Abstract
Objectives
To characterize patient profile and hemodynamic profile of those undergoing intra-aortic balloon pump (IABP) for cardiogenic shock and define predictors of hemodynamic failure of IABP support.
Background
Clinical characteristics of IABP support in cardiogenic shock not related to acute myocardial infarction (AMI) remain poorly characterized.
Methods
We retrospectively studied a cohort of 74 patients from 2010–2015 who underwent IABP insertion for cardiogenic shock complicating acute decompensated heart failure not due to AMI.
Results
In the overall cohort, which consisted primarily of patients with chronic systolic heart failure (89%), IABP significantly augmented cardiac index and lowered systemic vascular resistance (P<0.05). Despite this improvement, 28% of these patients died (24%) or require urgent escalation in mechanical circulatory support (MCS) (4%). Multivariable regression revealed that baseline left ventricular cardiac power index (LVCPI), a measure of LV power output derived from cardiac index and mean arterial pressure (P=0.01), and history of ischemic cardiomyopathy (P=0.003) were significantly associated with the composite adverse-event endpoint of death or urgent MCS escalation. An IABP Failure risk score using baseline LVCPI<0.28 W/m2 and ischemic history predicted 28-day adverse events with excellent discrimination.
Conclusion
Despite hemodynamic improvements with IABP support, patients with non-AMI cardiogenic shock still suffer poor outcomes. Patients with ischemic cardiomyopathy and low LVPCI fared significantly worse. These patients may warrant closer observation or earlier consideration of more advanced hemodynamic support.
Keywords: Intra-aortic balloon pump, cardiogenic shock, mechanical circulatory support, cardiac power, ischemic cardiomyopathy
INTRODUCTION
Cardiogenic shock complicating acute decompensated heart failure (ADHF) is a rapidly growing clinical problem.1 In addition to diuresis and inotropic support, percutaneous support devices such as intra-aortic balloon pump (IABP) and more advanced temporary mechanical circulatory support (MCS) devices such as Impella (Abiomed, Danvers, MA), veno-arterial extracorporeal membrane oxygenation (ECMO), or Tandem-Heart (TamdemLife, Pittsburgh, PA) have become an integral part of the treatment paradigm. When needed, these therapies have become critical for patients with refractory cardiogenic shock as a bridge to recovery, implantation of more durable MCS devices such as left ventricular assist device (LVAD), or orthotopic heart transplant (OHT).1
Among percutaneous devices, IABP is often considered early in the treatment of cardiogenic shock because of its relatively low cost, ease of implantation, and low complication rate.2 Clinically, IABP support provides adequate short-term support. However, over time it often proves insufficient, leading to need for escalation to more robust temporary MCS devices. Recent advances in the latter have led to their growing utilization in cardiogenic shock over the past few years.1 Furthermore, studies have demonstrated the hemodynamic superiority of Impella over IABP in the treatment of cardiogenic shock due to acute myocardial infarction (AMI),3 while other studies have shown earlier implementation of percutaneous MCS helps to improve patient outcomes in the treatment of cardiogenic shock.4 Thus, IABP support of cardiogenic shock, in particular when the patient condition calls for a more robust form of MCS, may lead to lost time and worsened patient outcomes.
Although IABPs have been well studied in cardiogenic shock complicating AMI,5,6 their hemodynamic effects in treating cardiogenic shock complicating non-AMI ADHF are less well understood. Furthermore, predictors of failure of IABP support are unknown in this population. Thus, given the potential opportunity cost of inadequate IABP support, understanding such predictors may help identify patients at risk of IABP support failure or improve up-front selection of temporary MCS devices over IABP support. Thus, the present study sought to characterize the hemodynamic changes following IABP implantation in cardiogenic shock complicating non-AMI acute decompensated heart failure, and find characteristics at the time of IABP implantation that predict worsened in-hospital outcomes and failure of IABP support.
METHODS
Patient Population and Outcomes
A retrospective analysis was performed of all consecutive adult patients (age ≥ 18 years) hospitalized at a single tertiary-care hospital between July 2010 and June 2015 that underwent placement of an intra-aortic balloon pump. The study was approved by the institutional review board of the Johns Hopkins Medical Institution.
The target study population comprised patients with known or newly diagnosed cardiomyopathy who required IABP for treatment of cardiogenic shock. Patients were excluded if they underwent IABP placement for AMI. Patients were also excluded if IABP was placed for refractory coronary ischemia, cardiac arrest, peri-procedural support (including post-cardiotomy), severe valvular disease, and acute stress cardiomyopathy. Patients were included if they had a known or newly diagnosed cardiomyopathy and underwent IABP placement for treatment of acute cardiogenic shock, which was defined as a systolic blood pressure < 90 mmHg for at least 30 minutes with evidence of poor end-organ perfusion or need for inotropic support. All included subjects had a pulmonary artery catheter placed prior to or at the time of IABP insertion for hemodynamic monitoring.
Baseline clinical, laboratory, and echocardiographic characteristics were recorded. Echocardiographic data were obtained from admission echocardiogram or echocardiogram obtained just prior to hospital admission. Hemodynamic parameters and mixed venous oxygen saturations from the pulmonary artery catheter were recorded from a baseline time point (defined as prior to IABP insertion) and at 12-hour intervals after IABP insertion up until 48 hours post IABP. Left ventricular cardiac power index (LVCPI), defined by the product of flow and the mean arterial pressure (MAP) against which the left ventricle contracts, is a variable that has been shown to predict mortality in AMI cardiogenic shock;7 we thus investigated this variable as well. LVCPI was calculated as CI × MAP divided by 451, whereas right ventricular CPI (RVCPI) was calculated as CI × mean pulmonary arterial pressure (mPAP) divided by 451. Units for both were expressed as W/m2.
Adverse events were defined as a composite of death despite IABP support, or need for emergent MCS escalation for refractory cardiogenic shock (defined as need for Impella, ECMO, or emergent LVAD for continued decompensation despite IABP support). Favorable clinical outcomes were defined as survival to hospital discharge or successful bridge to durable LVAD or OHT.
