Figure 6.
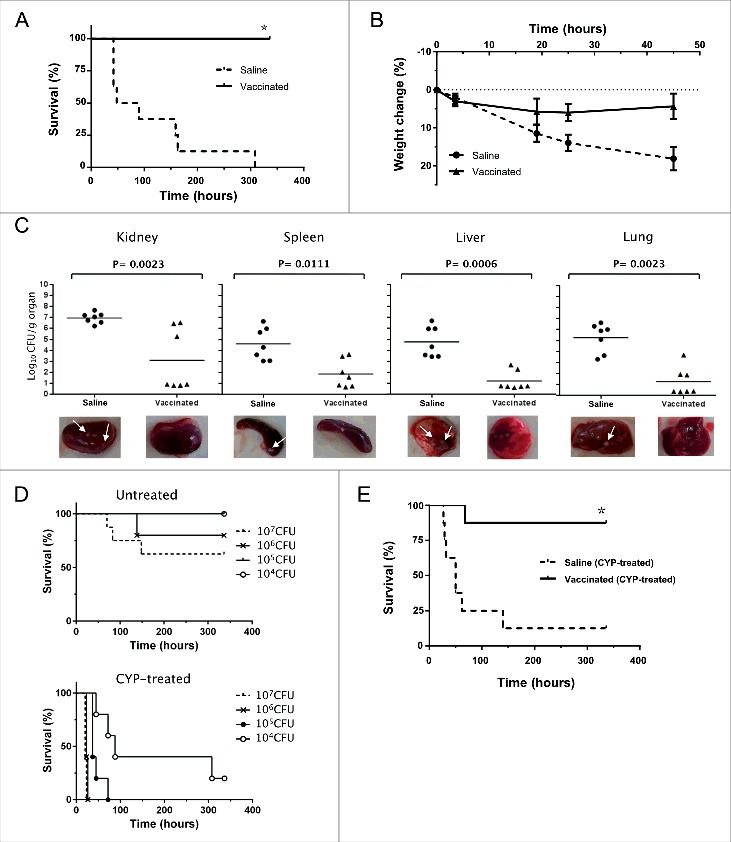
Vaccinated mice are protected against infection with S. aureus 132. A. Survival of BALB/c mice (n = 8/group) immunized via i.p. with S. aureus 132 Δdat Δalr1 Δalr2 (two dose-schedule, approximately 2.5 × 107 CFU) after challenge with a lethal dose of S. aureus 132 (1.2 × 108 CFU per mouse) *P < 0.0001 is significantly different from no vaccination control (Log-rank Mantel-Cox test). B. Percentage weight change relative to starting body weight (preinfection) in BALB/c mice (n = 6/group) after challenge with a sub-lethal dose of S. aureus 132 (2.4 × 107 CFU per mouse) on day 21. Vaccinated and control mice were injected via i.p. with S. aureus 132 Δdat Δalr1 Δalr2 (two dose-schedule, approximately 3 × 107 CFU per mouse) or saline, respectively. C. Bacterial loads in kidney, spleen, liver and lung obtained from vaccinated and control mice 5 days after infection with S. aureus 132. BALB/c mice (n = 7/group) were immunized via i.p. with S. aureus 132 Δdat Δalr1 Δalr2 (two dose-schedule, approximately 3 × 107 CFU per mouse) or saline, and challenged with a sub-lethal dose of S. aureus 132 (2 × 107 CFU per mouse). Each symbol represents an individual mouse, with the horizontal lines showing the mean for each group. P-value, according to log-rank (Mantel-Cox) test is significantly different from no vaccination control in all four organs. Representative images of those organs in each group are shown below the graphics and arrows indicate some bacterial aggregates and small abscess formation into the organs of saline group. D. S. aureus 132 infection in leukopenic mice. BALB/c mice (n = 5/group) untreated or treated with cyclophosphamide (CYP, three doses of 100 mg/kg via i.p. route) in 48-h intervals were inoculated via i.p. route with S. aureus 132 wild type strain at different bacterial doses (CFU), as indicated in the legend, and survival was recorded over 14 days post-infection. E. Vaccine protection of leukopenic mice against S. aureus 132 infection. Vaccinated and control BALB/c mice (n = 8/group) were injected via i.p. with S. aureus 132 Δdat Δalr1 Δalr2 (two dose-schedule, approximately 3 × 107 CFU per mouse) or saline, respectively. Animals were treated with CYP (three doses of 100 mg/kg via i.p. route) in 48-h intervals, one week before challenge by i.p. injection with S. aureus 132 with 7.5 × 104 CFU on day 21 following the first immunization. Survival of mice was recorded over 14 days. *P < 0.005 is significantly different from no vaccination control (Log-rank Mantel-Cox test).
