]) and analyzed the data (pigs–PET). Y.K. designed and conducted the study (rabbits and patients) and analyzed the data (rabbits and patients). T.Y. designed the study (rabbits, pigs–electrical impedance tomography [EIT], and patients), conducted the study (pigs–EIT and patients), analyzed the data (rabbits, pigs–EIT, and patients), wrote the manuscript, and revised the manuscript. G.M.P., S.G., C.A.S.L., O.P.S.R., and S.M.P. conducted the study (pigs–PET and –EIT). N.K. and H.Y. analyzed the data (rabbits). A.U. participated in the study design (rabbits and patients) and analyzed the data (rabbits and patients). J.B.B. and M.F.V.M. participated in the study design (pigs–PET). M.R.T. designed and conducted the study (pigs–PET and –EIT) and analyzed the data (pigs–PET and –EIT). M.B.P.A. designed the study (pigs–PET and –EIT), analyzed data (pigs–PET and –EIT), interpreted all data, and revised the manuscript. B.P.K. interpreted all data and revised the manuscript. E.V.L.C. designed and conducted the study (pigs–PET and –EIT), analyzed the data (pigs–PET and –EIT), interpreted all data, revised the manuscript, and organized the study as a supervisor (pigs–PET and –EIT). Y.F. designed the study (rabbits and patients), interpreted all data, and organized the study as a supervisor (rabbits and patients).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201706-1244OC on January 11, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Von Spiegel T, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. 2001;164:43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. 2004;170:626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 4.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. 2015;192:1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 5.Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- 6.Vassilakopoulos T, Divangahi M, Rallis G, Kishta O, Petrof B, Comtois A, et al. Differential cytokine gene expression in the diaphragm in response to strenuous resistive breathing. 2004;170:154–161. doi: 10.1164/rccm.200308-1071OC. [DOI] [PubMed] [Google Scholar]
- 7.Leray V, Bourdin G, Flandreau G, Bayle F, Wallet F, Richard JC, et al. A case of pneumomediastinum in a patient with acute respiratory distress syndrome on pressure support ventilation. 2010;55:770–773. [PubMed] [Google Scholar]
- 8.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, et al. Mechanical ventilation–induced diaphragm atrophy strongly impacts clinical outcomes. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. 2012;40:1578–1585. doi: 10.1097/CCM.0b013e3182451c40. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. The comparison of spontaneous breathing and muscle paralysis in two different severities of experimental lung injury. 2013;41:536–545. doi: 10.1097/CCM.0b013e3182711972. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Torsani V, Gomes S, De Santis RR, Beraldo MA, Costa EL, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. 2013;188:1420–1427. doi: 10.1164/rccm.201303-0539OC. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Roldan R, Beraldo MA, Torsani V, Gomes S, De Santis RR, et al. Spontaneous effort during mechanical ventilation: maximal injury with less positive end-expiratory pressure. 2016;44:e678–e688. doi: 10.1097/CCM.0000000000001649. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida T, Nakahashi S, Nakamura MAM, Koyama Y, Roldan R, Torsani V, et al. Volume-controlled ventilation does not prevent injurious inflation during spontaneous effort. 2017;196:590–601. doi: 10.1164/rccm.201610-1972OC. [DOI] [PubMed] [Google Scholar]
- 14.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. risks, mechanisms, and management. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 17.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 18.Bellani G, Laffey JG, Pham T, Madotto F, Fan E, Brochard L, et al. LUNG SAFE Investigators; ESICM Trials Group. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. 2017;195:67–77. doi: 10.1164/rccm.201606-1306OC. [DOI] [PubMed] [Google Scholar]
- 19.Hoppin FG, Jr, Green ID, Mead J. Distribution of pleural surface pressure in dogs. 1969;27:863–873. doi: 10.1152/jappl.1969.27.6.863. [DOI] [PubMed] [Google Scholar]
- 20.D’Angelo E, Agostoni E. Continuous recording of pleural surface pressure at various sites. 1973;19:356–368. doi: 10.1016/0034-5687(73)90039-x. [DOI] [PubMed] [Google Scholar]
