Abstract
Acclimatization to altitude has been shown to improve elements of performance. Use of simulated altitude is popular among athletes across the sports spectrum. This work was on a handheld, re-breathing device touted to enhance performance. Seven recreationally-trained athletes used the device for 18 hours over the course of the 37-day intervention trial. The elevations simulated were progressively increased from 1,524m to 6,096m. To ascertain potential efficacy, four performance trials were included (familiarization, baseline, and 2 follow-ups). Hematological (hematocrit, hemoglobin, and lactate), physiological (respiratory exchange ratio, heart rate, and oxygen consumption), and perceptual (Borg’s RPE) variables were monitored at rest, during two steady state running economy stages, and at maximal effort during each visit. The device is clearly capable of creating arterial hypoxemic conditions equating to high altitude. This fact is exemplified by average pulse oximetry values of approximately 78.5% in the final 6-day block of simulation. At the same time, there were no changes observed in any hematological (p>0.05), physiological (p>0.05), or perceptual (p>0.05) variable at either follow-up performance trial. Relative VO2 data was analyzed with a 15-breath moving average sampling frequency in accordance with our recent findings (Scheadler et al.) reported in Medicine and Science in Sports and Exercise. Effect sizes are reported within, but most were trivial (d=0.0–0.19). Overall, findings align with speculation that a more robust altitude stimulus than can be offered by short-term arterial hypoxemia is required for changes to be evidenced. The device has shown some promise in other work, but our data is not supportive.
Keywords: Simulated altitude training, hypoxia, red blood cell mass
INTRODUCTION
Empirical evidence indicates that most athletes who acclimatize to high altitude conditions realize improvements in aerobic performance events (4, 7, 10, 14). The positive response is not universal (8); the response may be blunted when an athlete has a high red cell mass prior to acclimatization (12). This last statement concurs with the notion that performance enhancement is mediated through hypoxia-inducible factor alpha which stimulates erythropoietin-induced augmentation in red cell mass (11). Since its introduction in the 1990’s, the live high and train low strategy of altitude acclimatization has gained popularity as the favored terrestrial option for improving performance (10). Athletes could, theoretically, incorporate terrestrial altitude training alongside physical training to enrich physiological adaptations and performance outcomes. Indeed, altitude training is popular across the sports spectrum (17).
Methodologies to simulate altitude at sea level include tents, hypobaric chambers, and various devices. These products are intended to offer the user the opportunity to create altitude-like conditions while circumventing the need for finding terrestrial locations to do so. The manufacturers of these products often point to a physiological underpinning for how their products work and many convey that their device changes red cell mass. To reduce the chance of bias, these claims need to be evaluated independently.
This study utilized the AltoLab (PharmaPacific, Inc.), a handheld re-breathing apparatus used to simulate altitude under seated, resting, normobaric conditions. The AltoLab re-breathing device consists of a mouthpiece and tube that attaches to a silo system (a single silo or a stack of silos). The system is constructed of two types of silos. There is always a single silo filled with soda lime attached to the tube and mouthpiece. Additional silos containing an antibacterial foam can be stacked beneath to increase respiratory dead space. In effect, the silo system varies in height based on the desired altitude stimulus. The adjustable stacking provides opportunity to simulate heights well above 6,000 meters (m). Regardless of height, the silo stack is open-ended at the bottom. The silo containing soda lime may be used alone, but when used with other silos, it is always positioned on top of the stack. This silo provides space for a limited quantity of normoxive air to mix with exhaled air. The normoxive air is drawn into the silo stack from the open end. The soda lime acts as a “scrubber” to remove exhaled carbon dioxide. The “scrubbing” is a chemical reaction of soda lime with carbon dioxide that produces water and heat (16). Thus, the AltoLab silo containing soda lime feels warm to the touch after several minutes of use. Most high-grade soda lime changes color (typically from a white granule to a purple granule) during the chemical reaction. This colorimetric change is an added feature to inform users when to replace granules. Overall, the system is constructed to blunt ventilatory reflexes, which would protect against arterial hypoxemia.
