Abstract
Our aim was to investigate the acute effects of a single bout of high intensity intermittent training (HIIT) on glucose tolerance and other physiological and metabolic markers in non-diabetic older adults. Fourteen healthy older adults (age, 64 ± 2 y; BMI, 25.7 ± 2.8 kg·m−2) performed two acute exercise trials: continuous moderate intensity exercise (MOD) and HIIT, with the response to an oral glucose tolerance test (OGTT) determined <24 hours after. Inflammatory, haematological, and lipid parameters were also assessed the day after each trial. There was an effect of the trials on the insulin response to an OGTT (P=0.047), but not the glucose response. Following an acute bout of HIIT, insulin concentration during an OGTT was elevated at 60 min compared to the control trial (P=0.045), indicating more insulin was secreted, but glucose concentration was unchanged in all trials. The study findings demonstrate that a single bout of HIIT affects the insulin response but not the glycaemic response to a glucose load, proffering a potential benefit for metabolic health in older adults.
Keywords: Glucose tolerance, high intensity intermittent training, type 2 diabetes mellitus, ageing
INTRODUCTION
Ageing is one of the biggest social issues in Europe. There is a disparity between life expectancy and healthy life expectancy which is being exacerbated by high levels of inactivity in the older population. An inactive lifestyle and sedentary behaviour are reported to be among the leading causes of chronic disease (3). Scotland has the highest proportion of people aged over 60 years in the UK and therefore finding innovative solutions to improve quality of life are needed. The most recent public health guidelines from the American Heart Association (AHA) and the American College of Sports Medicine (ACSM) advocate that regular physical activity is essential for healthy ageing (26).
The main pathophysiological events in the development of type 2 diabetes are a reduction in insulin secretion and/or the decline in insulin sensitivity (12). This can result in a decline in glucose tolerance and thus hyperglycaemic state, leading to the predisposed state for the development of diabetes. It is known that glucose tolerance deteriorates with age, which is of importance for older adults as diabetes is associated with increased mortality and demand on the National Health Service (NHS) for treatment. However, preventative measures should be adopted in order to improve glucose tolerance in older adult years (60–75 years) to prevent or delay the onset of diabetes in elderly years (75 years +).
Resistance and endurance exercise have been shown to improve functional outcomes (24), cognitive function (13), arterial stiffness (18), and glucose metabolism (4) in older adults. High intensity intermittent training (HIIT) involving repeated short bouts of high intensity exercise (above 85% of maximal capacity), has been shown to provide similar, and in some cases greater, physiological and metabolic adaptations that influence systemic glucose control compared to moderate intensity continuous exercise (MOD) (2, 5, 16, 25). Furthermore, 6 weeks of HIIT in 60–75 year olds has been shown to improve physical function, lower blood pressure and improve self-reported health (2). The response and adaptations have mainly been investigated using an oral glucose tolerance test (OGTT), which reports systemic insulin and glucose concentrations in response to a high glucose load, indicating insulin secretion in to the circulation from beta cells and glucose uptake from the circulation, thus systemic glucose tolerance.
Ageing is also associated with an increase in inflammatory proteins within the circulation, which can lead to chronic low-grade inflammation. This inflammatory state can be reflected by increased systemic concentration of some cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) concentration (28), and also underlies the development of type 2 diabetes. Previous research has shown that acute and chronic exercise can induce anti-inflammatory effects (14, 15). However, the acute response to HIIT has not yet been investigated in a non-diabetic older adult population.
The primary purpose of this study was to determine if there is a difference in the acute metabolic response to MOD and HIIT by examining the response to an OGTT and effect on key inflammatory markers.
METHODS
Participants
Sixteen healthy male (n=12) and female (n=4) volunteers with a body mass index (BMI) between 20–30 kg·m2 were recruited for the study, and of those, 14 participants completed the control trial and both exercise trials as prescribed (mean ± SD: age 64 ± 2 y, body mass 80.1 ± 11.2 kg, height 1.77 ± 0.09 m, BMI 25.7 ± 2.8 kg·m−2). Volunteers were excluded if they smoked, or were taking medications that would alter blood parameters, otherwise all participants were healthy and recreationally active. Informed consent was obtained from all individual participants included in the study. The study was approved by the School of Applied Sciences Research Integrity Approvals Group at Edinburgh Napier University and followed procedures in accordance with the ethical standards of the Helsinki Declaration.