Statistical Analysis
Comparison of continuous variables between patient groups was done using Student’s T-test, or rank sum tests when necessary. For categorical variables, either Chi square tests or Fisher’s exact test, when necessary, was used. Temporal trends in hemodynamic variables were assessed using repeated-measures analysis of variance (ANOVA). To determine predictors of adverse clinical events, univariate logistic regression was performed of all baseline variables (all variables in Tables 1 and ). All variables reaching a significance of P<0.2 with univariate analysis were identified as candidate variables. Candidate variables were eliminated from model building if collinear, which was defined by a variance inflation factor greater than 2. History of coronary artery bypass surgery was collinear with history of ischemic cardiomyopathy, while measures of RV cardiac power, cardiac output and index, and mean arterial blood pressure were collinear with LVCPI by nature of the mathematical calculation of LVCPI.
Table 1.
Baseline Demographic and Clinical Characteristics
| Total Cohort n = 74 |
Event-Free n = 53 |
Adverse Outcome n = 21 |
P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 54.8(14.1) | 54.1(13.8) | 56.5(14.9) | 0.52 |
| Race | ||||
| Caucasian (n,%) | 39 (53%) | 27 (51%) | 12 (57%) | 0.63 |
| African-American (n,%) | 30 (41%) | 23 (43%) | 7 (33%) | 0.43 |
| Other (n,%) | 5 (7%) | 3 (6%) | 2 (10%) | 0.62 |
| Sex | ||||
| Male (n,%) | 49 (66%) | 34 (64%) | 15 (71%) | 0.55 |
| Female (n,%) | 25 (34%) | 19 (36%) | 6 (29%) | 0.55 |
| BSA (m2) | 2.06 (1.99) | 2.07 (1.99) | 2.04 (1.90) | 0.74 |
| Comorbidities | ||||
| Clinical Presentation | ||||
| Chronic heart failure (n,%) | 66 (89%) | 49 (92%) | 17 (81%) | 0.21 |
| New heart failure (n,%) | 8 (11%) | 4 (8%) | 4 (19%) | 0.21 |
| Cardiomyopathy Type ICM (n, %) | 19 (26%) | 8 (15%) | 11 (52%) | 0.001 |
| Hypertension (n,%) | 29 (39%) | 23 (43%) | 6 (29%) | 0.24 |
| Diabetes Mellitus (n,%) | 32 (43%) | 23 (43%) | 9 (43%) | 0.97 |
| Dyslipidemia (n,%) | 26 (35%) | 20 (38%) | 6 (29%) | 0.46 |
| CAD (n,%) | 21 (28%) | 12 (23%) | 9 (43%) | 0.08 |
| Prior MI (n,%) | 19 (26%) | 9 (17%) | 10 (48%) | 0.007 |
| History of CAB (n,%) | 10 (14%) | 3 (6%) | 7 (33%) | 0.004 |
| History of VT (n,%) | 18 (24%) | 12 (23%) | 6 (29%) | 0.59 |
| Tobacco Use | ||||
| Former (n,%) | 28 (38%) | 23 (43%) | 5 (24%) | 0.18 |
| Active (n,%) | 7 (10%) | 4 (8%) | 3 (14%) | 0.40 |
| History of Stroke (n,%) | 6 (8%) | 5 (10%) | 1 (5%) | 0.67 |
| History of AF/AFL (n,%) | 28 (38%) | 18 (34%) | 10 (48%) | 0.28 |
| CKD (n,%) | 34 (46%) | 26 (49%) | 8 (38%) | 0.39 |
| Known COPD (n,%) | 4 (5%) | 3 (6%) | 1 (5%) | 1.00 |
| Home Medications | ||||
| Home Inotrope (n,%) | 10 (14%) | 9 (17%) | 1 (5%) | 0.23 |
| Beta-blocker (n,%) | 39 (53%) | 28 (53%) | 11 (52%) | 0.97 |
| ACEI/ARB (n,%) | 42 (57%) | 35 (66%) | 7 (33%) | 0.01 |
| Diuretic (n,%) | 59 (80%) | 44 (83%) | 15 (71%) | 0.26 |
| MRA (n,%) | 31 (42%) | 26 (49%) | 5 (24%) | 0.07 |
| Hydralazine ISDN (n,%) | 11 (15%) | 5 (10%) | 6 (29%) | 0.07 |
For continuous variables, Student’s T-test or Mann-Whitney Rank Sum Test where appropriate For categorical variables, Chi square test or Fisher’s exact test where appropriate. BSA, body surface area; NICM, non-ischemic cardiomyopathy; ICM, ischemic cardiomyopathy; CAD, coronary artery disease; MI, myocardial infarction; CAB, coronary artery bypass; VT, ventricular tachycardia; AF/AFL, atrial fibrillation and atrial flutter; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; MRA, mineralocorticoid receptor antagonist; ISDN, isosorbide dinitrate.
The final multivariable model was generated by stepwise regression using backward elimination of candidate variables, with a P-value of 0.05 required for final inclusion; it was confirmed using forward selection and the same P-value cutoff as well. Because of the known influence of age and sex on left ventricular (LV) cardiac power,7 although age and sex did not meet the univariate P-value cutoff, they were forced into the final model. Survival free of adverse events for the first 28 days was studied using Cox proportional hazards regression and Kaplan-Meier survival function analysis. An IABP failure risk score predicting 28-day adverse events was generated from the significant predictors from the aforementioned regressions; significance was determined by log rank testing. Statistical analyses were performed using Stata 11.0 (StataCorp, College Station, Texas).
