Abstract
Iron overload disorders may be treated by chelation therapy. This study describes a novel method for isolating iron chelators from complex mixtures including plant extracts. We demonstrate the one-step isolation of curcuminoids from turmeric, the medicinal food spice derived from Curcuma longa. The method uses iron-nitrilotriacetic acid (NTA)-agarose, to which curcumin binds rapidly, specifically, and reversibly. Curcumin, demethoxycurcumin, and bis-demethoxycurcumin each bound iron-NTA-agarose with comparable affinities and a stoichiometry near 1. Analyses of binding efficiencies and purity demonstrated that curcuminoids comprise the primary iron binding compounds recovered from a crude turmeric extract. Competition of curcuminoid binding to the iron resin was used to characterize the metal binding site on curcumin and to detect iron binding by added chelators. Curcumin-Iron-NTA-agarose binding was inhibited by other metals with relative potency: (>90% inhibition) Cu2+ ~ Al3+ > Zn2+ ≥ Ca2+ ~ Mg2+ ~ Mn2+ (<20% inhibition). Binding was also inhibited by pharmaceutical iron chelators (desferoxamine or EDTA) or by higher concentrations of weak iron chelators (citrate or silibinin). Investigation of the physiological effects of iron binding by curcumin revealed that curcumin uptake by cultured cells was reduced >80% by addition of iron to the media; uptake was completely restored by desferoxamine. Ranking of metals by relative potencies for blocking curcumin uptake agreed with their relative potencies in blocking curcumin binding to iron-NTA-agarose. We conclude that curcumin can selectively bind toxic metals including iron in a physiological setting, and propose inhibition of curcumin binding to iron-NTA-agarose for iron chelator screening.
Keywords: Iron, Metal chelation, Curcumin, Turmeric, Curcuma longa
Introduction
Iron is a physiologically essential metal in humans but can be toxic at high levels, likely due to oxidative stress from redox cycling of the free metal (Jomova and Valko 2011; Guo et al. 2016). High body iron is a risk factor for a number of diseases, in particular diseases of the liver, the primary storage organ for excess iron (Pietrangelo 2016). In humans, iron overload disorders can cause liver fibrosis, cirrhosis, and cancer; effective therapies include phlebotomy or administration of pharmaceutical iron chelators. Drugs such as EDTA and desferoxamine can sequester iron and facilitate its elimination (Mobarra et al. 2016). However, many iron chelation therapies must be injected and carry significant risks of adverse effects with prolonged use (Badria et al. 2015). Endogenous mechanisms for mitigating tissue injury from iron and other redoxactive metals (including Cu and Al) include antioxidant and metal sequestration defense mechanisms (e.g. glutathione, thioredoxin, ferritin and metallothionein). These may be supported by dietary antioxidants and weak metal chelators, compounds that likely contribute to the health benefits of a plant based diet (Fraga and Oteiza 2002).
The food spice turmeric, derived from the Asian plant Curcuma longa, has a number of purported health benefits, most ascribed to its high content of curcumin and related curcuminoids (Ammon and Wahl 1991; Jurenka 2009); C. longa is ~3–5% curcumin by weight (World Health Organization 1999). A large number of studies in recent years have identified biological activities and molecular targets of curcumin with anti-disease potential, including metal chelation (Kunnumakkara et al. 2016). Previous reports using spectroscopic techniques have characterized curcumin binding to iron and copper under non-physiological conditions (Sreejayan and Rao 1994; Baum and Ng 2004; Bernabe-Pineda et al. 2004). However, evidence that curcumin binds iron in cells and tissues is indirect. In mice, dietary curcumin decreased ferritin (Jiao et al. 2006, 2009) and iron (Chin et al. 2014; Badria et al. 2015) in the liver, both consistent with a reduction of body iron stores. In cell culture, curcumin reduced cytotoxic (Messner et al. 2009) and tumor promoting (Messner et al. 2017) effects of iron. Standardized turmeric extracts appeared more potent than curcumin alone in cells; whether this is due to additional bioactive compounds in turmeric (possibly iron chelators) is unknown.