An a priori power analysis using GPower software 3.1.9.2 was performed for sample size estimation based on an ANOVA: 3 (control, endurance, HIIT) × 5 (OGTT time-points) repeated measures, within factors statistical test (F test). The projected sample size required for the statistical test was n=12, to achieve power of 0.99 and effect size f of 0.50 (Cohen’s f).
Protocol
Baseline testing: Participants attended the laboratory between 0730–0900 following an overnight fast (≥10 h) for baseline testing, involving basic anthropometric measurements, a standard oral glucose tolerance test (OGTT), and a maximal exercise test (VO2peak test), and a familiarisation HIIT protocol. Participants were asked to abstain from consuming caffeine and alcohol in the 24 h prior to the first visit, and engaging in strenuous exercise in the 72 h prior to the first visit. On arrival, participant’s height (Harpenden Portable, Holtain Limited, UK), body mass (Seca, 808, Germany), and waist and hip circumference were measured. Arterial blood pressure was measured on the participant’s non-dominant arm (Omron, R5-1, Japan) following a 5 min rest in the supine position. Following anthropometric measurements, participants were seated on a laboratory bed to complete a standard 75 g OGTT. A cannula (20 G Venflon Pro Safety I.V. cannula, BD, Sweden) was inserted into the antecubital vein and fasted blood samples were collected into K3 ethylenediaminetetraacetic acid (EDTA) (approximately 1.0 mg) and sodium fluoride/potassium oxalate vacutainers, then participants consumed 82.5 g of dextrose monohydrate dissolved in 290 ml of water and 10 ml of lemon juice within 5 min. Further blood samples were subsequently taken every 30 min for a period of 2 h, with the cannula kept patent via regular flushing with 0.9% saline solution. Participants remained seated and rested during the 2 h test.
Participants performed a peak oxygen uptake test (VO2peak) using a continuous incremental exercise test on a stationary electromagnetically-braked cycle ergometer (Lode, Corival CPET, Netherlands). An on-line breath-by-breath gas analysis system (Cortex, MetaLyzer 3B, Germany) was used to measure expired air continuously throughout the test, and heart rate (HR) was monitored by a chest worn HR monitor (Polar, RS400, Finland) and the HR sensor linked to the gas analysis system. After a 5 min warm up at 50 W, the intensity increased by 25–30 W every 3 min until volitional exhaustion (30). Participants were instructed to maintain a pedaling rate of 70 revolutions per min (rpm) and were verbally encouraged to perform to volitional exhaustion whereby the test was terminated (pedaling rate fell below 55 rpm). The value used for VO2peak corresponded to the highest value averaged over 30 s during the test. In the same session, following a 30 min rest period, participants performed a familiarisation trial for the HIIT protocol, involving four 1 min intervals.
Exercise sessions: All participants performed two exercise trials in a randomised, counter balanced fashion: MOD and HIIT (Figure 1), separated by 7–8 days. Participants attended the lab to complete the exercise trial between 0730–0900. Body mass and arterial blood pressure were measured on arrival to the laboratory. Both protocols were performed on a cycle ergometer (Lode, Corival CPET, Netherlands), with expired air (Cortex, MetaLyzer 3B, Germany) and HR continuously throughout both exercise trials.
Figure 1.
Schematic diagram of the MOD and HIIT protocols.
The HIIT protocol was modelled on previous studies (16, 17, 20) consisting of a 5 min warm up at 50 W, then ten 1 minute intervals at a power output corresponding to 100% VO2peak, interspersed with 1 min at 50 W. This was concluded with a cool down of 5 min at 50 W. The MOD protocol matched the HIIT protocol for total duration (29 min) and workload (energy cost was not determined) and consisted of continuous cycling at ~65% of VO2peak.
The day after each exercise trial, participants attended the lab to repeat the OGTT between 0800–0930, which was dependent on the time they completed the exercise trial (ensuring it was 24 h after).
Blood sample analysis: All assays and analysis methods were performed in duplicate. Whole blood was analysed for full blood cell count using a haematology analyser (Sysmex, XS 1000i, USA). Whole blood was then centrifuged (Satorius Universal, 320R, Germany) within 30 min of collection at 1500 rpm for 15 min at 4°C, and the resulting plasma aliquoted into Eppendorf tubes and stored at −80°C for subsequent analysis.