RESULTS
Cohort Characteristics
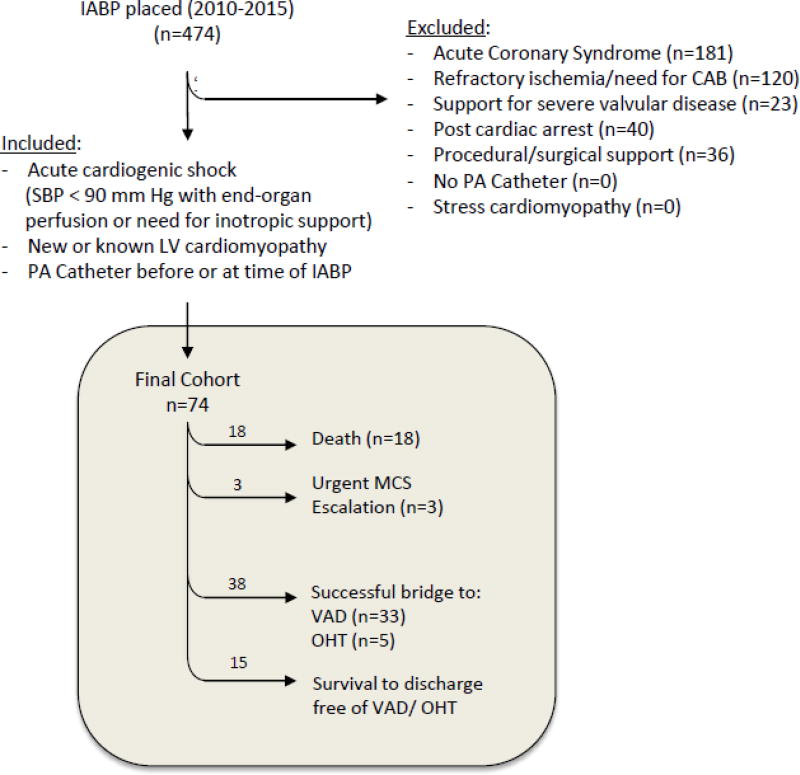
Out of the 474 patients who underwent IABP placement at Johns Hopkins Hospital between 2010–2015, 400 patients were excluded, yielding 74 patients in the final cohort. (Figure 1). These patients all had known or newly diagnosed cardiomyopathy and underwent IABP placement for the treatment of cardiogenic shock complicating non-AMI heart failure. The vast majority of these patients had cardiogenic shock complicating a known cardiomyopathy (89%). Of the 74 patients, 15 patients (20%) survived to hospital discharge after successful IABP wean, while 38 patients (51%) underwent successful bridge to a durable LVAD (n=33) or OHT (n=5). Conversely, 21 patients (28% of this cohort) suffered an adverse event: 18 died with IABP support prior to any possible intervention, while 3 required urgent escalation of MCS (ECMO, n=1; emergent LVAD, n=2) (Figure 1).
Figure 1. Flow Diagram of Retrospective Patient Review.
Over 5 years, 474 intra-aortic balloon pumps (IABP) were placed, of which 74 were placed for non-AMI cardiogenic shock. Of these 74, 18 died despite IABP support while 3 required urgent escalation to an advanced MCS. Of those who survived, 15 did so free of advanced therapies, 25 required LVAD, and 4 required OHT. CAB, coronary artery bypass; SBP, systolic blood pressure; PA, pulmonary artery.
Those who suffered an adverse event—death while on IABP support or urgent MCS—had similar baseline demographics and co-morbidities as those who did not, with the exception that the former group had a significantly higher prevalence of ischemic cardiomyopathy (52% versus 15%, P=0.001). This was concordant with a greater proportion of patients with a history of prior AMI and coronary artery bypass graft surgery in this group (Table 1). The final cohort was comprised of 26% ischemic cardiomyopathy (ICM) and 74% non-ischemic cardiomyopathy (NICM). Among those with NICM, approximately 50% were deemed idiopathic, while the other 50% comprised a range of causes including familial, autoimmune, and infectious. In the adverse event group, medication use was overall similar, except for less use of angiotensin converting enzyme inhibition and angiotensin receptor blockade (Table 1).
Echocardiographically, patients with adverse events had less ventricular dilation (6.1±1.3 versus 6.8±1.3 cm, P=0.03) and trended towards more right ventricular dysfunction (Table 2). From a hemodynamic perspective, those suffering adverse events had significantly lower cardiac index (CI, 1.44±0.41 vs. 1.86±0.62 L/min/m2, P=0.007), higher systemic vascular resistance (SVR, 1578±641 vs. 1246±555 dynes·s/cm5), and lower LV stroke work indices (LVSWI, 8.0±3.2 versus 11.6±6.4 gm·m/m2) at time of IABP implantation. The adverse event group had significantly lower left and right ventricular cardiac power indices (0.22±0.07 W/m2 versus 0.29±0.11 W/m2, P=0.009, and 0.12±0.05 versus 0.16±0.05 W/m2, P=0.007, respectively) (Table 2).
Table 2.
Baseline Echocardiographic, Laboratory, and Hemodynamic Characteristics
| Total n = 74 |
Event-Free n = 53 |
Adverse Event n = 21 |
P-value | |
|---|---|---|---|---|
| Admission Echo | ||||
| LV Ejection Fraction (%) | 14.6 (6.0) | 14.7 (6.3) | 14.5 (5.6) | 0.93 |
| LVEDd (cm) | 6.6 (1.4) | 6.8 (1.3) | 6.1 (1.3) | 0.03 |
| MR, ≥ moderate (n,%) | 33 (45%) | 23 (43%) | 10 (48%) | 0.74 |
| TR, ≥ moderate (n,%) | 28 (38%) | 23 (43%) | 5 (24%) | 0.18 |
| RV dilation, ≥ moderate (n,%) | 9 (12%) | 7 (13%) | 2 (9%) | 1.00 |
| RV dysfunction, ≥ moderate (n,%) | 34 (46%) | 21 (40%) | 13 (62%) | 0.12 |
| Laboratory Data | ||||
| Troponin I (ng/mL) | 1.2 (2.9) | 0.9 (2.6) | 1.9 (3.4) | 0.18 |
| Creatinine (mg/dL) | 2.1 (1.3) | 2.1 (1.3) | 2.2 (1.2) | 0.77 |
| Lactate (mmol/L) | 3.2 (3.8) | 2.8 (3.3) | 4.2 (4.9) | 0.16 |
| PA Oxygen Saturation (%) | 51.0 (14.8) | 51.2 (14.6) | 50.4 (15.5) | 0.83 |
| Pro-BNP (pg/mL) | 9849 (7332) | 8847 (6793) | 12253 (8238) | 0.13 |