The device has shown some promise in promoting performance improvements in cyclists (15) and runners (3, 18). In these studies, the device was used for 1 hour in cyclical fashion, 6 minutes (min) of use to stimulate arterial hypoxemia followed by 4 min of normoxia. There is empirical data to indicate the device does not interfere with mucosal immune defenses (3) and can be used acutely without instigating unfavorable cardiac ischemic events in healthy persons (2, 6). Despite previous publications, there remains a need for elucidation of the efficacy of the device (5) and the identification of the time-course of potential improvements when utilizing the device in-line with distributor-recommended usage patterns. The goal of this work was to focus on those two aspects. Our hypothesis was that improvements in variables would reach significance at the completion of the trial (follow-up #2) but not at the intermediate point (follow-up #1).
METHODS
Participants
The study was approved by the Institutional Review Boards at Abilene Christian University and Northern Kentucky University. All participants provided informed written consent prior to engaging in the study. Participants were recruited from local community running clubs and university settings. To recruit a sample of low-risk, recreationally-trained athletes, the primary inclusion criteria were: (a) age > 18 years; (b) physician approval for exposure to moderate to high altitude; (c) no acclimatization to simulated or terrestrial altitude within 6 months; (d) documentation of run training history for a minimum of 3 months prior to the study; (e) ability to maintain training volume and intensity; and, (f) low risk stratification from AHA/ACSM screening tool. The exclusion criteria included: (a) failure to meet criteria listed above; and (b) an inability to follow the 37-day training protocol.
Participant Characteristics – Seven recreationally-trained athletes (females=5; males=2) enrolled in the study. According to training logs, participants averaged 3.95 hours of run training per week prior to enrolling, and they had, on average, been training for 7.0 years (range 3.5 to 10). Table 1 provides descriptive characteristics of the participants at the four visits.
Table 1.
Descriptive characteristics and resting heart rate data of the participants at each visit (n=7).
| Familiarization (M±SD) | Baseline (M±SD) | Follow-up #1 (M±SD) | Follow-up #2 (M±SD) | Omnibus F or T Test | |
|---|---|---|---|---|---|
| Age, years | 21.9±2.7 | - | - | - | - |
| Height, centimeters | 168.7±7.9 | - | - | - | - |
| Weight, kilograms | 58.0±7.9 | 57.7±8.0 | 57.7±8.0 | 57.4±7.8 | p=0.097 |
| Resting HR, bpm | 64.1±8.9 | 60.9±7.2 | 64.9±12.0 | 62.9±7.3 | p=0.317 |
| Body comp., % Fat (n = 6) | - | 18.8±4.0 | 17.4±5.2 | - | p=0.260 |
M=mean; SD=standard deviation; HR=heart rate; bpm=beats per minute; comp.=composition
Protocol
Data collection occurred over the course of 37 calendar days. The protocol was rigorous and data was collected on 28 of the 37 days. The protocol included 4 performance test dates: familiarization, baseline, and 2 follow-up visits. The intent of the familiarization was to habituate participants to testing requirements and abate learning effects. The baseline performance was labeled as Day 0 and the variables collected at baseline were considered the criterion on which to monitor efficacy of the altitude device. On each performance test date, hematological [hematocrit (Hct), hemoglobin (Hgb), and lactate], physiological [respiratory exchange ratio (RER), steady state running economy, peak oxygen consumption, and heart rate (HR)], and perceptual [Borg’s Rating of Perceived Exertion (RPE)] variables were monitored at rest, during two steady state running economy stages, and/or at maximal effort. Diet was examined via records, which commenced 48 hours prior to each performance test date.