Plasma samples were thawed on ice prior to analysis. Commercially available enzyme-linked immunosorbent assays (ELISA) kits were used to determine plasma insulin concentrations (Mercodia, Uppsala, Sweden) and IL-6, TNF-α, and C-Reactive protein (CRP) (R&D Systems, Minneapolis, USA). Plasma glucose, total cholesterol, triglyceride, and high density lipid (HDL) cholesterol concentrations were analysed using a bench top clinical chemistry analyser (Randox, RX Monza, UK). Low density lipid (LDL) cholesterol concentration was estimated using the following equation: LDL cholesterol = Total cholesterol – (triglycerides/2.2) – HDL cholesterol (10). Insulin sensitivity and insulin resistance was calculated using the insulin sensitivity index (ISI) (22) and homeostatic model assessment-of insulin resistance (HOMA-IR) (23). Plasma and insulin concentrations were determined at the 5 time-points involved in the OGTT. All other blood parameters were determined at the baseline time-point of the OGTT (0 min).
Statistical Analysis
Data are presented as means ± SD. All statistical tests were performed using SPSS 24.0 statistical software (IBM Analytics, New York, USA). Data was checked for normal distribution using the Shapiro-Wilk test. All data was normally distributed and parametric tests were performed. A one-way analysis of variance (ANOVA) was used to compare means for the three trials, with post-hoc Tukey tests for multiple comparisons between groups. A 3 × 5 repeated measures ANOVA was also used to analyse the insulin and glucose response during the OGTT. If a significant effect was found, post-hoc analysis was performed to identify where significance exists between trials. If a main effect was observed when comparing two trials, paired sample t-tests were used to compare trials at each time-point. Significance was accepted at P<0.05.
RESULTS
Anthropometric and performance measurements: Participant characteristics and all performance outcomes from the VO2peak test (n=14, 2 females) are provided in Table 1. Participants were normotensive and had a BMI (25.7 ± 2.8 kg·m−2) just over the classification for overweight (>25 kg·m−2).
Table 1.
Baseline participant characteristics.
| Number or mean | |
|---|---|
| Males:females | 12:2 |
| Age (y) | 64 ± 2 |
| Height (m) | 1.77 ± 0.09 |
| Body mass (kg) | 80.1 ± 11.2 |
| BMI (kg·m−2) | 25.7 ± 2.8 |
| Waist circumference (cm) | 88 ± 10 |
| Hip circumference (cm) | 100 ± 6 |
| HR rest (bpm) | 64 ± 8 |
| Systolic blood pressure (mmHg) | 138 ± 9 |
| Diastolic blood pressure (mmHg) | 85 ± 6 |
| Absolute VO2peak (L·min−1) | 2.35 ± 0.52 |
| Relative VO2peak (ml·kg−1·min−1) | 29.5 ± 6.6 |
| Absolute peak power output (W) | 185 ± 32 |
| Relative peak power output (W kg−1) | 2.33 ± 0.40 |
| RER peak | 1.18 ± 0.07 |
| HR peak (bpm) | 159 ± 11 |
Data are presented as mean ± SD.
Effect of exercise on glycaemic outcomes: Three participants had high fasting blood glucose concentrations (>7.0 mmol·L−1) in the control trial, two in the HIIT trial, and none in the MOD trial, based on the American Diabetes Association (ADA) ‘Criteria for the diagnosis of diabetes’ (1). However, none of these participants had high fasting levels on more than one trial and thus the data was included in the analysis. Table 2 provides the biochemical analysis outcomes.
Table 2.
Blood parameters following the control, HIIT and MOD trials.