Thus although curcumin can bind iron, it is not known whether this occurs to a significant degree in the presence of other metals and/or weak iron chelators typically present in cells and tissues. Selective iron binding by curcumin in the presence of potential inhibitors has not been directly demonstrated, in part due to lack of suitable techniques. We address this issue in the present study using iron affinity chromatography; binding of curcumin to iron was selective enough to allow its purification directly from crude turmeric extracts. This approach allowed us to define the metal binding properties of curcumin and turmeric, and develop an efficient method for screening plant extracts for iron chelator activity.
Materials and methods
Materials
Turmeric (C. longa) and purified curcuminoids (C3 complex®) were from Sabinsa Corporation and characterized as described in the next section. Nickel-NTA-agarose was from Qiagen. Newborn calf serum was from Atlanta Biologicals and other cell culture reagents were from Thermo Fischer Scientific. Desferoxamine mesylate, disodium EDTA, sodium citrate, silymarin, ferric ammonium citrate, ferric chloride and other metal salts, and dimethylformamide were from Sigma-Aldrich.
Curcumin and turmeric quality
Pure curcuminoids (as C3 complex®) and turmeric powder were quality tested as to purity and contamination as described previously (Messner et al. 2017); specifications were in line with requirements of the World Health Organization (WHO) (World Health Organization. 1999) and the Product Integrity Working Group of the National Center for Complementary and Integrative Health (NCCIH, formerly NCCAM). Curcuminoids were prepared as a 50 mM stock solution in dimethylformamide and stored at −20 °C. Turmeric powder was extracted at room temperature for 30 min in dimethylformamide (0.62 g turmeric/ml) and filtered to remove particulates. The extract was diluted with dimethylformamide as required to achieve a total curcuminoid concentration of 50 mM and stored at −20 °C as for the purified curcuminoids. Curcuminoids stored in this way were stable for >1 year (Messner et al. 2017). Curcuminoid compositions were determined by reverse phase HPLC using an Inertsil ODS-3 column from Agilent, an isocratic mobile phase of tetrahydrofuran: 0.1% citric acid (40:60), with detection by absorbance at 425 nm and integration of peak areas (Table 1). Subsequent dilutions of purified curcuminoids or turmeric extracts in ethanol and/or cell culture media were completed just prior to use.
Table 1.
Relative curcuminoid composition of study test products (% of total)
| Purified curcuminoids (%) | Turmeric (%) | |
|---|---|---|
| Curcumin | 75 | 65 |
| Demethoxycurcumin | 22 | 21 |
| Bisdemethoxycurcumin | 3 | 15 |
Metal binding assays
Iron-nitrilotriacetic acid (NTA)-agarose was prepared by loading NTA-agarose with iron to its full capacity of 15 μmol iron per ml resin according to the manufacturer’s recommendations. Resin complexed with other metals or metal free (stripped) resin were used where indicated. Once loaded the resin was stored at 4 °C in 50% ethanol and washed 3× prior to use.
Binding experiments were performed in 10 mM Tris-HCl (pH 7) in 80% ethanol (assay buffer), generally for 30 min at room temperature. Purified curcuminoids or turmeric extract diluted in assay buffer (generally to 10 μM curcuminoids final concentration) were combined with metal salts and/or chelators prior to addition of iron-NTA-agarose. Unless specified otherwise, an amount of resin (20 μl) containing 300 nmol NTA ± bound metal was used per each 1 ml assay point and all points were done in duplicate. Curcuminoid concentrations were determined by absorbance at 425 nm. Bound curcumin was calculated by subtracting free curcumin remaining in solution (after centrifugation of the resin) from the total curcumin added, expressed as % of the total curcumin added to the assay. Where indicated bound material was eluted from iron-NTA-agarose by three successive washes with 0.1 M EDTA pH 7 in 80% ethanol. Non-specific binding was measured using iron-free stripped NTA-agarose in the assay. Additional experiment-specific details are described in the corresponding Figure legends.
