Abstract
Frontal EEG alpha asymmetry provides a promising index of depression risk, yet very little is known about the neural sources of alpha asymmetry. To identify these sources, this study examined alpha asymmetry using a distributed inverse solution: exact low resolution brain electromagnetic tomography (eLORETA). Findings implicated a generator in lateral mid-frontal regions that contributed to both surface asymmetry and depression risk. Participants with any lifetime history of depressive episodes were characterized by less left-than-right activity in the precentral gyrus and midfrontal gyrus. Anhedonia accounted for a significant portion of the relationship between alpha asymmetry and lifetime MDD. Results are suggestive of convergence between motivational and capability models of asymmetry and replicate results from experimental studies in a large resting-state dataset. The capability model of frontal alpha asymmetry is contextualized in terms of motor preparedness following emotional mobilization.
Keywords: EEG alpha asymmetry, eLORETA, depression, anhedonia, emotion, source estimation
Major depressive disorder (MDD) is a leading cause of disability in the world (Mathers, 2008), is one of the most prevalent psychological disorders (Kessler et al., 2003), and contributes to thousands of deaths by suicide (Nock, Hwang, Sampson, & Kessler, 2010). Resting frontal EEG alpha asymmetry is a widely-studied indicator of depression risk and emotional style (Thibodeau, Jorgensen, & Kim, 2006), and frontal alpha asymmetry may be useful for predicting response to anti-depressant medications (Arns et al., 2016).Yet, the frontal cortex is functionally and anatomically heterogenous, and the regions of the frontal cortex that give rise to frontal EEG alpha asymmetry and depression risk remain relatively unspecified. Uncovering the generators of alpha asymmetry may help predict risk for MDD, improve treatment selection, and inform theories of emotion and motivation.
The relationship between brain lateralization and risk for MDD is believed to result from a deficit in left frontal functioning and is based on neurological observations that left frontal lesions produce depressive episodes (Narushima, Kosier, & Robinson, 2003). Lesion-based study designs have disadvantages, however, especially due to a great deal of systematic and unsystematic noise (e.g., damage to fiber tracts, glia, and parenchyma may be complex, nonspecific, and/or unidentified). A measure of in vivo brain activity could attenuate some experimental noise, and can also be used to examine lateralization hypotheses. Alpha power (8Hz to 13Hz; i.e., the dominant rhythm in the human EEG) is an in vivo measure that indexes functional cortical inhibition; e.g., alpha-band activity inhibits cell assemblies from entraining to visual stimuli (Klimesch, 2012; Mathewson et al., 2011), and is correlated with reduced metabolic activity (Oakes et al., 2004). Alpha generators have historically been associated with occipital and thalamic regions, but more recently alpha power has also demonstrated covariation with metabolic activity in frontal-parietal and default-mode networks (Laufs et al., 2003, 2006; Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007; Sheeringa, Petersson, Kleinschmidt, Jensen, & Bastiannsen, 2012). EEG asymmetry scores are a measure of relative hemispheric activity and are calculated as the difference in left vs. right alpha power, with more left-than-right alpha power taken to indicate relatively less left-than-right activity. Less left-than-right activity reliably predicts depression status (Blackhart, Minnix, and Kline, 2006; Stewart, Bismark, Towers, Coan, and Allen, 2010; Nusslock et al., 2011), but most reports have examined the relative difference between the hemispheres and lower asymmetry scores can result from decreased left activity, increased right activity, or both. In other words, the left-hemisphere deficit hypothesis of depression is often not directly tested. Localizing generators of alpha asymmetry would be a more direct test of this hypothesis. Unfortunately, the available literature on alpha asymmetry sources is small and inconsistent. Some studies have found greater intracranial left frontal alpha power in MDD (less left frontal activity; Lubar, Congedo, and Askew, 2003), some have reported less intracranial right frontal alpha power in MDD (more right frontal activity; Saletu, Anderer, and Saletu-Zyhlarz, 2010), some have reported less right frontal alpha and more left frontal alpha (Arns, et al., 2016), yet others have found no resting-state frontal laterality effects in the alpha band related to MDD (Coutin-Churchman and Moreno, 2008; Pizzagalli et al., 2002). Inconsistent findings are possibly related to poor spatial sampling of the scalp and small sample sizes: montages with few channels (<64) may miss contributions from small patches of cortex situated between electrodes (Nunez and Srinivasan, 2006), and small sample sizes are prone to irreproducible results (Szucs and Ioannidis, 2016). Although researchers have examined alpha-band sources of depression status, no study has investigated the sources of scalp-level asymmetry scores (e.g., Allen, Coan, and Nazarian, 2004), and it remains to be seen what brain regions give rise to conventional scalp-level asymmetry metrics. Overall, depression is more frequent in patients with left frontal lobe lesions and in non-lesion participants with less relative left frontal activity in some EEG studies of intracranial alpha asymmetry (Arns, et al., 2016; Lubar et al., 2003), but no studies have yet directly linked depression, intracranial asymmetry, and scalp-level asymmetry metrics.