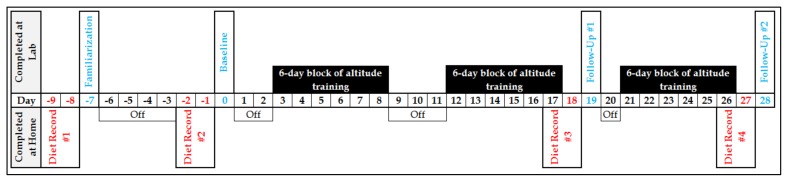
Eighteen days of simulated altitude were completed. Altitude training occurred during seated, resting conditions. Specifically, the altitude training took place in three, 6-day blocks after the baseline, but interspersed before the follow-up visits. Altitude exposure, monitored by pulse oximetry, intensified across the days and blocks. Twelve days of altitude training were completed before follow-up #1. The final six days of altitude training were completed after follow-up #1 but before follow-up #2. This research design was intended to allow for evaluation of the effectiveness of the device and elucidation of a crude time-course of changes, should they be realized. Figure 1 illustrates the schematic flow of the trial and may be helpful in illustrating how the altitude training was fitted within the performance testing dates. Each aspect of the trial is described in further detail below.
Figure 1.
Schematic representation of the 37-day simulated altitude study. Baseline is labeled as Day 0. Diet records commenced two days prior to each trial visit (familiarization, baseline, follow-up #1, and follow-up #2). The three, 6-day blocks of simulated altitude training via re-breathing occurred after the baseline trial but interspersed between the follow-up visits.
Descriptive Information – Age, height, weight, resting HR, and body composition were recorded. Age and height were recorded at the familiarization trial. Weight and resting HR were monitored each visit. Body composition was assessed by air displacement plethysmography (BodPod, Cosmed) at baseline and follow-up #1. Data was presented in Table 1.
Nutritional Analysis – Beginning two days prior to each performance testing date, participants were required to journal about food and drink intake (see Figure 1). All records were reviewed with study personnel. Verbal discussion assisted with accuracy of the food and drink descriptions. Analysis of the food and drink items occurred with use of MyFitnessPal (Under Armour, Inc.). The purpose was to identify the total consumption of kilocalories (kcal) as well as the macronutrient [carbohydrate (CHO), fat, and protein] combination of food and drink in the meals preceding each performance trial.
Hematological Variables – Finger prick analyses were conducted at rest to determine Hct, Hgb, and lactate levels. Finger pricks were also taken during the two steady state running economy stages and at maximal effort to determine lactate levels. Hemoglobin and Hct were determined by optical absorption photometry (HemePoint Analyzer, Stanbio), and lactate levels by the LactatePlus (Nova Biomedical) lactate sensor.
Performance Testing – Respiratory exchange ratio and oxygen consumption (VO2) were assessed during a single, continuous treadmill task. The treadmill task consisted of two steady state running economy stages and a maximal protocol. Collection and analysis of expired gases were completed with use of the ParvoMedics Metabolic Cart (Sandy, Utah). A 30-second block sampling average was utilized for analysis of the steady state VO2 data. However, a 15-breath moving average was used to analyze the peak VO2 values as we have recently reported this sampling frequency presents a higher VO2peak compared with commonly employed sampling frequencies regardless of the exercise protocol employed (13). All performance tests started as two, four-minute running economy stages and then transitioned to a more traditional maximal protocol. During the familiarization trial, the participants transitioned to the third stage of the traditional Bruce protocol and followed the standard protocol thereafter. The participants exercised until volitional fatigue. For the baseline and follow-up visits, the warm-up and running economy speeds were individualized based on the peak VO2 values obtained during the familiarization trial. More specifically, the running economy speeds were selected to elicit a workload that equated to 55% and 65% of the peak VO2. After the two running economy stages, the protocol transitioned to a modified-Astrand protocol. The speed from the second running economy phase was maintained and the grade was increased 2% every two minutes. The test continued in this fashion until volitional fatigue.