| VS/Hemodynamic Data | ||||
| Systemic Oxygen Saturation (%) | 96.4 (3.9) | 96.7 (3.2) | 95.9 (5.2) | 0.42 |
| Intubated (n,%) | 7 (10%) | 2 (4%) | 5 (24%) | 0.02 |
| On Inotrope (n,%) | 65 (88%) | 48 (91%) | 17 (81%) | 0.26 |
| Heart Rate (min−1) | 102 (20) | 99 (21) | 110 (17) | 0.04 |
| Systolic BP (mm Hg) | 93 (13) | 93 (13) | 92 (12) | 0.78 |
| Diastolic BP (mm Hg) | 60 (10) | 61 (10) | 59 (10) | 0.39 |
| Mean Arterial Pressure (mm Hg) | 72 (10) | 72 (10) | 71 (9) | 0.79 |
| Right Atrial Pressure (mm Hg) | 18 (7) | 18 (7) | 18 (7) | 0.93 |
| PA Systolic Pressure (mm Hg) | 53 (13) | 53 (11) | 51 (16) | 0.70 |
| Mean PA Pressure (mm Hg) | 38 (10) | 38 (8) | 38 (12) | 0.82 |
| PA Diastolic Pressure (mm Hg) | 29 (8) | 29 (8) | 29 (8) | 0.75 |
| PCWP (mm Hg) | 28 (8) | 29 (7) | 27 (9) | 0.34 |
| Cardiac Index (L/min/m2) | 1.74 (0.59) | 1.86 (0.62) | 1.44 (0.41) | 0.007 |
| SVR (dyn·s·cm−5) | 1342 (596) | 1246 (555) | 1578 (641) | 0.03 |
| PVR (Wood units) | 3.2 (2.5) | 2.8 (1.8) | 4.2 (3.6) | 0.14 |
| Stroke Volume Index (ml/m2/beat) | 17.8 (7.8) | 19.5 (8.1) | 13.5 (4.7) | 0.003 |
| LV Stroke Work Index (g/m2/beat) | 10.6 (5.8) | 11.6 (6.4) | 8.0 (3.2) | 0.016 |
| RV Stroke Work Index (g/m2/beat) | 4.8 (3.1) | 5.3 (3.2) | 3.6 (2.5) | 0.05 |
| LV Cardiac Power Index (W/m2) | 0.28 (0.10) | 0.29 (0.11) | 0.22 (0.07) | 0.009 |
| RV Cardiac Power Index (W/m2) | 0.14 (0.05) | 0.16 (0.05) | 0.12 (0.05) | 0.007 |
For continuous variables, Student’s T-test was used to compare, or Mann--Whitney Rank Sum Test where appropriate. For categorical variables, Chi square test was used to compare, or Fisher’s exact test where appropriate. LVEDd, LV End-Diastolic dimension; MR, mitral regurgitation; TR, tricuspid regurgitation; PA, pulmonary artery; Pro-BNP, pro-brain natriuretic peptide; BP, blood pressure; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance.
Hemodynamic Trends with IABP Support
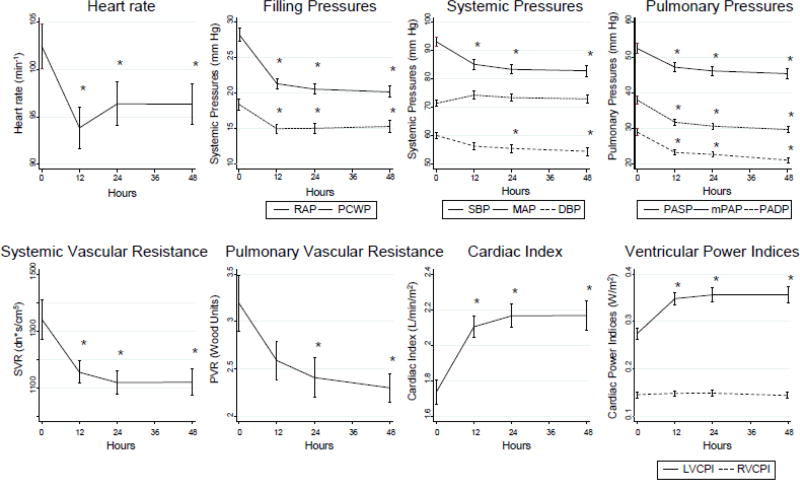
Hemodynamic parameters in the overall cohort were measured at baseline and at 12-hour increments for the first 48 hours after IABP placement (Figure 2). By 12 hours, there was a significant decrease in heart rate and systemic vascular resistance and significant increase in cardiac index (P<0.05). There was also a slight but significant decrease in systemic systolic and un-augmented diastolic blood pressures (P<0.05); as expected with IABP support, this was accompanied by a slight trend towards increased augmented MAP. In concordance, there was a significant improvement in both right and left-sided filling pressures and pulmonary pressures (P<0.05). When trended over time, there was also a significant, early, and sustained improvement in LVCPI (given the significant improvement in CI and preserved MAP) but no significant change in RVCPI (since the rise in CI was countered by a fall in mPAP) (P<0.05) (Figure 2).
Figure 2. Overall Hemodynamic Trends.
Hemodynamic parameters were obtained just prior to IABP insertion (0 hours) as well as 12, 24, and 48 hours after insertion. Mean ± standard deviation at each time point is noted; time points were compared by one-way repeated measures analysis of variance. * significant difference versus 0 hour time point (P < 0.05). After IABP insertion, there were significant decreases in heart rate, ventricular filling pressures, systemic and pulmonary pressures, and systemic and pulmonary vascular resistance. Cardiac index improved, as did left ventricular cardiac power index, but not right ventricular cardiac power index. RAP, right atrial pressure; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; MAP, mean arterial pressure; DBP, diastolic blood pressure; PASP, pulmonary artery systolic pressure; mPAP, mean pulmonary artery pressure; PADP, pulmonary artery diastolic pressure; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; LVCPI, left ventricular cardiac power index; RVCPI, right ventricular cardiac power index.