Cell culture and curcuminoid uptake assays
T51B rat liver epithelial cells, a non-neoplastic cell line developed for carcinogenesis and tumor promotion experiments, were maintained as described previously (Messner and Kowdley 2008). They display density-inhibited and anchorage-dependent proliferation that makes them convenient for curcuminoid uptake assays. They have characteristics of liver oval cells, rather than hepatocytes, and enzymatic metabolism of curcuminoids or other xenobiotics by these cells is minimal (Swierenga et al. 1980). Curcuminoids (20 μM) diluted in pre-warmed cell culture media in the presence of specified metals and/or chelators were added to confluent T51B cells in 60 mm dishes and placed in the CO2 incubator at 37 °C for 60 min. At the end of the incubation (uptake) period, the cells were rinsed twice with ice-cold 0.1% BSA in PBS pH 7.4, twice with PBS only, and then scraped in 1 ml 90% ethanol on ice. Cell debris was removed by centrifugation and the relative amount of curcuminoids was determined by measuring absorbance at 425 nm after subtracting the absorbance of extracts from untreated control cells not given curcuminoids. Previous work confirmed by HPLC assay that all three curcuminoids are taken up to a similar degree by T51B cells (Messner et al. 2017). Uptake was expressed as a percentage of that measured in control cells given purified curcuminoids (100%).
Statistics
Unless specific otherwise, experimental values represent the mean ± standard error of the mean (sem) of at least three independent replicates, using a student t test to determine significance at *p < 0.05 or **p < 0.01. Curve fitting and determination of kinetic constants utilized the Prism Graph Pad® software suite.
Results
Curcumin (diferuloylmethane) is a weak iron chelator that can be extracted from solution using iron affinity chromatography (Messner et al. 2009). Figure 1 shows other common curcuminoids also bind iron. HPLC analysis of the iron binding compounds isolated from a crude turmeric extract revealed a curcuminoid composition nearly identical to the starting extract (curcumin: demethoxycurcumin: bisdemethoxycurcumin = 69:18:13 after elution versus 65:21:15 in the starting extract). Binding was rapid and selective; gallium, but not copper or nickel, could substitute for iron on NTA-agarose resin (not shown). Thus the three curcuminoids found in turmeric reversibly and selectively bound iron-NTA-agarose with comparable affinities.
Fig. 1.
Recovery of curcuminoids from iron-NTA-agarose. a Curcuminoid structures. b Curcuminoids isolated from C. longa. A dimethylformamide extract of turmeric powder was incubated with iron-NTA-agarose; the bound material was eluted with EDTA and analyzed by HPLC as described in Methods. A representative HPLC profile (absorbance at 425 nm) illustrates recovery of curcumin (diferuloylmethane, 20 min), demethoxycurcumin (23.5 min), and bisdemethoxy-curcumin (27 min)
Figure 2 illustrates the concentration dependence of curcuminoid binding to 300 nmol/ml iron-NTA-agarose. Metal-free NTA-agarose was used to assess non-specific binding. Scatchard analysis indicated a single binding site for curcuminoids having an apparent KD near 500 μM and a stoichiometry near 1 under these conditions, reflecting formation of a 1:1:1 ternary complex between curcuminoids, iron, and NTA-agarose. Disruption of this ternary complex is predicted for other molecules that bind either curcuminoids (e.g. selected metals) or iron-NTA-agarose (e.g. iron chelators). Figure 3 shows the effect of 100× excess metal salts added to the binding reaction. Under these conditions copper and aluminum blocked cur-cuminoid binding nearly completely (>80%), while zinc was less effective (45% reduction). There were no significant effects of 1 mM calcium, manganese or magnesium (Fig. 3). The monovalent cations sodium and potassium similarly did not inhibit curcumin binding to the resin (not shown). Iron remained bound to NTA-agarose in the presence of these other metals as shown by chemical assay, indicating that inhibition was due to disruption of iron-curcuminoid binding (and not to displacement of iron from NTA-agarose). These data indicate curcuminoids preferentially bind more toxic metals iron, copper and aluminum, with little or no binding to physiological metals typically found at higher free concentrations in blood and tissues.
Fig. 2.
Purified curcuminoid binding to iron-NTA-agarose. Increasing amounts of purified curcuminoids were added to 300 nmol iron-NTA-agarose (total binding, circles) or an equivalent amount of metal-free NTA-agarose (non-specific binding, squares). Bound curcuminoids were determined as described in Methods. Means (±SEM) of three replicate experiments are shown. A Scatchard analysis of specific curcuminoid binding is presented in the inset
Fig. 3.