Simulated Altitude Re-breathing Sessions – With the baseline testing date being considered Day 0, the 3, 6-day training sessions to stimulate arterial hypoxemia occurred on days 3–8, 12–17, and 21–26 via usage of the AltoLab. The actual re-breathing sessions were just under one hour long. Six-min phases of usage to stimulate arterial hypoxemia were separated by four-min normoxia phases (breathing room air). Participants were seated during the entire process. The usage pattern of six-min on and four-min off during the resting condition is in accordance with device recommendations. Pulse oximetry was used constantly to safeguard against an unsafe arterial hypoxemia stimulus. The amount of time to return to 97% saturation of hemoglobin during the intervening normoxia phases was also recorded. To our knowledge, this latter piece of data has not been presented. The mouthpiece and tube were attached to the open-ended silo containing soda lime while a nose clip prevented nasal breathing. Silos containing the antibacterial foam were used to adjust the altitude stimulus. After 4 or 5 hours of altitude training, the participants were taught to record their own data while trial staff monitored. This process of passing responsibility from the trial staff to the study participants was incorporated to maintain internal validity while augmenting reception of the work (external validity); the device can be purchased online and used by anyone without guidance. The altitude equivalent stimulus, based on pulse oximetry, was progressively increased from 1,524m on days 1–2 up to 6,096m after day 10 of usage. Those altitude exposures result in pulse oximetry values that approximate 89–91% and 75–80%, respectively, according to the distributor.
Statistical Analysis
Separate repeated measures analysis of variance (ANOVA) were conducted to examine differences with dependent variables (weight, kcal, HR, Hct, Hgb, lactate, RER, VO2, and RPE). Effect sizes are reported as Cohen’s d (d) with accompanying meaning of trivial (0–0.19), small (0.20–0.49), medium (0.50–0.79), and large (>0.80). Sphericity was checked with Mauchly’s W. Means (M) and standard deviations (SD) are presented for descriptive purposes. Paired t-tests were utilized to compare body composition at the two assessment points and to compare familiarization and baseline outcomes. A level of 0.05 denoted significance. Analysis was conducted with the IBM SPSS Statistics package (Version 24, New York).
RESULTS
Participant Characteristics and Resting Data – There were no changes across the trial in body weight (p>0.05) or body composition (p>0.05), nor were resting HR (p>0.05) different between trial visits, as was shown in Table 1. The effect sizes for weight (range d=.01–.07), resting HR (range d=.07–.41), and body composition (d=.31) were trivial to small.
Comparison of Familiarization and Baseline Testing Outcomes – To compare familiarization to baseline to explore potential differences, the values for weight (d=.04), resting HR (d=.40), Hct (d=.03), Hgb (d=.14), resting lactate (d=.05), maximal HR (d=.07), maximal lactate (d=.04), and maximal rating of perceived exertion (RPE) (d=.40) were compared. There were no significant differences found (p>0.05).
Nutritional Data – Within-subjects analysis revealed no significant differences (p>0.05) for consumption of kcal in the meals proceeding each performance trial. Moreover, there were no significant differences for kcal from CHO, fat, or protein (p>0.05).
Simulated Altitude Re-breathing Sessions – The device is clearly capable of creating arterial hypoxemic conditions equating to moderate to high altitude. As examples, during the first two days of training, participants experienced an altitude stimulus, which resulted in desaturation of hemoglobin to 87.6%. From days 11–18, the average lowest value for the participants was 78.5%. Aggregated data for the 18 days of altitude training, the distributor’s suggested altitude acclimatization schedule, and the time it took for participants to return to 97% saturation of hemoglobin after the 6-min exposure are all shown in Table 2.
Table 2.