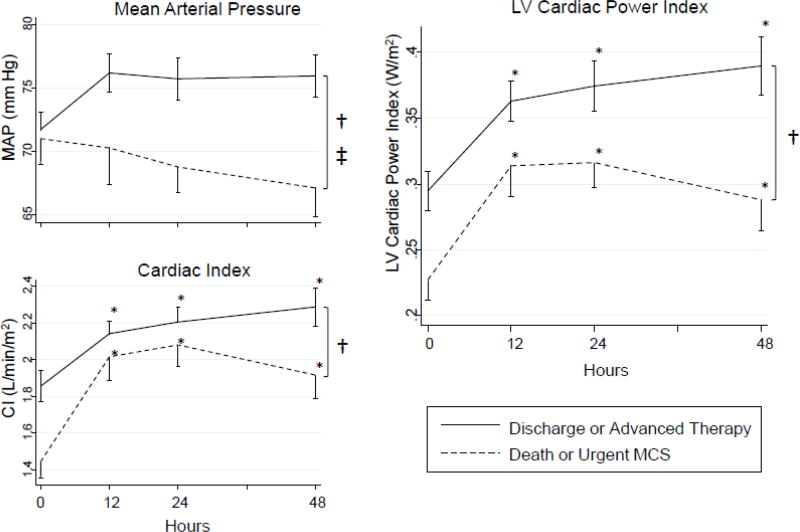
Despite hemodynamic improvement, 28% of this cohort suffered an adverse event. Separating the cohort into those who did and did not develop adverse events revealed several important differences in hemodynamic trends. There was a significant difference in MAP over time between those who suffered adverse in-hospital events and those who were free of adverse events (i.e. successful bridging to LVAD/OHT or successful hospital discharge free of advanced therapies) (Figure 3). Additionally, although both groups saw a significant early increase in CI, the event-free group sustained this improvement whereas the adverse event group saw a decline in CI between 24–48 hours; as a result, CI was also significantly different between both groups (Figure 3). LVCPI, which is the product of MAP and CI, was thus significantly different between groups across the 48-hour time period (Figure 3). This mirrored the significant difference seen between groups just prior to IABP implantation.
Figure 3. LVCPI Trend by Adverse Outcome Status.
Mean ± standard deviation at each time point is noted; time points were compared by two-way repeated measures analysis of variance. * significant difference versus 0 hour time point (P < 0.001); † significant difference between groups (P < 0.0001); ‡ significant effect of time/interaction term (P < 0.05). Those who suffered an adverse outcome (death or urgent MCS) had significantly lower mean arterial pressure (MAP) and cardiac index (CI) despite IABP insertion when compared to those who survived to hospital discharge or advanced therapies (LVAD/OHT). As a result, LVCPI, which is calculated from the product of MAP and CI, was significantly lower in those who suffered an adverse outcome than in those who survived to hospital discharge or advanced therapies.
Predictors of Adverse Events Despite IABP Support
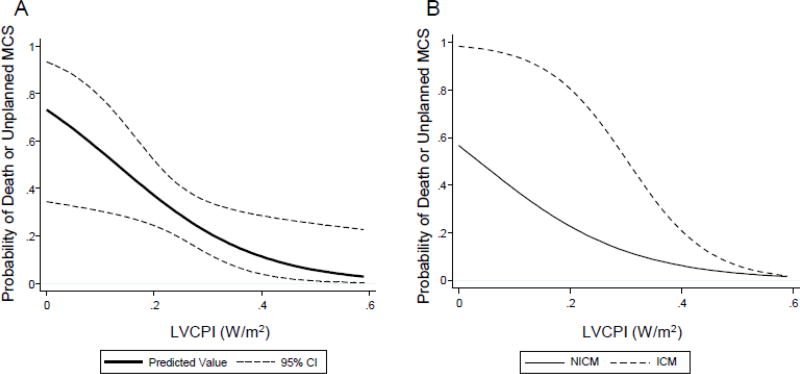
We next sought to identify predictors of failure of IABP support from among baseline clinical and hemodynamic variables. Univariable regression with a threshold of P<0.2 revealed several baseline variables predictive of adverse in-hospital events (Table 3). Testing for collinearity eliminated several variables from inclusion in multivariable modeling (Table 3). The final multivariable regression model revealed that history of ischemic cardiomyopathy (ICM) and low baseline LVCPI were the most powerful predictors of adverse in-hospital events. These remained significant even with inclusion of age and sex in the model, included because of their known effects on cardiac power. In the final multivariable model (Table 4), for every 0.10 W/m2 improvement in LVCPI, there was a 0.37 (95% 0.18, 0.79) decrease in adverse events (P=0.01). Similarly, a history of ICM conferred a 10.4 (95% 2.2, 48.7) risk of adverse event (P=0.003). Several comorbidities would complicate outcomes or candidacy for advanced therapies, such as history of hypertension, diabetes, chronic kidney disease, history of stroke, chronic obstructive pulmonary disease, and peripheral artery disease. These variables themselves were not significant enough to influence outcomes in our cohort, but by influencing candidacy for advanced therapy, they may have influenced the predictive power of ischemic heart disease. We tested this by regressing ischemic CM and adjusting for these variables specifically (Supplemental Table 1), but still found that ischemic CM was predictive of adverse outcomes in spite of these comorbidities. Hazard plots illustrate the probability of adverse events as a function of LVCPI in Figure 4. Decrease in LVCPI at time of implantation had a steady and linear effect on increasing probability of adverse events (Figure 4A). Additionally, history of ICM magnified this probability over much of the LVCPI range (Figure 4B).
Table 3.
Univariable Logistic Regression for In-hospital Adverse Event
| Variable | OR [95% CI] | P-value |
|---|---|---|
| History of CAD* | 2.56 [0.87, 7.52] | 0.087 |
| Prior MI* | 4.44 [1.45, 13.58] | 0.009 |
| Prior CABG* | 8.33 [1.9, 36.49] | 0.005 |
| Prior PCI* | 2.25 [0.67, 7.53] | 0.189 |
| Ischemic Cardiomyopathy | 6.19 [1.98, 19.34] | 0.002 |
| Aspirin* | 2.20 [0.79, 6.15] | 0.133 |
| Hydralazine-ISDN | 3.84 [1.02, 14.39] | 0.046 |
| Mineralocorticoid Receptor Antagonist | 0.03 [0.01, 1.01] | 0.053 |
| ACEI/ARB* | 0.43 [0.16, 1.15] | 0.094 |
| RV systolic dysfunction (≥ moderate) | 2.47 [0.88, 6.99] | 0.087 |
| Heart rate (min−1)† | 1.03 [1.00, 1.05] | 0.048 |
| Cardiac Output (L/min)† | 0.51 [0.30, 0.86] | 0.012 |
| SVR (dyn·s·cm−5) | 1.00 [1.00, 1.001] | 0.044 |
| PVR (Wood Units) | 1.24 [0.99, 1.54] | 0.055 |
| Stroke volume index (ml/m2/beat)† | 0.86 [0.78, 0.96] | 0.005 |
| LVSWI (g/m2/beat)† | 0.87 [0.76, 0.98] | 0.024 |
| RVSWI (g/m2/beat)† | 0.81 [0.66, 0.99] | 0.036 |
| RVCPI (per 0.10 W/m2)† | 0.23 [0.07, 0.70] | 0.010 |
| LVCPI (per 0.10 W/m2) | 0.44 [0.23, 0.84] | 0.013 |
Candidate variables from univariable logistic regression (P<0.2). CAD, coronary artery disease; MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; ISDN, isosorbide dinitrate; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; RV, right ventricular; SVR, systemic vascular resistance; PVR, pulmonary vascular resistance; LVSWI, LV stroke work index; RVSWI, RV stroke work index; RVCPI, RV cardiac power index; LVCPI, LV cardiac power index; OR, Odds ratio; W, Watts.
collinear with ischemic cardiomyopathy,
collinear with LVCPI.