Inhibition of curcuminoid binding to iron-NTA-agarose by free metals. The binding of 10 μM purified curcuminoids to 300 μM iron-NTA-agarose was measured alone (Iron-NTA-resin) or in the presence of 1 mM of the indicated metal salt. Curcuminoid binding to metal-free NTA-agarose (NTA- resin) is also displayed. Composite data representing the means (±SEM) of at least three replicate experiments are shown for each condition. The metals tested were: cupric sulfate (Cu), zinc sulfate (Zn), manganese chloride (Mn), magnesium chloride (Mg), calcium chloride (Ca), and aluminum chloride (Al). Inhibition of curcuminoid binding by 1 mM (100× excess) metals was significant at *p < 0.05 or **p < 0.01
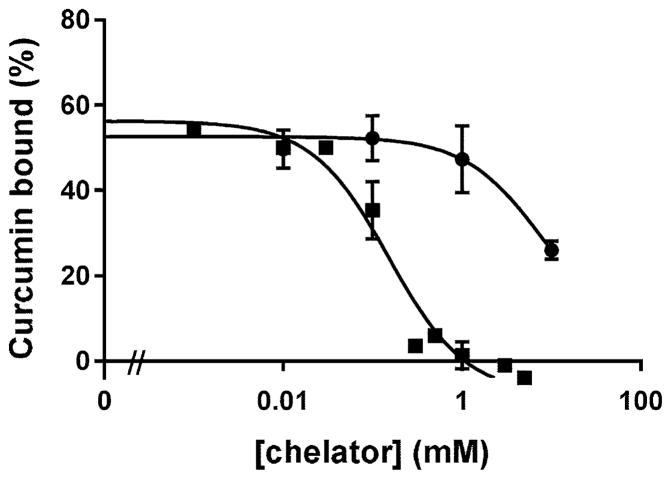
Figure 4 illustrates effects of known iron chelators on curcuminoid binding to iron-NTA-agarose. Two of these compounds (EDTA and desferoxamine) are known to bind ferric (3+) iron with high affinity, while one binds with low affinity (o-phenanthroline), relative to NTA (Smith and Arthur 2003). Both EDTA and desferoxamine prevented curcuminoid binding to iron-NTA-agarose (Fig. 4). Under conditions of this assay (300 μM iron) the apparent IC50s for EDTA and desferoxamine were near 100 μM, while o-phenanthroline had no inhibitory effect at concentrations up to 10 mM.
Fig. 4.
Maximal inhibition of curcuminoid binding to iron-NTA-agarose by strong iron chelators. Increasing concentrations of the iron chelators desferoxamine (squares), EDTA (circles), and o-phenanthroline (triangles) were added to 10 μM purified curcuminoids prior to measuring binding to 300 μM iron-NTA-agarose as described in Methods. The means (±SEM) of three replicate experiments are shown
Figure 5a illustrates the concentration dependence of iron-NTA-agarose binding by curcuminoids added as a crude turmeric extract. These data were consistent with a single binding site for turmeric curcuminoids having an apparent KD near 500 μM, comparable to that observed for purified curcuminoids (Fig. 2). Compared to purified curcuminoids, non-specific (iron-free) binding to the resin increased slightly, likely due to other organic material in the crude extracts. Non-specific binding was not decreased by EDTA (not shown) arguing against the presence of metals in the extract that might link curcuminoids to free NTA-agarose.
Fig. 5.
Analysis of iron binding molecules in crude turmeric extracts. a Binding of curcuminoids from turmeric. Increasing amounts of standardized turmeric extract were added to 300 nmol iron-FTA-agarose (total binding, circles) or an equivalent amount of metal-free NTA-agarose (non-specific binding, squares). Curcuminoid amounts were determined as described in Methods. Means (±SEM) of three replicate experiments are shown. a Scatchard analysis of specific curcuminoid binding is presented in the inset. b. Purity of turmeric curcuminoids eluted from iron-NTA-agarose. Absorbance ratios (A425/A240) were determined for material bound and eluted with EDTA from iron-NTA-agarose after correcting for absorbance of the elution buffer. The mean values (±SEM) from three preparations each of purified curcuminoids and crude turmeric extracts are displayed
Non-curcuminoid iron chelators, if present in turmeric extracts, would decrease both the amount and purity of turmeric curcuminoids recovered on iron-NTA-agarose. This was not observed; binding curves (Figs. 2, 5a) indicated the binding capacity of the resin was comparable for crude (turmeric extract) and pure curcuminoids. Under near saturating conditions (2000 nmol curcuminoids applied to ~300 nmol iron resin), 251 ± 32 nmol curcuminoids were retained using turmeric (total binding, mean ± sem, n = 3), indistinguishable (p = 0.58) from the 267 ± 33 nmol curcuminoids retained using purified curcuminoids (total binding, mean ± sem, n = 6). Figure 5b shows the purity of the curcuminoids obtained was similar regardless of whether a crude turmeric extract (A425/A240 = 3.169 ± 0.177, mean ± sem, n = 3) or pure curcuminoids (A425/ A240 = 3.701 ± 0.049, mean ± sem, n = 3) was used as the starting material (p = 0.12). Together these data suggest that curcuminoids are the main iron binding compounds in the turmeric extracts.
