To the Editor:
Nonanatomical traits contribute to obstructive sleep apnea (OSA) in certain individuals (1) and can predict response to therapies beyond continuous positive airway pressure (CPAP) (2, 3). Unstable ventilatory control (high loop gain) is one such trait that is useful for personalizing treatment (4). Measuring loop gain has traditionally required the use of specialized equipment and labor-intensive techniques (5); however, recently developed methods can determine loop gain from polysomnography (PSG) using a model-fitting technique (6). Because clinical testing for OSA is shifting to home sleep testing (HST), our aim in this study was to determine whether we could estimate loop gain using this limited dataset, which might facilitate personalized OSA treatment in clinical practice.
Subjects with untreated OSA (apnea–hypopnea index [AHI] ≥ 5/h) who underwent both PSG and HST within a 3-month period were included in the study. The research was approved by the institutional review board (#141272 and #150465), and the subjects provided written informed consent. The exclusion criteria were use of sedatives, hypnotics, or narcotics, ongoing OSA treatment, and/or prior airway surgery.
In-laboratory attended PSG was performed in the standard fashion with the subjects supine. HST was performed with a type III device (ApneaLink Plus/Air; ResMed). Results were scored using American Academy of Sleep Medicine Chicago criteria (3% desaturation) by a technologist blinded to outcomes.
Loop gain analysis was performed using a model-fitting procedure in MATLAB (The MathWorks) as previously described (6). Briefly, the total ventilatory drive for any given breath is modeled as the sum of the chemical drive and arousal drive, if arousal is present. The loop gain relates to the chemical drive and is the input–output function of the feedback loop that controls ventilation, quantifying the magnitude of the ventilatory response that follows a ventilatory disturbance (e.g., hypopnea or apnea). The ventilatory and chemical drives are considered equivalent to ventilation except during obstructive events (when drive exceeds ventilation) and arousal (when the ventilatory drive exceeds the chemical drive). The model iteratively adjusts to fit the drive to the observed data. The dynamic loop gain is assessed at a frequency of one cycle per minute (LG1) based on the kinetics of OSA (6, 7). Analysis is performed in 7-minute windows to allow the use of breath-by-breath uncalibrated ventilation. Median LG1 values from across the entire recording are reported.
As standard analysis, PSG loop gain was measured from windows occurring in non-REM sleep only. In addition, we sequentially adjusted the analysis to mimic the data available in HST, effectively stripping the PSG of 1) arousals, 2) sleep stage, and 3) both arousals and sleep stage. For the HST recordings, analyses were performed without knowledge of arousals and sleep stage.
The primary outcome of this study was the correlation between HST and PSG LG1 measurements. Statistical significance was defined at P < 0.05.
PSG and HST recordings were obtained in 27 subjects with OSA (age 56 [43–60] yr, 81% male, body mass index 30.0 ± 4.8 kg/m2, AHI 50 ± 21/h, time interval 28 ± 22 d). Pairwise comparisons revealed a lower AHI in HST recordings than in PSG recordings (24 ± 3/h vs. 50 ± 4/h; P < 0.001) and higher nadir saturation (81 ± 1% vs. 77 ± 2%; P = 0.009). Otherwise, there were no significant differences in event types or oxygenation.
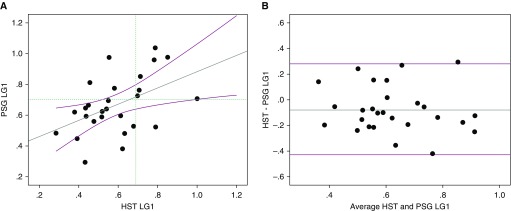
PSG and HST LG1 measurements correlated strongly without substantial bias (Table 1 and Figure 1). The intraindividual difference between HST and PSG was not associated with demographics, time difference, or AHI.
Table 1.
Comparison of Loop Gain Values Obtained by Home Sleep Test and Polysomnogram
| Correlation Coefficient | HST LG1 | Comparator LG1 | Mean Difference (HST − Comparator) | Limits of Agreement of LG1 (95% CI for HST − Comparator) | |
|---|---|---|---|---|---|
| Standard PSG | 0.470* | 0.59 ± 0.04 | 0.66 ± 0.04 | −0.08 ± 0.04* | −0.43 to 0.28 |
| Stripped-back PSG | |
||||
| Without arousals | 0.637† | 0.59 ± 0.04 | 0.74 ± 0.04 | −0.16 ± 0.03† | −0.47 to 0.16 |
| Without sleep state | 0.491* | 0.59 ± 0.04 | 0.57 ± 0.03 | 0.01 ± 0.03 | −0.31 to 0.34 |
| Without arousals or sleep state | 0.659† | 0.59 ± 0.04 | 0.65 ± 0.03 | −0.06 ± 0.03* | −0.34 to 0.22 |
Definition of abbreviations: CI = confidence interval; HST = home sleep test; LG1 = loop gain at frequency 1/min; PSG = polysomnogram.
Measurements were obtained under standard conditions (incorporating arousals and only non-REM sleep) and stripped back of various EEG-derived measures that are not available in HST. Paired differences are shown as mean ± SEM.
P < 0.05.
P < 0.001.
Figure 1.
