Abstract
The purpose of this study was to determine the effects of a pneumatic compression device (PCD) compared to a continuously-worn compression sleeve (CS) during a five-day recovery period from delayed-onset muscle soreness (DOMS) of the elbow flexors. Eight college-aged students participated in this crossover design study. The muscle-damage protocol consisted of four sets of 25 repetitions of isokinetic concentric elbow flexion followed by eccentric elbow extension at 60°/second. Immediately following the muscle-damage protocol, subjects either wore a CS continually for five days or completed daily, 20-minute PCD treatments for five days. Swelling, range of motion (ROM), and pain were measured daily during the five-day recovery period. Subjects rested for seven additional days before completing another muscle-damage protocol and the remaining treatment. Treatment order was randomized and balanced. Muscle swelling, assessed via changes in upper arm circumference, was significantly lower in the PCD treatment (1.7 vs. 2.0 cm in CS, p = 0.012), however there was no difference in lower arm circumference (p = 0.091). ROM disturbances during the PCD treatment were lower (mean peak reduction in ROM −9.04 degrees in PCD compared to −17.25 degrees in CS, p < 0.05) and peak pain was lower by 39% (27.5 mm in PCD compared to 45.2 mm in CS, p < 0.05) when compared to the CS treatment. These findings suggest that daily treatments using a PCD further reduce peak disturbance and recovery time from DOMS of the elbow flexors when compared to a continuously-worn CS.
Keywords: Muscle damage, muscle inflammation, soreness, arm sleeves, delayed onset muscle soreness
INTRODUCTION
Compression garments have received attention due to their purported abilities to improve exercise performance and speed muscle recovery and are available from a multitude of manufacturers for almost any sport imaginable. Previous research has demonstrated little to no benefit to while wearing compression garments while exercising (10). However, recovery from strenuous exercise resulting in delayed-onset muscle soreness (DOMS) has significantly improved when wearing such garments. It is thought that compressive garments aid in prevention of excessive muscle swelling and enhance blood flow and the removal of waste products and muscle metabolites, which result in greater range of motion (ROM) about the affected joint, less severe decrements in muscular strength and power, and reduced pain (4).
While the use of compression garments during recovery has shown to be beneficial, other forms of compression, such as pneumatic compression, have received little attention when directly compared to these garments. Pneumatic compression devices (PCDs) provide compression to the limbs via inflatable cuffs. Dynamic PCDs, such as the device used in this study, have up to five separate compartments which inflate sequentially from the most distal part of the limb towards the center of the body. The sequential nature of inflating, holding, and deflating cuffs returns blood, and thereby wastes and metabolites, from the periphery to the core.
Pneumatic compression devices, which offer short-term but more intense dynamic compression, have been used in the treatment of diseases such as lymphedema (3). However, little is known regarding the use of these devices in the recovery from DOMS, particularly in comparison to compression garments. Further, most previous research investigating PCDs have used a single treatment. Thus, the rate of DOMS recovery from multiple treatments is unknown.
A few studies have been conducted that investigate the use of a PCD in the recovery from strenuous exercise. In theory, PCDs promote faster blood flow, nutrient delivery, and waste removal from the compressed muscles. For example, when the legs were treated for 30 minutes following two 30-second Wingate anaerobic tests, Martin el al. found lower blood lactate at the end of the recovery period (by approximately 25%) but no significant difference in a subsequent Wingate test performance (8). Additionally, Keck et al. found no differences in muscle glycogen synthesis between the PCD and the control treatment at any point four hours after glycogen-depleting exercise and two, 60-min treatments (6). These findings suggest only minor changes in variables partially dependent upon blood flow to the affected muscles.
When evaluating changes in performance and perceptions of DOMS, no differences were detected in jump height, peak torque production, perceived recovery, or muscle soreness following fatiguing resistance exercise and either a 45-min PCD treatment, 12 minutes of vascular occlusion, or passive rest (9). Hoffman et al. examined the impact of one 20-min PCD treatment following an ultramarathon when compared to a 20-min massage or rest. Results indicated minor improvements in subjective ratings of pain and fatigue in the PCD condition when compared to rest, but no difference between the massage and PCD conditions (5). Together, these results suggest little benefit to short-term use of a PCD during recovery from strenuous exercise. However, the effectiveness of daily treatments, as opposed to short-term treatments, has yet to be explored. Further, all previous studies have only utilized lower-body protocols as opposed to an upper-body protocol. For some athletes, such as baseball players and weight lifters, upper body recovery is especially important for training and competition.