We next tested the ability of the binding assay to detect moderate affinity iron chelators, typically more common than high affinity chelators in human cells and tissues as well as plant extracts. Citrate is a recognized physiological iron chelator (Grootveld et al. 1989) while silibinin is a natural product from milk thistle (Silybum marianum) that binds iron (Borsari et al. 2001). Both citrate and silibinin inhibited curcuminoid binding in a concentration dependent fashion (Fig. 6). The concentration required to inhibit curcumin binding by 50% (apparent IC50) reflects in part the affinity of the inhibitor for iron-NTA-agarose. For citrate, the apparent IC50 was near 100 μM, while for silibinin the apparent IC50 was approximately 10 mM. When combined with Fig. 4, these data show the curcuminoid binding assay can detect a wide variety of iron chelators, and so represents a useful approach to screening crude plant extracts for novel compounds.
Fig. 6.
Differential inhibition of curcuminoid binding to iron-NTA-agarose by weak iron chelators. Increasing concentrations of the iron chelators citrate (squares) and silibinin (circles) were added to 10 μM purified curcuminoids prior to measuring curcuminoid binding to 300 μM iron-NTA-agarose as described in Methods. The means (±SEM) of three replicate experiments are shown
Inhibition of curcuminoid binding to iron-NTA-agarose by selected metals or weak physiological chelators argues that iron binding by curcumin may not be relevant in a physiological setting (like blood or cells) in which potential competitors are present at higher concentrations. To assess this more directly we measured binding in a cell culture assay. As shown in Fig. 7, curcumin uptake by cells was quantitatively reduced by addition of free iron to the media. This was prevented by the high affinity iron chelator desferoxamine, but not citrate, a relatively abundant physiological iron chelator (Fig. 7). Similar effects on curcumin uptake were observed for selected other metals (e.g. copper and aluminum) roughly in proportion to their potency revealed in binding experiments (Fig. 3). Notably, physiological levels of non-toxic metals (including calcium, magnesium, and manganese) had no effect. Thus relatively low concentrations of curcumin can bind iron in a biological setting that includes higher concentrations of physiologically abundant metals and weak iron chelators.
Fig. 7.
Effects of metals on curcuminoid uptake by cells in culture. Uptake of 20 μM purified curcuminoids by rat liver epithelial cells in culture was measured in the absence (control) or presence of 200 μM (light shaded bars) or 2 mM (dark shaded bars) of the indicated metal salt as described under Methods. Uptake is expressed relative to cells given curcuminoids only (set at 100%); composite data representing mean values (±SEM) from at least three independent replicates are shown for each condition. The conditions tested were: ferric chloride (Fe), cupric chloride (Cu), zinc chloride (Zn), manganese chloride (Mn), magnesium chloride (Mg), calcium chloride (Ca), aluminum chloride (Al), ferric chloride with 2.5-fold molar excess (500 μM) desferoxamine (Fe + dfo), and ferric chloride with tenfold molar excess (2 mM) sodium citrate (+citrate). Inhibition of curcuminoid uptake was judged significant at *p < 0.05 or **p < 0.01
Discussion
We describe a novel iron affinity chromatography (iron-NTA-agarose) approach to isolating iron chelators from crude plant extracts. Formation of a ternary curcumin-iron-NTA complex allowed development of a solid phase binding assay used to characterize interactions between curcuminoids, iron, and competing molecules. Under our standard assay conditions curcumin bound iron-NTA-agarose with a stoichiometry of one and an apparent KD ~ 500 μM, consistent with previous reports of two curcumin molecules bound per free iron in solution, where the second curcumin bound with a KD ~100 μM (Baum and Ng 2004).