Table 4.
Multivariable Logistic Regression for In-hospital Adverse Event
| Variable | OR [95% CI] | P-value |
|---|---|---|
| LVCPI (per 0.10 W/m2) | 0.37 [0.18, 0.79] | 0.010 |
| Ischemic CM | 10.4 [2.2, 48.7] | 0.003 |
| Age | 0.99 [0.95, 1.04] | 0.766 |
| Female Sex | 1.3 [0.3, 5.2] | 0.699 |
LVCPI and history of ischemic CM remained predictors of in--hospital adverse event after adjustment using multivariable logistic regression. Age and sex were kept in the model given their known effects on cardiac power. OR, Odds ratio; LVCPI, left ventricular cardiac power index; W, Watts; CM, cardiomyopathy.
Figure 4. Probability of Adverse Events based on LVCPI.
(A) Hazard function was plotted of probability of adverse event (with 95% confidence interval) versus left ventricular cardiac power index (LVCPI). Adverse events were inversely related to LVCPI at time of IABP insertion. (B) Hazard function plot stratified by history of ischemic cardiomyopathy (ICM) versus non-ischemic cardiomyopathy (NICM). ICM history led to significantly higher probability of adverse events over a wide range of LVCPI when compared to NICM.
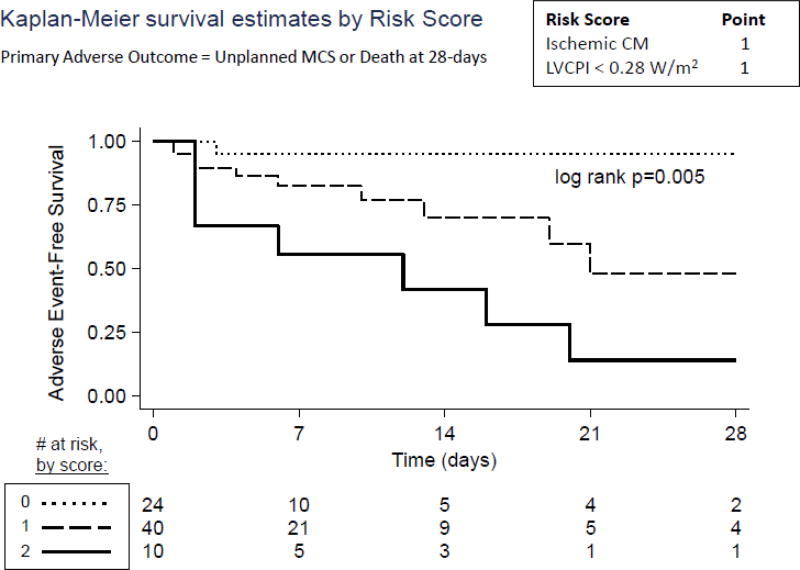
Given these findings, we next assessed the ability of LVCPI and ischemic history to predict 28-day survival free of adverse events. Using Cox proportional hazards regression, we found that LVCPI strongly predicted survival free of adverse events over the 28-days post IABP implantation (Hazard ratio 0.36 for every 0.1 W/m2 increase in LVCPI, 95%-CI 0.18, 0.71, P=0.003); meanwhile, ICM was a significant predictor of adverse events as well (Hazard Ratio 3.3, 95%-CI 1.0, 10.7, P=0.046; Table 5). LVCPI and ICM history were next incorporated into a simple scoring system to predict adverse events following IABP support, with 1 point designated to a history of ICM and 1 point designated to an LVCPI < 0.28 W/m2 (for a possible total of 2 points). These two variables were chosen because they were the only variables predictive of adverse outcomes from Tables 3 and 4. The LVCPI cut point of 0.28 W/m2 was chosen because it was the median baseline LVCPI in our cohort. As shown in Figure 5, this simple IABP failure risk score strongly predicted 28-day adverse event-free survival in our cohort (P=0.005). A score of 0 predicted over 95% adverse event-free survival at 28 days while a score of 2 predicted a steep drop in event-free survival within the first week (to approximately 50% by one week) and less than 20% survival at 4 weeks.
Table 5.
Cox Proportional Hazards Regression for 28-day Adverse Event
| Variable | HR [95% CI] | P-value |
|---|---|---|
| LVCPI (per 0.10 W/m2) | 0.36 [0.18, 0.71] | 0.003 |
| Ischemic CM | 3.3 [1.0, 10.7] | 0.046 |
| Age | 0.99 [0.96, 1.03] | 0.74 |
| Female Sex | 1.8 [0.6, 5.8] | 0.32 |
LVCPI and history of ischemic CM predicted survival free of adverse events at 28 days, after adjustment using Cox proportional hazards regression. Age and sex were left in the model given their known effects on cardiac power. HR, Hazard ratio; LVCPI, left ventricular cardiac power index; W, Watts; CM, cardiomyopathy.
Figure 5. Adverse Events as Predicted by IABP Failure Risk Score.
An IABP Failure Risk Score was generated for each patient at time of IABP insertion, with 1 point for a history of ischemic cardiomyopathy and 1 point for LVCPI<0.28 W/m2 (score range from 0 to 2 points). A score of 0 powerfully predicted success of IABP support, while a score of 2 predicted poor outcomes with IABP support (log rank P=0.005).
DISCUSSION
IABP counter-pulsation remains poorly studied in the setting of cardiogenic shock in the non-AMI setting. The current study identifies a cohort of non-AMI patients requiring IABP for cardiogenic shock and finds that despite initial hemodynamic improvement, nearly 30% of patients failed IABP support and went on to die or require urgent escalation in MCS. Low LVCPI (< 0.28 W/m2) at time of IABP implantation and history of ischemic cardiomyopathy proved to be the most powerful predictors of IABP failure. Moreover, a simple risk score incorporating these two variables powerfully predicted 28-day event-free survival. Given the ongoing advances in more robust forms of temporary MCS and the cost of using IABP support when more robust MCS options may be called for, these results suggest that an IABP failure risk score may identify patients with cardiogenic shock that may be at risk of further decompensation on IABP support. This group may warrant closer monitoring for the potential need for early escalation in MCS, or up-front consideration of more advanced MCS.