Descriptive information about recommendations for altitude exposure, approximate pulse oximetry for the recommended altitude exposure and the mean pulse oximetry data for the 18 days of simulated altitude training via rebreathing (n=7).
| Days of study | Distributor recommended altitude exposure | *Pulse oximetry for recommended altitude exposure | *Mean; Low oximetry values of participants (M±SD) | Time (seconds) back to 97% (M±SD) |
|---|---|---|---|---|
| Days 1–2 | 3,048m (10,000ft) | 89–91% | 87.6±3.8; 82.8±7.3 | 59.2±37.1 |
| Days 3–4 | 3,658m (12,500ft) | 86–88% | 86.6±3.2; 82.2±6.0 | 72.8±41.2 |
| Days 5–6 | 4,572m (15,000ft) | 83–85% | 87.8±2.9; 83.4±4.9 | 52.8±34.8 |
| Days 7–10 | 5,486m (18,000ft) | 80–82% | 83.0±2.9; 78.1±5.2 | 61.6±38.7 |
| Days 11+ | 6,096m (22,000ft) | 75–80% | ^83.9±3.5; 78.5±5.3 | 52.5±35.3 |
Percent saturation of hemoglobin;
M=mean; SD=standard deviation;
On days 11–18, for protective reasons, participants were instructed to take a small sip of normoxive air when values fell below 77–78% saturation during an on-going usage phase. Accordingly, pulse oximetry values moved up slightly before falling again, which may explain the discrepancy between mean recommended and achieved values.
Hematological Variables at Rest – Values for Hct (%), Hgb [grams (g)/deciliter (dl)], and lactate [millimoles (mMol)/liter (l)] were recorded at rest during each visit. There were no significant differences (p>0.05) for Hct (range d=.03–.18), Hgb (range d=.10–.39), or lactate (range d=.05–1.0), results are shown in Table 3.
Table 3.
Values for hematocrit, hemoglobin, and resting lactate at each visit (n=7).
| Familiarization (M±SD) | Baseline (M±SD) | Follow-up #1 (M±SD) | Follow-up #2 (M ±SD) | Omnibus F Test | |
|---|---|---|---|---|---|
| Hct, % | 41.1±5.4 | 41.3±4.3 | 41.7±3.1 | 42.1±5.3 | p=0.817 |
| Hgb, g/dl | 13.8±2.1 | 14.1±1.5 | 13.6±1.6 | 14.3±1.8 | p=0.625 |
| Lactate, mMol/l | 1.7±0.6 | 1.7±0.5 | 1.5±0.5 | 1.3±0.3 | p=0.173 |
M=mean; SD=standard deviation; Hct=hematocrit; Hgb=hemoglobin; g=grams; dl=deciliter; mMol=millimoles; l=liter
Physiological Variables – Lactate, RER, HR, and VO2 were analyzed during two steady state running economy stages set at 55% and 65% of each participant’s peak aerobic ability (determined at familiarization). Lactate, HR, RER, and VO2 were also analyzed at maximal effort. The steady state and maximal effort data are shown in Table 4. There were no significant effects for any of the 12 repeated measures ANOVAs (p>0.05). All F values were low (range=.061–1.112). Effect sizes for lactate (range d=.11–.67), RER (range d=.04–.10), HR (range d=.01–.24), and VO2 (range d=.04–.23) at 55% were primarily trivial to small. Likewise, effect sizes for lactate (range d=.10–.20), RER (range d=.07–.51), HR (range d=.10–.36), and VO2 (range d=.03–.16) at 65% were primarily trivial to small. Finally, effect sizes for lactate (range d=.04–.15), RER (range d=.08–.23), HR (range d=.03–.26), and relative peak VO2 (range d=.02–.12) at maximal effort were trivial to small. Maximal RPE was not different (range d=.16–.54, p>0.05).
Table 4.
Values for lactate, RER, HR, VO2, and/or RPE at 55% and 65% of peak VO2 and at maximal effort (n=7).