| Control | HIIT | MOD | ANOVA P value | |
|---|---|---|---|---|
| Fasting glucose, mmol·L−1 | 6.2 ± 0.9 | 5.9 ± 0.9 | 5.5 ± 0.6 | 0.072 |
| Fasting insulin, mU·L−1 | 5.6 ± 2.4 | 6.9 ± 4.8 | 5.3 ± 3.3 | 0.496 |
| Glucose AUC, mmol·h−1·L−1 | 910 ± 197 | 904 ± 216 | 865 ± 142 | 0.786 |
| Insulin AUC, mU·h−1·L−1 | 4781 ± 3856 | 5373 ± 5212 | 4576 ± 3681 | 0.879 |
| ISI | 10.4 ± 6.0 | 10.5 ± 7.6 | 11.6 ± 4.8 | 0.865 |
| HOMA-IR | 1.57 ± 0.82 | 1.87 ± 1.48 | 1.27 ± 0.66 | 0.329 |
| IL-6, pg·ml−1 | 2.23 ± 0.68 | 2.26 ± 0.76 | 2.18 ± 0.81 | 0.963 |
| TNF-alpha, pg·ml−1 | 1.27 ± 0.42 | 1.13 ± 0.35 | 1.13 ± 0.34 | 0.556 |
| CRP, mg·L−1 | 1.61 ± 0.94 | 1.63 ± 0.87 | 2.14 ± 1.78 | 0.464 |
| Cholesterol, mmol·L−1 | 5.5 ± 0.8 | 5.3 ± 0.9 | 5.6 ± 0.8 | 0.588 |
| Triglycerides, mmol·L−1 | 1.59 ± 0.42 | 1.57 ± 0.35 | 1.60 ± 0.38 | 0.968 |
| HDL-chol, mmol·L−1 | 1.52 ± 0.42 | 1.50 ± 0.37 | 1.49 ± 0.38 | 0.979 |
| LDL-chol, mmol·L−1 | 3.27 ± 0.83 | 3.08 ± 0.78 | 3.40 ± 0.71 | 0.556 |
Data are presented as mean ± SD.
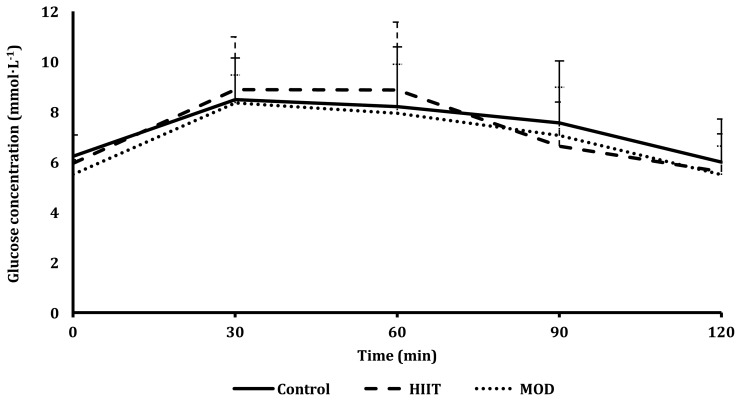
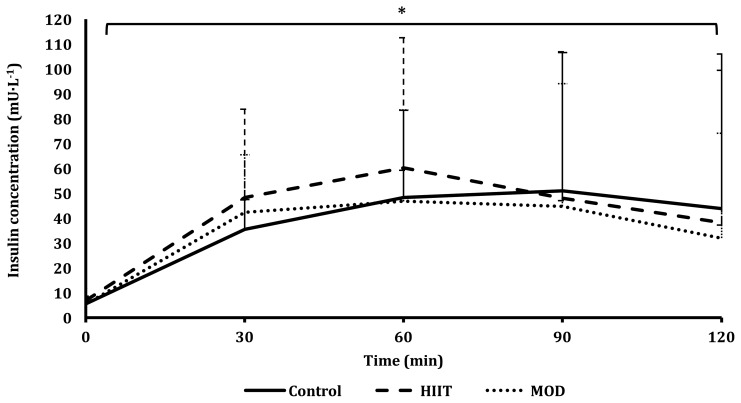
There was no difference observed between trials for the glucose response to the OGTT (Figure 2). There was a differing response between the three trials for the insulin response to the OGTT (P=0.047; Figure 3), with a difference found when comparing the control vs HIIT trial (P=0.045) but not when comparing the control vs MOD trials (P=0.065) or the HIIT vs MOD trials (P=0.140). This indicates that more insulin was produced during the HIIT trial, as shown in Figure 3. Specifically, 24.6% more insulin was produced at 60 min during OGTT following the HIIT trial compared to after the control trial. Insulin sensitivity as assessed by ISIMatsuda and HOMA-IR did not differ between trials (P=0.865; P=0.329; Table 2).
Figure 2.
Plasma glucose concentrations in response to a 75 g OGTT for all three trials: control, MOD, and HIIT.
*Denotes a significant difference in the OGTT between trials; P<0.05.
Figure 3.
Plasma insulin concentrations in response to a 75 g OGTT for all three trials: control, MOD, and HIIT.
*Denotes a significant difference in the OGTT between trials; P<0.05.