(A) Scatterplot of PSG versus HST LG1. Circles denote individual subjects. Regression line with confidence intervals shown as solid lines. The horizontal dotted line represents high versus low PSG LG1 using a predefined cutoff of 0.07. The vertical dotted line represents high versus low HST LG1 using a cutoff of 0.69 as determined by receiver operating characteristic curve analysis. (B) Bland-Altman analysis of LG1 determined from HST and PSG. Circles denote individual subjects. Mean difference of LG1 −0.08 ± 0.04 (dimensionless; P = 0.040). Limits of agreement (i.e., 95% confidence interval for SD of the difference) −0.43 to +0.28. No substantial bias was noted across the range of LG1 values. HST = home sleep test; LG1 = loop gain at frequency 1/min; PSG = polysomnogram.
Compared with standard PSG, removal of arousals from the PSG recordings resulted in higher LG1 (difference 0.08 ± 0.02; P < 0.001), whereas inclusion of REM events resulted in lower LG1 (difference −0.09 ± 0.02; P < 0.001). The net effect of removing both arousals and sleep stage from PSG was neutral (difference −0.01 ± 0.02; P = 0.558). These stripped-back PSGs demonstrated a higher correlation with HST LG1 than standard PSGs (Table 1).
To determine whether HST LG1 could be used to classify subjects into high or low loop gain categories, we performed a receiver–operator characteristic analysis using a previously defined PSG LG1 cutoff of 0.7 (6). The area under the curve was 0.853 (P < 0.001). At an HST LG1 cutoff of 0.69, the sensitivity was 70% and specificity was 94% (Cohen’s κ = 0.669; P < 0.001). Leave-one-out cross-validation was performed, resulting in a conservative sensitivity of 70% and specificity of 71% (κ = 0.390; P = 0.040).
The major finding of this study is that HST can be used to estimate the loop gain obtained from PSG. For the purpose of classifying subjects with high loop gain, HST performs well and therefore might be useful clinically, although further validation data would be welcome.
There are several likely sources of the discrepancy between HST and PSG loop gains, including the lack of EEG data. Arousal may transiently increase the overall ventilatory drive independently of the chemical drive as a brief transition to waking (8) or as a distinct ventilatory component akin to a startle response (9). Without sleep-stage data, events that presumably occurred during REM sleep were included in the HST measures, biasing the loop gain downward (8). Overall, our finding that the HST LG1 measures were most similar to the stripped-back PSG values (without arousals and/or sleep stage) is consistent with these influences. Differences in supine sleep might contribute, although the effect is likely small (10). Effects from the use of intrinsic sensors and night-to-night changes also likely play a role.
We acknowledge a number of limitations to our study. First, this was a small study with primarily obese men, which might limit its generalizability. Second, studies were performed on different nights. Although no substantial differences in health status were reported, we did not closely control all factors during HST, such as position. The study reflects HST use in the “real world.” The similar loop gains obtained despite the substantial differences in AHI between the modalities and test conditions used attest to the robustness of this measure. Third, in accordance with our study design, we did not perform more traditional loop gain assessments (e.g., CPAP drops and proportional assist ventilation). Our method demonstrated only a slightly lower correlation than was previously reported for PSG versus the CPAP drop method (6). However, these traditional techniques have limitations, and no clear gold standard has been defined. The optimal technique to identify patients who would be responsive to loop gain–lowering therapies is unknown. Despite these caveats, we believe our study represents an important step forward in bringing a personalized approach to OSA into clinical practice.
Footnotes
J.E.O. is supported by NIH grant F32 HL131306. B.A.E. was supported by a CJ Martin Overseas Biomedical Fellowship (1035115) from the National Health and Medical Research Council of Australia and is now supported by a Heart Foundation of Australia Future Leader Fellowship (101167). A.M. is the principal investigator on NIH grants RO1 HL085188, K24 HL132105, and T32 HL134632, and coinvestigator on NIH grants R21 HL121794, RO1 HL119201, and RO1 HL081823. As an Officer of the American Thoracic Society, A.M. has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to University of California San Diego in support of a sleep center.
Author Contributions: Conception, analysis, interpretation, and manuscript preparation: J.E.O., R.L.O., and A.M. Analysis, interpretation, and manuscript preparation: S.A.S. and B.A.E. Data acquisition and manuscript preparation: P.N.D., N.D., R.J., and Y.L. All authors approved the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201707-1357LE on November 30, 2017
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Owens RL, Edwards BA, Eckert DJ, Jordan AS, Sands SA, Malhotra A, et al. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. 2015;38:961–970. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Ye J, Han D, Cao X, Ding X, Zhang Y, et al. Physiology-based modeling may predict surgical treatment outcome for obstructive sleep apnea. 2017;13:1029–1037. doi: 10.5664/jcsm.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr JE, Edwards BA, Malhotra A. CrossTalk opposing view: loop gain is not a consequence of obstructive sleep apnoea. 2014;592:2903–2905. doi: 10.1113/jphysiol.2014.271841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellman A, Edwards BA, Sands SA, Owens RL, Nemati S, Butler J, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. 2013;114:911–922. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, et al. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efken C, Bitter T, Prib N, Horstkotte D, Oldenburg O. Obstructive sleep apnoea: longer respiratory event lengths in patients with heart failure. 2013;41:1340–1346. doi: 10.1183/09031936.00082212. [DOI] [PubMed] [Google Scholar]
- 8.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. 1982;126:758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- 9.Horner RL, Rivera MP, Kozar LF, Phillipson EA. The ventilatory response to arousal from sleep is not fully explained by differences in CO(2) levels between sleep and wakefulness. 2001;534:881–890. doi: 10.1111/j.1469-7793.2001.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. 2015;38:1469–1478. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]