| Familiarization (M±SD) | Baseline (M±SD) | Follow-up #1 (M±SD) | Follow-up #2 (M±SD) | Omnibus F Test | ||
|---|---|---|---|---|---|---|
| Lactate, mMol/l | 55% | - | 2.8±1.2 | 2.7±0.8 | 2.2±0.7 | p=0.464 |
| 65% | - | 2.7±0.7 | 2.6±0.7 | 2.5±0.7 | p=0.887 | |
| Max. | 10.4±2.6 | 10.2±3.5 | 10.7±2.8 | 10.5±3.2 | p=0.903 | |
|
| ||||||
| RER | 55% | - | 0.84±0.03 | 0.85±0.05 | 0.85±0.05 | p=0.941 |
| 65% | - | 0.90±0.03 | 0.91±0.03 | 0.90±0.05 | p=0.443 | |
| Max. | 1.15±0.08 | 1.11±0.09 | 1.10±0.09 | 1.12±0.09 | p=0.361 | |
|
| ||||||
| HR, bpm | 55% | - | 126.4±13.1 | 129.7±14.0 | 126.6±11.7 | p=0.574 |
| 65% | - | 141.1±21.3 | 146.3±20.9 | 148.1±17.3 | p=0.404 | |
| Max. | 193.1±8.7 | 192.6±8.0 | 191.0±6.8 | 192.9±8.0 | p=0.404 | |
|
| ||||||
| VO2, ml/kg/min | 55% | - | 21.4±5.0 | 21.0±4.4 | 21.8±6.1 | p=0.900 |
| 65% | - | 25.7±6.9 | 25.6±4.5 | 26.5±5.2 | p=0.894 | |
| Peak | 53.1±7.1 | 53.0±7.5 | 53.4±7.4 | 53.0±8.4 | p=0.925 | |
|
| ||||||
| RPE, Borg | Max. | 17.7±1.7 | 18.4±1.7 | 18.7±1.8 | 18.1±1.9 | p=0.162 |
M=mean; SD=standard deviation; mMol=millimoles; l=liter; Max.=maximal; RER=respiratory exchange ratio; HR=heart rate; bpm=beats per minute; VO2=oxygen consumption; min=minute; kg=kilograms; RPE=Borg’s Rating of Perceived Exertion
DISCUSSION
Contrary to published research (3, 15, 18) and in opposition to our hypothesis, our data failed to show differences in measured variables after 18 hours of simulated altitude re-breathing training with the AltoLab. Clearly, the device has potential, through the re-breathing, to create arterial hypoxemia as our altitude stimulus averaged above 3,658m. At the same time, the stimulus failed to change any of the investigated variables associated with performance, and nearly all effect sizes were trivial to small. The dearth of evidence on the device leaves us with few comparative studies. While the comparative works offer slightly better statistical power [n=18 (15), n=20 (3), and n=29 (18)], power analysis indicated that we would have needed 227, 441, and 1,123 participants to detect differences in Hct, Hgb, and peak relative VO2, respectively (Alpha=0.05, Beta=0.20, and Power=0.80). Our low participant number does not appear to be the origin of the lack of effectiveness.
As a starting point, it must be recognized that our data represent performance-related variables and not a performance test itself. However, the same is true of the studies with which we have discordance. It is practical to consider the lack of a performance testing as a shortcoming; after all, laboratory testing removes natural stimuli, such as competition, motivation, and strategy. We controlled our work in order to elucidate a potential time-course for changes.
Presently, participants were given intentional instructions on the use of the device and control of breathing (rate and depth) in order to achieve a specific pulse oximetry value. To our best knowledge, this is the only work that reports the approximate pulse oximetry values during the re-breathing sessions and the amount of time to return to normal saturation levels at normobaric normoxia. Consequently, we cannot compare our stimulus with others who only make general statements such as, “followed manufacturers suggested protocol for use” or “oxygen saturation dropped from 90% on Day 1 to 77% on Day 6–15” (3, 15). Maintaining prescribed pulse oximetry values is not as simple as might be thought. Our participants had some struggle in maintaining the recommended arterial hypoxemia exposure, even when stacking silos were adjusted and when there was intentional education and monitoring in a controlled setting. On days 11–18, when the lowest pulse oximetry values were targeted, participants were instructed to take a small sip of normoxive air when values fell below 77–78% saturation during an on-going usage phase. This was solely a protective response for the participants. Even when small sips of air were included, oximetry values slid into the upper 60’s for several participants. Overall, however, the small sips of air caused pulse oximetry values to jump up before slowly falling again. That is the reason that values in Table 2 show a slight discrepancy between mean values recommended on days 11–18 and the mean values achieved by the participants. It is not unreasonable to consider that our exposures, which were equal to altitudes above 3,658m for 18 days, would be capable of initiating hematological changes in the terrestrial realm. Therefore, we surmise the lack of effect was not the result of the inability to follow the prescribed guideline more closely.