Recently, moderately-priced PCDs have been marketed for purchase directly to athletic teams and individuals. Are these systems worth the additional expense or do less-expensive compression garments offer the same benefits? The purpose of this study was to compare recovery from DOMS when receiving daily PCD treatments when compared to a continuously-worn CS. It was hypothesized that all variables (muscular swelling, joint range of motion, and pain) would demonstrate less change from baseline in the PCD treatment when compared to the CS, and that a return to baseline would be achieved faster in the PCD treatment.
METHODS
Participants
Eight college students (Table 1) participated in this study to evaluate recovery from DOMS when wearing a continuously-worn compression sleeve or receiving daily, 20-minute treatments using a PCD. All subjects were considered recreationally fit in that no subjects were currently in season for a collegiate sport nor were any subjects considered untrained. Participants were recruited via personal contact.
Table 1.
Descriptive Statistics of Participants*
| Age (years) | Height (cm) | Weight (kg) | |
|---|---|---|---|
| Female Subjects (n = 4) | 22.0±0.8 | 162±4 | 63±5 |
| Male Subjects (n = 4) | 21.2±1.7 | 181±2 | 83±9 |
| All Subjects (n = 8) | 21.6±1.3 | 172±10 | 73±12 |
Values are mean ± SD
Protocol
Measurements regarding muscle swelling, range of motion (ROM), and pain were recorded prior to the muscle-damaging protocol (as described later), immediately after, and for the five following days at approximately the same time of day. Immediately following the muscle-damaging protocol, subjects were randomized into either the compression sleeve (CS) or pneumatic compression device (PCD) treatment. The order of treatments was randomized and balanced and each participant completed both treatments. A minimum of one week separated the ending of one treatment to the beginning of the other treatment. This study was approved by the Hanover College Institutional Review Board.
Upon arrival to the laboratory, subjects read and completed the informed consent. Pre-test measurements of muscle swelling were recorded by measuring bicep circumference at 3 cm above the elbow crease (lower arm circumference) and 12 cm above the elbow crease (upper arm circumference) using a tape measure. Flexion range of motion (ROM) was measured using a plastic goniometer when the elbow was fully flexed. Extension ROM was measured when the arm was fully extended. Pain was measured using a 100 mm visual analog scale during elbow flexion, extension, and palpation by the investigator. During palpation the investigator placed the index and middle fingers 3 cm above the elbow crease and applied enough pressure to elicit pain from a sore muscle, but not so much to cause pain from the palpation itself. While efforts were made to apply similar amounts of pressure each time, there was no method for standardizing the pressure applied (such as using a pressure algometer).
Following the pre-test measures, the muscle-damaging protocol was performed using the HUMAC NORM isokinetic dynamometer (CSMi, Stoughton, MA, USA) to induce soreness in the non-dominant elbow flexors. To begin the protocol, subjects completed three warm-up repetitions of concentric elbow flexion. Following a 10-second rest, subjects began the first of four sets of 25 repetitions of concentric elbow flexion and eccentric elbow extension at 60°/second. There was a one-minute rest between sets. Immediately following the muscle-damaging protocol all variables were measured again. At this point, subjects either applied the compression sleeve or received the first PCD treatment.
Approximately 24 hours later and for the following four days, subjects completed one of two treatments: continuous wear of a CS (Adidas, Germany) or daily, 20-minute treatments of pneumatic compression (NormaTec, Newton Center, MA, USA) on the non-dominant arm. During the CS treatment, subjects were instructed to wear the sleeve continuously except during showering and study measurements. The sleeve covered most the subjects’ arms from approximately the deltoid insertion to the wrist. During the PCD treatment, subjects received a 20-minute treatment of intermittent pneumatic compression once per day. The PCD had five separate compartments that sequentially inflated, compressed (at 100 mmHg), and released beginning in the most distal portion of the arm and moving towards the center of the body. For example, the compartment for the hand and wrist inflated and pulsed for one minute. After this minute, the next compartment inflated and pulsed as pressure in the first compartment was held constant. When the third compartment inflated, the first compartment released. Thus, this series of pressure pulses, holds, and releases created a massaging action to move fluids out of the limb. A complete cycle of inflating, holding, and releasing in all five compartments lasted five minutes and was repeated four times. Swelling, ROM, and pain were measured immediately following the 20-minute treatment. The same investigator collected all study measurements.
Statistical Analysis
Results were analyzed using SPSS 24 (IBM Corporation, Armonk, NY) and a 2 (treatment) by 7 (time) mixed ANOVA with repeated measures to identify significant differences between treatments. Significance was set at p ≤ 0.05.