DISCUSSION
The main findings of the present study are that less than 24 hours after an acute bout of HIIT, insulin secretion is increased, compared to the control trial, with 24.6% more insulin in the blood at 60 mins post-glucose loading. There was no effect of either exercise trial on insulin sensitivity or inflammatory markers. Although not significant, there was a physiological finding on fasting plasma glucose concentration after the exercise trials compared to the control trial. Similarly, following the HIIT trial, fasting plasma insulin concentration was reduced physiologically but was not statistically significantly. All of the participants were healthy and active, however due to a relatively small sample size with only 2 females, gender comparisons could not be investigated. To our knowledge, this is the first study to investigate the effects of a single bout of exercise on glycaemic control, assessed through the response to a standard 75 g OGTT, in older adults that are free from diabetes and metabolic disease.
The findings extend the observations of exercise intervention studies involving resistance and aerobic exercise training that have induced improvements in glycaemic control (6) and insulin sensitivity (8, 9), and decreases in fasting glucose and insulin concentrations (11). However, the study populations differ to the current study and thus are not largely comparable. Furthermore, chronic adaptations do not necessarily reflect expectations for acute effects/response.
In order to improve insulin sensitivity, it is ideal for less insulin to be produced to effectively maintain a normal glucose concentration, or for the insulin secretion response to remain unchanged whilst glucose concentrations decline. These scenarios would thus indicate that the uptake of glucose is increased and cells sensitivity to insulin action to actively uptake glucose is improved. However, our findings demonstrate that following a single bout of HIIT, more insulin was secreted to maintain the same systemic glucose concentration. This indicates that the acute metabolic response following HIIT is to secrete more insulin in a post-prandial state, possibly suggesting that Beta cell action is elevated (19).
The prevalence of diabetes or a metabolic disease means that baseline glycaemic indices and inflammatory markers will be disturbed. Therefore, exercise strategies will be prescribed as a treatment, as opposed to the current study, which aims to employ exercise as a preventative tactic. Since fasting values for insulin and glucose in non-diabetics are classified as normal, it is not considered an aim of exercise to reduce fasting insulin or glucose concentrations. The aim of the acute effect of exercise in persons exhibiting euglycaemia is thus to improve tolerance to the glucose load through insulin secretion and action. This may explain the lack of effect on indices of insulin sensitivity as they take into account changes in fasting concentrations. In order to assess glycaemic control, it is suggested that insulin secretion may be a better predictor than insulin sensitivity (29). In an intervention study in older adults, following 8 weeks of HIIT, the response to an OGTT and fasting values remained unchanged in the non-diabetic group, but significantly improved in the diabetic group (19). They also reported that HIIT can ameliorate pancreatic beta cell function, however this was only in the diabetic group, again demonstrating the effect that exercise could have as a treatment strategy. Although, following exercise, healthy pancreatic function is normally associated with a decrease in insulin secretion relative to improvements in insulin sensitivity. Therefore, the elevation in insulin secretion following an acute bout of HIIT observed in the current study is controversial.
Long-term exposure to HIIT may induce an adaptation in beta cell function and subsequently insulin secretion, and thus the acute response may merely predict that beta cells have the capacity to respond rapidly and differently to glucose loading. A recent study has hypothesized that the acute response to exercise may induce an early metabolic remodeling mechanism (19), explaining the rise in systemic insulin despite the unaltered glucose response. Although, the chronic adaptations to regular HIIT in older adults would have to be investigated to confirm this alongside the reports of the acute effects. A study investigating the longer term effects of endurance exercise (12–16 weeks) on pancreatic beta cell function and glycaemic control found that glucose-stimulated insulin secretory capacity prior to initiating training predicts training-induced adaptations (29). This supports our finding that a single bout of HIIT induced higher insulin secretion following glucose stimulation and thus may be ascertained to glucose-stimulated insulin secretory capacity. In addition, advancing age is associated with poorer beta cell function and thus glycemic control, although insulin sensitivity was not affected by ageing (7).
Although the present study addresses the acute response, the short term effects could then expand to have a chronic adaptation, which future studies should investigate. If ageing is associated with increasing fasting glucose concentration thus disposing older adults to hyperglycaemic risk, the ability to secrete more insulin when challenged with a high glucose load may be beneficial to effectively modulate euglycaemia.
Exercise has been reported to be a protective tool against chronic inflammation-associated diseases (21), which is ascribed to the anti-inflammatory effect that exercise can induce. One of the main possible mechanisms by which exercise can induce this anti-inflammatory environment is though the increased production and release of specific cytokines from contracting skeletal muscle (27), known as myokines, which are designed to reduce inflammation. However, these myokines are released at differing time-points after exercise. Therefore, measuring concentrations of IL-6, TNF-α, and CRP at 24 h after exercise may modestly omit the acute response. It should be considered that if only a relatively short term effect is observed, then it may not establish change in the inflammatory environment that could influence disease risk.