Selected metals inhibited curcumin binding to iron-NTA-agarose. This may be attributed to the metal occupying the iron binding site of curcumin, since iron was retained by the resin. Of the metals investigated, curcumin bound most strongly to copper and aluminum, two potentially toxic redoxactive metals. Binding was specific in that inhibition was not seen with comparable (100×) concentrations of calcium, zinc, magnesium, or manganese. The extent of inhibition allowed us to rank the affinities of curcumin binding to metals (tightest to weakest) as: iron >copper ~ aluminum >zinc ~ calcium ~ manganese ~ magnesium. Binding of iron or other toxic metals in the presence of more physiologically abundant metals argues for metal chelation as a biologically (and potentially therapeutically) relevant mechanism for curcuminoids.
Analyses of curcumin uptake by cells in culture provided more direct insight to iron binding by curcumin in a physiological setting. Curcumin uptake was significantly reduced by iron (added as iron chloride or FAC). This occurred at low (microM) concentrations of curcumin in the presence of apotransferrin and other potential iron binding molecules found in serum. Selected other metals also displayed this effect in a concentration-dependent way, allowing us to deduce a relative affinity of curcuminoids for metals in cell culture as iron >copper ~ aluminum > zinc > calcium ~ manganese ~ magnesium. The mechanism by which iron (or other metal) binding inhibits curcumin uptake to cells is unknown; it is possible that the curcumin-metal complex binds more tightly to carrier proteins in serum, so that the concentration of free curcumin available to enter cells is decreased. A similar effect in the GI tract may contribute to the low bioavailability of curcuminoids reported in humans (Garcea et al. 2004). A reduction in cell uptake of the curcumin-iron complex can explain previously published reports of reduced curcumin cytotoxicity in the presence of iron (Nakano et al. 2004; Ishihara and Sakagami 2005; Minear et al. 2011; Khalil et al. 2013).
We were initially concerned that the biological relevance of iron binding by curcumin may be reduced by other small iron binding molecules known to be present in cells and tissues. At high enough concentrations, even very weak chelators could minimize or negate any added effect of small amounts of curcumin. Citrate, for example, is an important physiological iron chelator and the major transporter of non-transferrin bound iron in blood (Grootveld, Bell et al. Grootveld et al. 1989). In iron-NTA-agarose binding experiments, we found that citrate partially inhibited curcumin binding at concentrations (0.2 mM) comparable to the 0.1 mM citrate reported in blood (Tomisek et al. 1975). However in cell culture, even 2 mM citrate did not eliminate the ability of 0.2 mM iron to block 20 μM curcumin uptake. In contrast, the pharmaceutical iron chelator deferoxamine restored curcumin uptake in iron-treated cultures (as did the non-specific metal chelator EDTA). No/low inhibition by physiological iron chelators or common physiologically abundant metals supports the proposal that iron binding by curcumin is physiologically relevant. These direct observations are consistent with previous estimates of the formation constant (log β110) for curcumin-iron of 22 (Bernabe-Pineda et al. 2004) that is comparable to other iron chelators known to be biologically active (Martell and Smith 1974; Jiao et al. 2006). Significant iron binding by curcumin is predicted given estimates of labile iron in cells of 5–10 μM (Petrat et al. 1999). Our demonstration of a multi-component iron complex (curcumin-iron-NTA) indicates curcumin may form ternary complexes with other iron binding small molecules (citrate) or proteins (transferrin, ferritin, IRP1 and IRP2) in cells and tissues. Disruption of normal iron homeostasis is predicted.
Curcuminoids are widely acknowledged to have poor bioavailability (Hatcher et al. 2008). Mechanistic effects of curcuminoids observed in vitro typically require concentrations near 10 μM, which is difficult to reconcile with pharmacokinetic studies of humans and animals fed curcumin that show levels in blood or tissues to be significantly less (Ireson et al. 2001; Garcea et al. 2004). However, serum levels of curcumin metabolites (e.g. curcumin glucuronide) can approach 10 μM, and many metabolites retain the ketoenol structure important for iron binding (Baum and Ng 2004; Ferrari et al. 2009). Thus extracellular iron chelation (by curcumin metabolites in blood) may be expected in humans or experimental animals given curcumin in their diet. Importantly, iron binding is most likely to occur when labile iron levels in blood are high, as has been reported for iron overload patients (Grootveld et al. 1989).