RESULTS
In general, DOMS-related swelling, ROM limitations, and pain ratings were lower and returned to baseline faster in the PCD treatment when compared to the CS treatment (p < 0.05). The only exceptions were lower arm circumference and pain during palpation, in which there was no main effect of treatment (p = 0.091 and 0.102, respectively). The main effect of time was significant for all variables (p < 0.05), as would be expected following the onset of DOMS and the gradual return to baseline in both treatments. Treatment by time interactions were not significant (p > 0.05) suggesting that the time course of changes in swelling, ROM, and pain were similar between treatments, however the magnitude of the disturbances were lower in the PCD treatment.
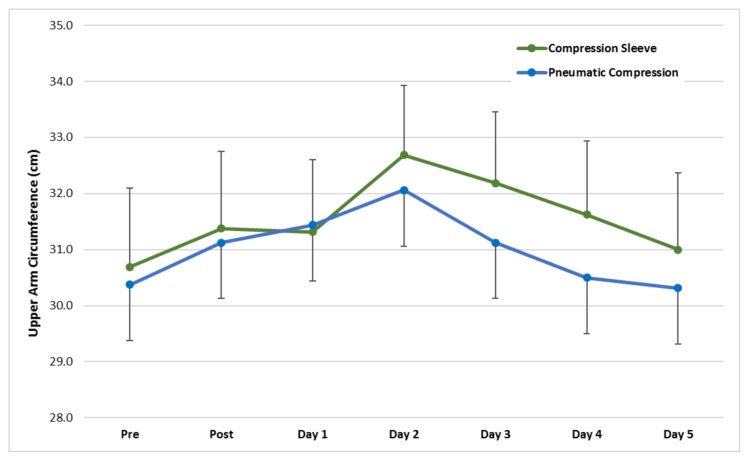
Swelling, as determined by arm circumference (Figure 1), was significantly greater in the CS treatment when compared to the PCD treatment at the upper arm location (χ̄ difference = 0.56 cm, p = 0.012, Cohen’s d effect size (ES) = 0.16). Swelling had returned to baseline by Day 3 in the PCD treatment and by Day 5 in the CS treatment. At the lower arm location, there was no significant difference between treatments (main effect of treatment p = 0.091).
Figure 1.
Mean (±SE) upper arm circumference pre, post, and five days following the DOMS-inducing protocol. The main effects of time (p = 0.007) and treatment were significant (p = 0.012). The time × treatment interaction was not significant (p = 0.408).
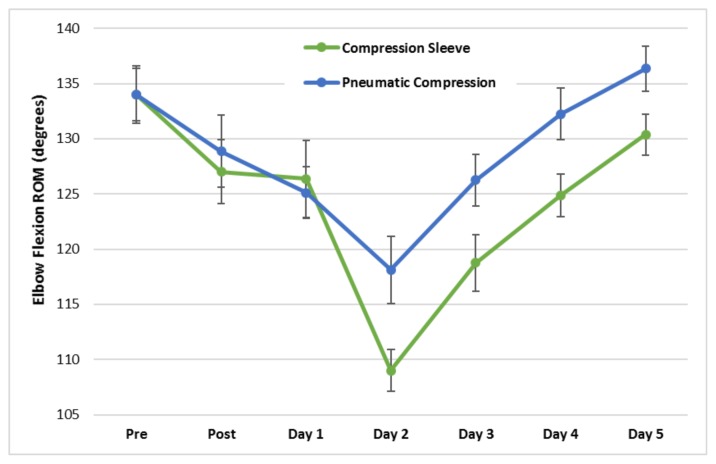
In both treatments, range of motion about the elbow joint was reduced following the DOMS-inducing protocol and gradually returned to baseline over the five-day recovery period. Range of motion during elbow extension (χ̄ difference = 1.0 degree, p = 0.005, Cohen’s d ES = 0.64) and elbow flexion (χ̄ difference = 4.38 degrees, p = 0.002, Cohen’s d ES = 0.46) was significantly greater in the PCD treatment (Figure 2).
Figure 2.
Mean (±SE) elbow flexion range of motion pre, post, and five days following the DOMS-inducing protocol. The main effects of time (p = 0.042) and treatment were significant (p=0.002). The time × treatment interaction was not significant (p = 0.183).