There were no changes in the hematological variables investigated. A lack of change suggests there is no greater ability for oxygen delivery to the active tissues. This is in line with previous findings where changes in Hct, lactate, and red blood cell volume were not evidenced with use of the device (15). It has been suggested that change in red cell mass is blunted when a priori mass is high prior to acclimatization (12). The Hct levels at baseline in this work (41.3%) were similar to baseline values (43.25%) found by Swain et al. (15). Perhaps these initial levels are high enough to impede change. This may be particularly true in our case considering that we primarily enrolled females, who naturally have lower average and ceiling effects for Hct. We cannot rule out that timing of measurement influenced one or more of our results.
The current ideology is that hypoxia (which would be evidenced as arterial hypoxemia) induces performance changes through regulation of hypoxia-inducible factors (HIF)-1 and 2 (1). In the absence of hypoxia, there is rapid proteasomal degradation and the half-life of the HIF-1alpha subunit is less than five minutes (9). In hypoxic conditions, the change in HIF levels is prompt, increasing in the nucleus in less than two minutes (9). The HIF targets genes which stimulate protein synthesis (among them – erythropoietin) (1). Intermittent hypoxia may fail to result in significant changes in hematological variables for this very reason—it simply does not activate the changes in HIF long enough to overcome the short half-life experienced during the overwhelming majority of the time when living in normobaric settings. If such were the case, any change in performance from use of the AltoLab would have to come from non-hematological mechanisms.
Performance improvements in the works by Blazek, Swain, Wood and their respective colleagues, were between 1.3–4.5% after use of the AltoLab (3, 15, 18). A non-hematological mechanism, specifically a more efficient delivery of oxygen, is suggested by Swain and colleagues (15) as the underlying reason for a reduction in HR and VO2 at the same given workload after usage of the device. None of the present results corroborates those findings. Presently, we failed to show any changes in lactate, HR, RER, or VO2 at different running economy stages or at maximal effort.
Conclusion – The AltoLab is a simplistic device that is capable of simulating moderate to high altitude for users via re-breathing. The ability of the device to improve performance-testing outcomes in aerobic events is exhibited in some published work, but not supported by the present investigation where there were no changes in any hematological or physiological variable investigated. That finding does align with the knowledge that a HIF-mediated erythropoiesis is predicated on a more robust altitude stimulus than can be offered by a simulation device, such as the AltoLab. At this time, we are not able to support the efficacy of the device, and we are not able to comment on the potential time-course of the adaptations that have been described to occur with usage.
ACKNOWLEDGEMENTS
This study would not have been possible without cooperation and involvement of the participants. Thanks for your dedication to this intervention protocol. The researchers note the generosity of the ACU Pursuit Grant Team for their funding. Also, thank you to Taylor G. Flowers and Heather Johnston for their assistance with data collection.