The curcumin inhibition binding assay may be generally applied to the task of screening crude extracts for the presence of novel iron chelators. High affinity chelators (desferoxamine or EDTA) extracted iron from iron-NTA-agarose, while weak chelators (citrate or silibinin) competed with curcumin for binding. These effects are consistent with the relative ferric iron affinities of these compounds: (strongest) desferoxamine >EDTA > NTA > citrate ~ silibinin (weakest) (Motekaitis and Martell 1994; Motekaitis et al. 1996; Borsari et al. 2001; Smith and Arthur 2003). As a screening assay, inhibition of curcumin binding would indicate the presence of iron chelator. Subsequent assay of the iron content of the resin would further distinguish low affinity (iron retained) from high affinity (iron removed) chelators. Additional information may be derived for low affinity chelators. Silibinin, for example, had an apparent IC50 in this assay of 7.9 mM (Fig. 6), greater than 300 μM required to saturate the iron-NTA resin, and roughly 800× fold higher than the 10 μM curcumin used in the assay. Clearly silibinin has a lower affinity for iron-NTA-agarose than curcumin. Nevertheless, we calculate that the iron chelating activity in a milk thistle (Silybum marianum) extract standardized to 20 mg/ml silibinin (equivalent to 40 mM silibinin) would be readily detected by this approach. Biological iron chelation has been reported in humans given silibinin-containing preparations (Bares et al. 2008). Compounds such as silibinin or curcumin that are isolated on iron-NTA-agarose may be easily eluted from the resin for identification and further study.
Acknowledgments
We gratefully acknowledge Cheryl Wong and Brian S. Kunakom, Bastyr University, for technical assistance and supporting experiments. We thank Dr. Muhammed Majeed and colleagues at Sabinsa Corporation for generously providing the purified curcuminoids (C3 complex®) and turmeric used in this study. Preliminary aspects of this work appeared in abstract form (Messner et al. FASEB J April 2015 29:773.7). Supported by the National Center for Complementary and Alternative Medicine (Grant AT3448 to DJM).
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Donald J. Messner, Bastyr University, 14500 Juanita Drive NE, Kenmore, WA 98028, USA
Christine Surrago, Bastyr University, 14500 Juanita Drive NE, Kenmore, WA 98028, USA.
Celia Fiordalisi, Bastyr University, 14500 Juanita Drive NE, Kenmore, WA 98028, USA.
Wing Yin Chung, Bastyr University, 14500 Juanita Drive NE, Kenmore, WA 98028, USA.
Kris V. Kowdley, Swedish Medical Center, Seattle, WA, USA
References
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57(1):1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Badria FA, Ibrahim AS, et al. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE. 2015;10(7):e0134156. doi: 10.1371/journal.pone.0134156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bares JM, Berger J, et al. Silybin treatment is associated with reduction in serum ferritin in patients with chronic hepatitis C. J Clin Gastroenterol. 2008;42(8):937–944. doi: 10.1097/MCG.0b013e31815cff36. [DOI] [PubMed] [Google Scholar]
- Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004;6(4):367–377. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- Bernabe-Pineda M, Ramirez-Silva MT, et al. Spectrophotometric and electrochemical determination of the formation constants of the complexes curcumin-Fe(III)-water and Curcumin-Fe(II)-water. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60(5):1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- Borsari M, Gabbi C, et al. Silybin, a new iron-chelating agent. J Inorg Biochem. 2001;85(2–3):123–129. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Chin D, Huebbe P, et al. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2014;2:563–569. doi: 10.1016/j.redox.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E, Arezzini B, et al. Synthesis and characterization of glucosyl-curcuminoids as Fe3 + suppliers in the treatment of iron deficiency. Biometals. 2009;22(5):701–710. doi: 10.1007/s10534-009-9213-8. [DOI] [PubMed] [Google Scholar]
- Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180(1):23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- Garcea G, Jones DJ, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90(5):1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootveld M, Bell JD, et al. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989;264(8):4417–4422. [PubMed] [Google Scholar]