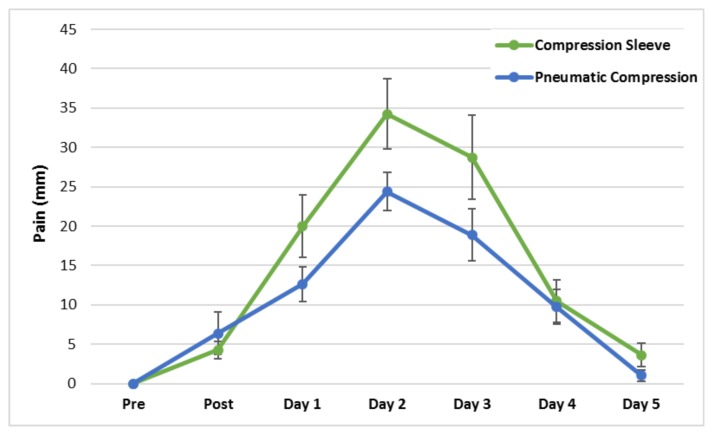
Subjective ratings of pain during elbow flexion (Figure 3) were higher in the CS condition (χ̄ difference = 4.1 mm, p = 0.015, Cohen’s d ES = 0.31) as well as during elbow extension (χ̄ difference = 5.6 mm, p = 0.031, Cohen’s d ES = 0.34). These differences were evident especially during the first three days of the recovery period. By Day 5, pain ratings had returned to baseline in both treatments for flexion pain. Pain during elbow extension remained significantly elevated from baseline (p = 0.040) in the CS treatment on Day 5 whereas pain ratings had returned to baseline in the PCD treatment by this time. Pain ratings during palpation by the investigator were not significantly different between treatments (p = .102).
Figure 3.
Mean elbow flexion pain pre, post, and five days following the DOMS-inducing protocol. The main effects of time (p = 0.017) and treatment were significant (p = 0.015).
DISCUSSION
The main finding of this study was that daily treatments using a PCD provided superior recovery from DOMS when compared to a continuously-worn CS. Almost all measured variables demonstrated significantly less disturbance from baseline using the PCD, apart from lower arm circumference and palpation pain. Additionally, there were no significant differences from baseline values by Day 4 in two variables (upper arm circumference and extension pain) during the PCD treatment, suggesting a complete recovery from DOMS during that time. In the CS treatment, these variables returned to baseline a day later.
Results for two variables, lower arm circumference and pain during palpation, were not significantly different between treatments (p = 0.091 and 0.102, respectively). Although not statistically significant, the mean values follow the same pattern found in other variables indicating less disturbance from baseline and a faster return to baseline in the PCD treatment. Results from these variables would have likely been significant with a larger sample size, and thus, more power.
While the compression pressures created by the two treatments were not measured, the PCD generated substantially higher pressures than a commercially-available CS. Previous authors have either measured or estimated pressures ranging from 10 mmHg – 17 mmHg in similar CSs (2,7) and the manufacturer of the PCD in this study reported pressures of 100 mmHg at the device setting used in this study. Previous research has indicated that greater external pressure increased forearm blood flow two-fold as pressure increased from 13–23 mmHg using a series of increasingly compressive sleeves (1). Thus, the reduced swelling, ROM disturbance, and pain found in the PCD treatment may be due to greater arterial blood flow promoting increased removal of metabolites, wastes, and excessive interstitial fluid in the affected arm.
Previous investigations using PCDs have found little benefit when compared to other treatments. The lack of previous significant findings may be due to how the PCD was used, most commonly for a single treatment ranging from 20 – 60 minutes in duration (5, 8, 9) whereas in the current study treatments occurred daily. Additionally, the studies cited examined the lower body as opposed to the upper body investigated in this study. During the recovery period in the current study, swelling, range of motion, and pain were recorded immediately following treatment with the PCD when the effects of the treatment would likely be the greatest. Results may differ if measurements were taken a later time following treatments.
The athleticism of the subject group also contributes to the level of perceived fatigue and soreness experienced from a given protocol. In the study by Northey et al. subjects included 12 strength-trained males currently training at least three times per week. Following the fatiguing exercise, 10 sets of 10 repetitions of back squat at 70% of 1RM, peak soreness reached approximately 5.5 out of 10 (9). Peak muscle pain and soreness was 8.5 out of 10 in a group of well-trained runners following 161-km ultramarathon. Neither study found PCD treatments to be effective in reducing perceived pain and soreness. In the current study involving recreationally-active subjects, peak pain ranged from 34 – 39 out of 100mm, comparatively less than the previous studies. Perhaps athletic subjects can more accurately describe their soreness than less-trained subjects which may explain why PCD treatments were effective only in the current study. Another possibility is that once soreness reaches beyond a certain level, no treatment will be effective.