REFERENCES
- 1.Adams J, Difazio L, Rolandelli R, Lujan J, Hasko G, Csoka B, Selmeczy Z, Németh Z. HIF-1: A key mediator in hypoxia (Review) Acta Physiologica Hungarica. 2009;96(1):19–28. doi: 10.1556/APhysiol.96.2009.1.2. [DOI] [PubMed] [Google Scholar]
- 2.Bell JD, Garver MJ, McCurley LE, Kerrigan DJ, Jessup RK, Geeslin JB, Priest NE, White AM, West EB. Short-term, moderately-high, simulated altitude hypoxia does not evoke cardiac ischemia. Med Sci Sports Exerc. 2013;45(5):s207. [Google Scholar]
- 3.Blazek AD, Anderson PJ, Brichler JG, Slawinski MK, Rose MT, Kirby TE, Swain CB. Effects of a simulated altitude device on endurance performance and mucosal immunity. J Exerc Phys Online. 2014;17(6):45–57. [Google Scholar]
- 4.Brugniaux JV, Schmitt L, Robach P, Nicolet G, Fouillot J-P, Moutereau S, Lasne F, Pialoux V, Saas P, Chorvot MC. Eighteen days of “living high, training low” stimulate erythropoiesis and enhance aerobic performance in elite middle-distance runners. J Appl Physiol. 2006;100(1):203–211. doi: 10.1152/japplphysiol.00808.2005. [DOI] [PubMed] [Google Scholar]
- 5.Flowers TG, Garver MJ, Scheadler CM, Taylor SJ, Smith LM, Harbach CM, Johnston H. The impact of simulated altitude on selected elements of running performance. Int J Exerc Sci: Conf Abstr. 2015;2(7):36. [Google Scholar]
- 6.Geeslin JB, Priest NE, White AM, Garver MJ, McCurley LE, Bell JD, Jones SA, Kerrigan DJ. Benign conduction abnormalities in response to acute, moderately-high, simulated altitude exposure. Int J Exerc Sci: Conf Abstr. 2013;2(5):58. [Google Scholar]
- 7.Green H, Roy B, Grant S, Hughson R, Burnett M, Otto C, Pipe A, McKenzie D, Johnson M. Increases in submaximal cycling efficiency mediated by altitude acclimatization. J Appl Physiol. 2000;89(3):1189–1197. doi: 10.1152/jappl.2000.89.3.1189. [DOI] [PubMed] [Google Scholar]
- 8.Hamlin M, Manimmanakorn A, Creasy R, Manimmanakorn L. Live high-train low altitude training: responders and non-responders. J Athl Enhanc. 2015;8(4):1545–1650. [Google Scholar]
- 9.Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J. 2001;15(7):1312–1314. [PubMed] [Google Scholar]
- 10.Levine BD, Stray-Gundersen J. Living high-training low: effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol. 1997;83(1):102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Levine BD, Stray-Gundersen J. Point: Positive effects of intermittent hypoxia (live high: train low) on exercise performance are mediated primarily by augmented red cell volume. J Appl Physiol. 2005;99(5):2053–2055. doi: 10.1152/japplphysiol.00877.2005. [DOI] [PubMed] [Google Scholar]
- 12.Lundby C, Millet GP, Calbet JA, Bärtsch P, Subudhi AW. Does altitude training increase exercise performance in elite athletes? Br J Sports Med. 2012;46(11):792–795. doi: 10.1136/bjsports-2012-091231. [DOI] [PubMed] [Google Scholar]
- 13.Scheadler CM, Garver MJ, Hanson NJ. The gas sampling interval effect on VO2peak is independent of exercise protocol. Med Sci Sports Exerc. 2017;49(9):1911–1916. doi: 10.1249/MSS.0000000000001301. [DOI] [PubMed] [Google Scholar]
- 14.Stray-Gundersen J, Chapman RF, Levine BD. “Living high-training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol. 2001;91(3):1113–1120. doi: 10.1152/jappl.2001.91.3.1113. [DOI] [PubMed] [Google Scholar]
- 15.Swain C, Kirby T, Altshuld J. Simulated altitude via re-breathing improves performance in well-trained cyclists. J Exerc Physiol Online. 2010;13(6):21–34. [Google Scholar]
- 16.Waters RM. Carbon dioxide absorption from anaesthetic atmospheres. J R Soc Med. 1936;30(1):11–22. doi: 10.1177/003591573603000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilber RL. Application of altitude/hypoxic training by elite athletes. J Human Sport Exerc. 2011;6(2):271–286. [Google Scholar]
- 18.Wood MR, Dowson MN, Hopkins WG. Running performance after adaptation to acutely intermittent hypoxia. Eur J Sport Sci. 2006;6(3):163–172. [Google Scholar]