The key finding of the study was the increase in insulin secretion the day following the bout of HIIT, which was accompanied with no change in the glycaemic response. This indicates that the higher intensity nature of the HIIT bout may proffer an exercise-induced increase in insulin production or insulin secretion. However, the mechanisms surrounding the lack of change in glucose concentration in the presence of elevated insulin concentrations should be further investigated.
REFERENCES
- 1.ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–590. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.Adamson S, Lorimer R, Cobley JN, Lloyd R, Babraj J. High intensity training improves health and physical function in middle aged adults. Biology. 2014;3(2):333–344. doi: 10.3390/biology3020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286(10):1218–1227. doi: 10.1001/jama.286.10.1218. [DOI] [PubMed] [Google Scholar]
- 5.Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, et al. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25(12):2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Lee NP, Cohan P, Chuang LM. Beta cell function declines with age in glucose tolerant Caucasians. Clin Endocrinol. 2000;53(5):569–575. doi: 10.1046/j.1365-2265.2000.01132.x. [DOI] [PubMed] [Google Scholar]
- 8.Davidson LE, Hudson R, Kilpatrick K, Kuk JL, McMillan K, Janiszewski PM, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169(2):122–131. doi: 10.1001/archinternmed.2008.558. [DOI] [PubMed] [Google Scholar]
- 9.Frank LL, Sorensen BE, Yasui Y, Tworoger SS, Schwartz RS, Ulrich CM, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13(3):615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 10.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 11.Hersey WC, 3rd, Graves JE, Pollock ML, Gingerich R, Shireman RB, Heath GW, et al. Endurance exercise training improves body composition and plasma insulin responses in 70- to 79-year-old men and women. Metabolism. 1994;43(7):847–854. doi: 10.1016/0026-0495(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 12.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46(1):3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 13.Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leggate M, Carter WG, Evans MJ, Vennard RA, Sribala-Sundaram S, Nimmo MA. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J Appl Physiol. 2012;112(8):1353–1360. doi: 10.1152/japplphysiol.01080.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leggate M, Nowell MA, Jones SA, Nimmo MA. The response of interleukin-6 and soluble interleukin-6 receptor isoforms following intermittent high intensity and continuous moderate intensity cycling. Cell Stress Chaperones. 2010;15(6):827–833. doi: 10.1007/s12192-010-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, et al. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. J Appl Physiol. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 17.Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588(6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden KM, Lockhart C, Cuff D, Potter TF, Meneilly GS. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Diabetes Care. 2009;32(8):1531–1535. doi: 10.2337/dc09-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen SM, Thorup AC, Overgaard K, Jeppesen PB. High Intensity Interval Training Improves Glycaemic Control and Pancreatic beta Cell Function of Type 2 Diabetes Patients. PLoS One. 2015;10(8):e0133286. doi: 10.1371/journal.pone.0133286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancilla R, Torres P, Alvarez C, Schifferli I, Sapunar J, Diaz E. High intensity interval training improves glycemic control and aerobic capacity in glucose intolerant patients. Revista Medica De Chile. 2014;142(1):34–39. doi: 10.4067/S0034-98872014000100006. [DOI] [PubMed] [Google Scholar]
- 21.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008(109502) doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalfe RS, Babraj JA, Fawkner SG, Vollaard NBJ. Towards the minimal amount of exercise for improving metabolic health: beneficial effects of reduced-exertion high-intensity interval training. Eur J Appl Physiol. 2012;112(7):2767–2775. doi: 10.1007/s00421-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 26.Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 27.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Solomon TP, Malin SK, Karstoft K, Kashyap SR, Haus JM, Kirwan JP. Pancreatic beta-cell function is a stronger predictor of changes in glycemic control after an aerobic exercise intervention than insulin sensitivity. J Clin Endocrinol Metab. 2013;98(10):4176–4186. doi: 10.1210/jc.2013-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon BK, Kravitz L, Robergs R. VO2max, protocol duration, and the VO2 plateau. Med Sci Sports Exerc. 2007;39(7):1186–1192. doi: 10.1249/mss.0b13e318054e304. [DOI] [PubMed] [Google Scholar]