Our study had some limitations. First, the subject pool was relatively small (n=8) and it is unknown whether these results would extend to other subject groups. However, previous unpublished work from our lab has found similar results when comparing a CS to no compression with a similarly-sized subject group. Second, the investigator measuring the outcome variables was not blinded to the subject treatment. As treatments were performed just before measurements were made, blinding of the treatment would have been difficult to accomplish. Third, it is possible that a placebo effect occurred during the PCD treatment as the PCD is novel and sophisticated in appearance and provides a greater intensity of pressure, although only during the 20-minute treatment time. The presence of a placebo effect could explain at least part of the superiority of the PCD treatment in the alleviation of DOMS in this subject group when compared to a CS. Neither a placebo treatment nor a no-treatment control were included in this study due to demands on subjects’ time (five days per treatment plus an additional seven days’ rest) and the occurrence of the repeated bout effect in which the damaged muscle groups adapt to the exercise stimulus and demonstrate less DOMS-related symptoms with each successive bout.
Future research should address the possibility of a placebo effect when using a PCD. Various subject groups, highly trained or unfit subjects, may also respond differently to daily use of a PCD. How well-trained athletes, engaged in daily training and frequent competition, may use this device most effectively, including how frequently and for how long, remains unknown. Lastly, whether daily use of a PCD significantly impacts performance has yet to be examined.
Overall, daily treatments using a PCD significantly decreased DOMS-related muscular swelling, disturbances in ROM, and subjective pain when compared to a continuously-worn CS in a group of recreationally-active subjects. Other investigations have generally supported improvements in DOMS symptoms and the recovery from muscle damage while using compression garments when compared to a no-treatment control (4). Thus, advantages from daily treatments using a PCD are likely to be even greater than no treatment at all.
ACKNOWLEDGEMENTS
We would like to thank the participants in the study who generously provided their time.
REFERENCES
- 1.Bochmann R, Seibel W, Haase E, Hietschold V, Rodel H, Deussen A. External compression increases forearm perfusion. J Appl Physiol. 2005;99:2337–2344. doi: 10.1152/japplphysiol.00965.2004. [DOI] [PubMed] [Google Scholar]
- 2.Carling J, Francis K, Lorish C. The effects of continuous external compression on delayed-onset muscle soreness (DOMS) Int J Rehab Health. 1995;1(4):223–235. [Google Scholar]
- 3.Chen A, Frangos S, Kilaru S, Sumpio B. Intermittent pneumatic compression devices- physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001;21:383–392. doi: 10.1053/ejvs.2001.1348. [DOI] [PubMed] [Google Scholar]
- 4.Hill J, Howatson G, van Someren K, Leeder J, Pedlar C. Compression garments and recovery from exercise-induced muscle damage: a meta-analysis. Br J Sports Med. 2014;48:1340–1346. doi: 10.1136/bjsports-2013-092456. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman M, Badowski N, Chin J, Stuempfle K. A randomized controlled trial of massage and pneumatic compression for ultramarathon recovery. J Orthop Sports Phys Ther. 2016;46(5):320–326. doi: 10.2519/jospt.2016.6455. [DOI] [PubMed] [Google Scholar]
- 6.Keck N, Cuddy J, Hailes W, Dumke C, Ruby B. Effects of commercially available pneumatic compression on muscle glycogen recover after exercise. J Strength Cond Res. 2015;29(2):379–385. doi: 10.1519/JSC.0000000000000772. [DOI] [PubMed] [Google Scholar]
- 7.Kraemer W, Bush J, Wickham R, Denegar C, Gomez A, Gotshalk L, Duncan N, Volek J, Putukian M, Sebastianelli W. Influence of compression therapy on symptoms following soft tissue injury from maximal eccentric exercise. J Orthop Sports Phys Ther. 2001;31(6):282–290. doi: 10.2519/jospt.2001.31.6.282. [DOI] [PubMed] [Google Scholar]
- 8.Martin J, Friedenreich Z, Borges A, Roberts M. Acute effects of peristaltic pneumatic compression on repeated anaerobic exercise performance and blood lactate clearance. J Strength Cond Res. 2015;29(10):2900–2906. doi: 10.1519/JSC.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 9.Northey J, Rattray B, Argus C, Etxebarria N, Driller M. Vascular occlusion and sequential compression for recovery after resistance exercise. J Strength Cond Res. 2016;30(2):533–539. doi: 10.1519/JSC.0000000000001080. [DOI] [PubMed] [Google Scholar]
- 10.Stanek J. The effectiveness of compression socks on athletic performance and recovery. J Sport Rehabil. 2017;26(1):109–114. doi: 10.1123/jsr.2015-0048. [DOI] [PubMed] [Google Scholar]