IABP counter-pulsation has been a mainstay in the treatment of cardiogenic shock for decades.2 However, data supporting its use in cardiogenic shock is somewhat limited. Although commonly used in the setting of cardiogenic shock complicating AMI, the utility of IABP in this setting has been called into question by several randomized controlled studies.5,6,8 That said, the significant crossover in some studies5,9 from the control arm to IABP limits such conclusions. Data regarding IABP use in cardiogenic shock in the non-AMI setting is sparse. One retrospective study of LVAD patients supported with IABP prior to LVAD implantation found that a significant proportion still worsened clinically prior to LVAD implantation.10 Furthermore, this study did not capture patients with cardiogenic shock supported with IABP that did not go on to LVAD. Another single-center study described the single-centered experience of the large volume IABP in cardiogenic shock due to a variety of causes, but did not draw conclusions about those that failed to derive sustained benefit with IABP alone.11
The current study thus sought to study IABP use for cardiogenic shock outside of the AMI setting. The vast majority of patients in the present study underwent IABP placement to stabilize cardiogenic shock complicating known, chronic systolic heart failure. The 48-hour hemodynamic improvements in our cohort were concordant with the known hemodynamic improvements of IABP support in AMI cohorts.12 However, despite these improvements, a third of our cohort suffered an adverse in-hospital event. These rates are comparable to existing studies of cardiogenic shock in both the AMI and non-AMI setting, which generally quote a 40% mortality in cases of cardiogenic shock.5,13 Stratifying our cohort based on adverse events revealed that those who went to suffer an adverse event exhibited waning mean arterial pressure and cardiac index over their first 48-hours post IABP. Although potentially useful, such trends are arguably not surprising and are already commonly incorporated into existing clinical assessments. Furthermore, 48-hour trends do nothing to help predict IABP failure at the time of implantation.
Instead, predicting who might fail IABP support prior to implantation would more greatly aid in the treatment strategy of cardiogenic shock complicating chronic heart failure. The recent advancement of more advanced temporary MCS devices has revolutionized our ability to support patients in cardiogenic shock.1 Even if IABP is used first, which is understandable given its relative ease of implantation and lower cost and complication rate, identifying a cohort at risk for faring poorly would allow for close monitoring and early consideration of MCS escalation if hemodynamic trends do not improve. However, choice of hemodynamic support often depends heavily on clinical expertise and judgment, as there are no clinical tools available to help choose between IABP and more advanced MCS devices.
Our data suggest that LVCPI and history of ICM together are useful predictors of IABP hemodynamic failure. Power is equal to the product of flow and pressure; thus the LVCPI, measured as W/m2, is defined as the product of cardiac index, or flow, and the mean systemic arterial pressure, divided by the constant 451. Although not clinically used, cardiac power has been shown to be a powerful predictor of clinical events.14 In fact, LVCPI was the most powerful predictor of outcome in the Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock (SHOCK) Trial cohort.7 It was also shown to be a useful predictor of decompensation prior to LVAD in the aforementioned study by Sintek and colleagues.10 In the outpatient setting, cardiac power has been shown to help prognosticate patients with heart failure.15 Ischemic cardiomyopathy (ICM) history, the other predictive variable in our cohort, has also long been known to predict worse outcomes in heart failure and is in fact a predictive variable in the original Heart Failure Survival Score.16 It was also shown to be predictive of poor outcomes in those supported by peripheral ventricular assist device.16,17 The reason for poor in-hospital outcomes among ICM patients in our cohort was not entirely clear, as ICM history remained predictive despite adjustment in our cohort for atherosclerotic co-morbidities. Regardless, it is possible that ICM patients do poorly due to the sum of their age, prior surgeries, comorbidities and relative contraindications to advanced therapies, which would lead not only to worsened outcomes but also reduce the likelihood that they would be candidates for advanced therapies.
An IABP failure risk score utilizing LVCPI and history of ICM in our cohort helped to predict not only those who would fare well with IABP support (score of 0), but also at high risk of failing IABP support (score of 2). Identifying patients in the latter group would help to identify a cohort better served by up-front consideration of more advanced therapies. Indeed, one retrospective study showed that early initiation of MCS leads to better survival than when MCS is delayed.4 Whether this latter group would fare better with more advanced MCS remains speculative, as there are few data exploring this question. Studies comparing Impella to IABP in the treatment of cardiogenic shock complicating AMI have shown mixed results.3,18,19 Furthermore, this group may just be a cohort of patients that would do poorly despite the intervention chosen. Alternatively, if IABP is pursued first, this risk score could help identify the cohort at high risk of IABP failure, thus prompting closer hemodynamic monitoring for potential MCS escalation. Regardless, our IABP Failure risk score would contribute to the clinical decision-making at the time of IABP insertion for cardiogenic shock, as there are presently no objective tools used to assess such patients. Importantly, data remain scant in this clinical space, and while the current study helps shed some light on this question, further work is necessary to validate this score in other populations and improve clinical decision-making in cardiogenic shock.
Limitations
This was a single-center retrospective study of IABP patients and thus limited by incomplete data, the particular patient population at this center, as well as the practice patterns of the center. IABP hemodynamic data were well recorded in our electronic record system and free of missing values, but still subject to error due to the lack of prospective standardization of data collection. Patients who suffered an adverse event on average had higher heart rates, which may have reduced IABP efficiency;20 however, causality was difficult to discern retrospectively, as heart rate may have been a reflection of a less responsive cohort instead. Inflammation can play a significant role in outcomes in cardiogenic shock,21 but unfortunately data on inflammatory markers were only sporadically available. Patients were studied from a 5-year period during which there was evolution in the use of advanced MCS as well as durable LVAD support in the treatment of cardiogenic shock. Given the retrospective nature of the study, reasoning for IABP implantation could not be systemically assessed. Finally, reasoning for advanced therapy candidacy could not be determined. As such, although we tried to adjust for comorbidities, it is possible that history of ICM was not itself the risk factor for poor outcomes but still a surrogate for comorbidities that precluded advanced heart failure therapies.