- 21.D’Angelo E, Sant’Ambrogio G, Agostoni E. Effect of diaphragm activity or paralysis on distribution of pleural pressure. 1974;37:311–315. doi: 10.1152/jappl.1974.37.3.311. [DOI] [PubMed] [Google Scholar]
- 22.Borges JB, Okamoto VN, Matos GF, Caramez MP, Arantes PR, Barros F, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. 2006;174:268–278. doi: 10.1164/rccm.200506-976OC. [DOI] [PubMed] [Google Scholar]
- 23.Maeda Y, Fujino Y, Uchiyama A, Matsuura N, Mashimo T, Nishimura M. Effects of peak inspiratory flow on development of ventilator-induced lung injury in rabbits. 2004;101:722–728. doi: 10.1097/00000542-200409000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Martin CJ, Young AC, Ishikawa K. Regional lung mechanics in pulmonary disease. 1965;44:906–913. doi: 10.1172/JCI105207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa EL, Borges JB, Melo A, Suarez-Sipmann F, Toufen C, Jr, Bohm SH, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. 2009;35:1132–1137. doi: 10.1007/s00134-009-1447-y. [DOI] [PubMed] [Google Scholar]
- 26.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 27.Borges JB, Costa EL, Suarez-Sipmann F, Widström C, Larsson A, Amato M, et al. Early inflammation mainly affects normally and poorly aerated lung in experimental ventilator-induced lung injury. 2014;42:e279–e287. doi: 10.1097/CCM.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 28.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. 2006;174:279–289. doi: 10.1164/rccm.200506-1006OC. [DOI] [PubMed] [Google Scholar]
- 29.Bellani G, Messa C, Guerra L, Spagnolli E, Foti G, Patroniti N, et al. Lungs of patients with acute respiratory distress syndrome show diffuse inflammation in normally aerated regions: a [18F]-fluoro-2-deoxy-D-glucose PET/CT study. 2009;37:2216–2222. doi: 10.1097/CCM.0b013e3181aab31f. [DOI] [PubMed] [Google Scholar]
- 30.Bellani G, Guerra L, Musch G, Zanella A, Patroniti N, Mauri T, et al. Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. 2011;183:1193–1199. doi: 10.1164/rccm.201008-1318OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borges JB, Costa EL, Bergquist M, Lucchetta L, Widström C, Maripuu E, et al. Lung inflammation persists after 27 hours of protective acute respiratory distress syndrome network strategy and is concentrated in the nondependent lung. 2015;43:e123–e132. doi: 10.1097/CCM.0000000000000926. [DOI] [PubMed] [Google Scholar]
- 32.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. 1994;149:1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 33.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. 2004;32:168–174. doi: 10.1097/01.CCM.0000104203.20830.AE. [DOI] [PubMed] [Google Scholar]
- 34.Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, et al. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. 2014;18:505. doi: 10.1186/s13054-014-0505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans CL, Hill AV. The relation of length to tension development and heat production on contraction in muscle. 1914;49:10–16. doi: 10.1113/jphysiol.1914.sp001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pengelly LD, Alderson AM, Milic-Emili J. Mechanics of the diaphragm. 1971;30:797–805. doi: 10.1152/jappl.1971.30.6.797. [DOI] [PubMed] [Google Scholar]
- 37.Kim MJ, Druz WS, Danon J, Machnach W, Sharp JT. Mechanics of the canine diaphragm. 1976;41:369–382. doi: 10.1152/jappl.1976.41.3.369. [DOI] [PubMed] [Google Scholar]
- 38.Rossi A, Brandolese R, Milic-Emili J, Gottfried SB. The role of PEEP in patients with chronic obstructive pulmonary disease during assisted ventilation. 1990;3:818–822. [PubMed] [Google Scholar]
- 39.Haberthür C, Guttmann J. Short-term effects of positive end-expiratory pressure on breathing pattern: an interventional study in adult intensive care patients. 2005;9:R407–R415. doi: 10.1186/cc3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Troyer A, Leduc D, Cappello M, Mine B, Gevenois PA, Wilson TA. Mechanisms of the inspiratory action of the diaphragm during isolated contraction. 2009;107:1736–1742. doi: 10.1152/japplphysiol.00753.2009. [DOI] [PubMed] [Google Scholar]
- 41.Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. 2017;43:408–418. doi: 10.1007/s00134-016-4653-4. [DOI] [PubMed] [Google Scholar]