- Guo S, Frazer DM, et al. Iron homeostasis: transport, metabolism, and regulation. Curr Opin Clin Nutr Metab Care. 2016;19(4):276–281. doi: 10.1097/MCO.0000000000000285. [DOI] [PubMed] [Google Scholar]
- Hatcher H, Planalp R, et al. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireson C, Orr S, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61(3):1058–1064. [PubMed] [Google Scholar]
- Ishihara M, Sakagami H. Re-evaluation of cytotoxicity and iron chelation activity of three beta-diketones by semiempirical molecular orbital method. Vivo. 2005;19(1):119–123. [PubMed] [Google Scholar]
- Jiao Y, Wilkinson JT, et al. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40(7):1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wilkinson JT, et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood. 2009;113(2):462–469. doi: 10.1182/blood-2008-05-155952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283(2–3):65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- Khalil MI, Al-Zahem AM, et al. Synthesis, Characterization, Mossbauer Parameters, and Antitumor Activity of Fe(III) Curcumin Complex. Bioinorg Chem Appl. 2013;2013:982423. doi: 10.1155/2013/982423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnumakkara AB, Bordoloi D, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. 2016;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AE, Smith RM. Critical stability constants. Plenum Press; New York: 1974. [Google Scholar]
- Messner DJ, Kowdley KV. Neoplastic transformation of rat liver epithelial cells is enhanced by non-transferrin-bound iron. BMC Gastroenterol. 2008;8:2. doi: 10.1186/1471-230X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner DJ, Sivam G, et al. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009;29(1):63–72. doi: 10.1111/j.1478-3231.2008.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner DJ, Robinson T, et al. Curcumin and turmeric modulate the tumor-promoting effects of iron in vitro. Nutr Cancer. 2017;69(3):481–489. doi: 10.1080/01635581.2017.1274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear S, O’Donnell AF, et al. Curcumin inhibits growth of Saccharomyces cerevisiae through iron chelation. Eukaryot Cell. 2011;10(11):1574–1581. doi: 10.1128/EC.05163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobarra N, Shanaki M, et al. A review on iron chelators in treatment of iron overload syndromes. Int J Hematol Oncol Stem Cell Res. 2016;10(4):239–247. [PMC free article] [PubMed] [Google Scholar]
- Motekaitis RJ, Martell AE. The iron(III) and iron(II) complexes of nitrilotriacetic acid. J Coord Chem. 1994;31(1):67–78. [Google Scholar]
- Motekaitis RJ, Rogers BE, et al. Stability and structure of activated macrocycles. ligands with biological applications. Inorg Chem. 1996;35(13):3821–3827. doi: 10.1021/ic960067g. [DOI] [PubMed] [Google Scholar]
- Nakano K, Nakayachi T, et al. Induction of apoptosis by beta-diketones in human tumor cells. Anticancer Res. 2004;24(2B):711–717. [PubMed] [Google Scholar]
- Petrat F, Rauen U, et al. Determination of the chelatable iron pool of isolated rat hepatocytes by digital fluorescence microscopy using the fluorescent probe, phen green SK. Hepatology. 1999;29(4):1171–1179. doi: 10.1002/hep.510290435. [DOI] [PubMed] [Google Scholar]
- Pietrangelo A. Iron and the liver. Liver Int. 2016;36(Suppl 1):116–123. doi: 10.1111/liv.13020. [DOI] [PubMed] [Google Scholar]
- Smith RMAM, Arthur E. NIST critically selected stability constants of metal complexes database. N. I. o. S. a. Technology; Gaithersburg: 2003. [Google Scholar]
- Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46(12):1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Swierenga SH, Whitfield JF, et al. Regulation of proliferation of normal and neoplastic rat liver cells by calcium and cyclic AMP. Ann N Y Acad Sci. 1980;349:294–311. doi: 10.1111/j.1749-6632.1980.tb29534.x. [DOI] [PubMed] [Google Scholar]
- Tomisek AJ, Winkler EM, et al. Fluorometry of citrate in serum, with use of citrate (pro-3S)-lyase. Clin Chem. 1975;21(6):730–734. [PubMed] [Google Scholar]
- World Health Organization. WHO monographs on selected medicinal plants. World Health Organization; Geneva: 1999. [Google Scholar]