Conclusions
Despite significant hemodynamic improvement from IABP support in the treatment of cardiogenic shock in the non-AMI setting, many patients still fare poorly. In our cohort of cardiogenic shock patients undergoing IABP, low LVCPI (< 0.28 W/m2) at the time of IABP implantation and history of ICM helped to predict significantly poorer 28-day outcomes. This may represent a cohort that warrants up-front consideration of more advanced MCS support, or at least close monitoring and consideration of MCS escalation in the event that they do indeed fail IABP support. Further studies are needed to validate these predictors and improve clinical decision-making surrounding the best choice of mechanical support for refractory cardiogenic shock.
Supplementary Material
Acknowledgments
Financial Support
This study was supported by the National Institutes of Health-National Heart, Lung, and Blood Institute (T32-HL007227-40, S.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author disclosures: All authors have no relationships with industry.
References
- 1.Shekar K, Gregory SD, Fraser JF. Mechanical circulatory support in the new era: an overview. Crit Care. 2016;20(1):66. doi: 10.1186/s13054-016-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Nunen LX, Noc M, Kapur NK, Patel MR, Perera D, Pijls NHJ. Usefulness of Intra-aortic Balloon Pump Counterpulsation. Am J Cardiol. 2016;117(3):469–476. doi: 10.1016/j.amjcard.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 3.Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R, Dirschinger J, Kastrati A, Schömig A. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;5219:1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Basir MB, Schreiber TL, Grines CL, Dixon SR, Moses JW, Maini BS, Khandelwal AK, Ohman EM, O'Neill WW. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. Am J Cardiol. 2017;119(6):845–851. doi: 10.1016/j.amjcard.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 5.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 6.Unverzagt S, Buerke M, de Waha A, Haerting J, Pietzner D, Seyfarth M, Thiele H, Werdan K, Zeymer U, Prondzinsky R. Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock. Cochrane Database Syst Rev. 2015;(3):CD007398. doi: 10.1002/14651858.CD007398.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fincke R, Hochman JS, Lowe AM, Menon V, Slater JN, Webb JG, LeJemtel TH, Cotter G SHOCK Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44(2):340–348. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Zeymer U, Neumann F-J, Ferenc M, Olbrich H-G, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Böhm M, Ebelt H, Schneider S, Werdan K, Schuler G Intraaortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial investigators. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382(9905):1638–1645. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, Chew D, Cohen M, French J, Perera D, Ohman EM. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA. 2011;306(12):1329–1337. doi: 10.1001/jama.2011.1280. [DOI] [PubMed] [Google Scholar]
- 10.Sintek MA, Gdowski M, Lindman BR, Nassif M, Lavine KJ, Novak E, Bach RG, Silvestry SC, Mann DL, Joseph SM. Intra-Aortic Balloon Counterpulsation in Patients With Chronic Heart Failure and Cardiogenic Shock: Clinical Response and Predictors of Stabilization. Journal of Cardiac Failure. 2015;21(11):868–876. doi: 10.1016/j.cardfail.2015.06.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visveswaran GK, Cohen M, Seliem A, DiVita M, McNamara JKR, Dave A, Wasty N, Baran DA. A single center tertiary care experience utilizing the large volume mega 50cc intra-aortic balloon counterpulsation in contemporary clinical practice. Catheter Cardiovasc Interv. 2017;367:1287. doi: 10.1002/ccd.26908. [DOI] [PubMed] [Google Scholar]
- 12.Santa-Cruz RA, Cohen MG, Ohman EM. Aortic counterpulsation: a review of the hemodynamic effects and indications for use. Catheter Cardiovasc Interv. 2006;67(1):68–77. doi: 10.1002/ccd.20552. [DOI] [PubMed] [Google Scholar]
- 13.Werdan K, Gielen S, Ebelt H, Hochman JS. Mechanical circulatory support in cardiogenic shock. Eur Heart J. 2014;35(3):156–167. doi: 10.1093/eurheartj/eht248. [DOI] [PubMed] [Google Scholar]
- 14.Popovic B, Fay R, Cravoisy-Popovic A, Levy B. Cardiac power index, mean arterial pressure, and Simplified Acute Physiology Score II are strong predictors of survival and response to revascularization in cardiogenic shock. Shock. 2014;42(1):22–26. doi: 10.1097/SHK.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 15.Lang CC, Karlin P, Haythe J, Lim TK, Mancini DM. Peak cardiac power output, measured noninvasively, is a powerful predictor of outcome in chronic heart failure. Circ Heart Fail. 2009;2(1):33–38. doi: 10.1161/CIRCHEARTFAILURE.108.798611. [DOI] [PubMed] [Google Scholar]
- 16.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95(12):2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 17.Berg DD, Sukul D, O'Brien M, Scirica BM, Sobieszczyk PS, Olenchock BA, Bohula EA, Morrow DA. Outcomes in patients undergoing percutaneous ventricular assist device implantation for cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2016;5(2):108–116. doi: 10.1177/2048872615584079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouweneel DM, Eriksen E, Seyfarth M, Henriques JPS. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump for Treating Cardiogenic Shock: Meta-Analysis. J Am Coll Cardiol. 2017;69(3):358–360. doi: 10.1016/j.jacc.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Ouweneel DM, Eriksen E, Sjauw KD, van Dongen IM, Hirsch A, Packer EJS, Vis MM, Wykrzykowska JJ, Koch KT, Baan J, de Winter RJ, Piek JJ, Lagrand WK, de Mol BAJM, Tijssen JGP, Henriques JPS. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. J Am Coll Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 20.Papaioannou TG, Terrovitis J, Kanakakis J, Stamatelopoulos KS, Protogerou AD, Lekakis JP, Nanas JN, Stamatelopoulos SF. Heart rate effect on hemodynamics during mechanical assistance by the intra-aortic balloon pump. Int J Artif Organs. 2002;25(12):1160–1165. doi: 10.1177/039139880202501207. [DOI] [PubMed] [Google Scholar]
- 21.Rigamonti F, Graf G, Merlani P, Bendjelid K. The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: a retrospective cohort study*. Crit Care Med. 2013;41(11):2484–2491. doi: 10.1097/CCM.0b013e3182982ac3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.