The present study had two primary goals. The first goal was to identify generators of alpha asymmetry that contribute to alpha asymmetry scores at the scalp. The second goal was to identify alpha generators that contribute jointly to surface alpha asymmetry and MDD. Resting state EEG source imaging reports as well as meta-analyses of neuroradiological findings have previously found diminished left-lateralized dorsal prefrontal activity in depressed participants (Fitzgerald, Laird, Maller, and Daskalakis, 2008; Hamilton et al., 2012; Lubar et al., 2003). Dorsal-lateral PFC (dlPFC) also lies underneath frontal channels often used to calculate scalp asymmetry metrics (Okamoto et al., 2004). Thus, it was hypothesized that left dlPFC would be identified as a source of alpha generators that jointly contribute to surface asymmetry metrics and depression risk.
Present report
This report examined resting state data using 60-channel montages from 306 participants. Surface data metrics were calculated using data that were transformed to the surface laplacian for improved spatial specificity and mitigation of volume conduction (Stewart et al., 2010). Correlations between scalp asymmetry and eLORETA-modeled intracranial asymmetry were calculated to reveal putative generators of surface asymmetry. Intracranial asymmetry sources were also examined in relation to depression status. Finally, intracranial asymmetry voxels that were jointly related to surface asymmetry and MDD status were identified. These voxels were used for post-hoc analyses with continuous measures of mood, anxiety, and sociodemographic variables (to evaluate generalizability). Compared to other investigations, the use of the current source density (CSD) transformation improved the spatial specificity of surface asymmetry metrics, and a higher electrode density provided an improvement in terms of source estimation performance.
Method
Participants
Beck Depression Inventory scores were collected from undergraduate students in introductory psychology courses at the University of Arizona, and from online surveys. Participants had scores across the entire range of the BDI, and the sample consisted of participants with depression severity ranging from absent to severe (BDI scores were averaged across four EEG recording days and ranged 0-45.5, M = 10.9, MDN = 8.63, SD = 10.4). All participants were strongly right-handed (at least 36 of a possible 39, Chapman and Chapman, 1986). Participants were excluded if they had any history of neurological impairment, disease, or injury (e.g., loss of consciousness > 10 minutes, epilepsy), history of electroconvulsive therapy, were currently using psychotropic medications, met DSM-IV criteria for an Axis-I disorder besides depression or dysthymia, or were actively suicidal. These data have been published elsewhere (Stewart et al., 2010). The source estimates of EEG recordings were never calculated and are presented here for the first time.
All participants were evaluated for DSM-IV Axis-I criteria using the Structured Clinical Interview for DSM-IV (SCID) by graduate students trained in the administration of the SCID, and supervised by the senior author (J.J.B.A.). Participants meeting DSM-IV criteria for any previous lifetime major depressive episode or current major depressive episode made up the lifetime MDD group (N = 143, 39 males, 74.85% Caucasian, 17.5% Hispanic). Participants never meeting criteria for any Axis-I disorder made up the healthy control group (N = 163, 56 males, 68.55% Caucasian, 23% Hispanic). Among the participants in the lifetime MDD group, 44% also met criteria for MDD at the first EEG recording session (current MDD group; N = 62; 18 males). Participants meeting criteria for current dysthymia, or a lifetime history of dysthymia, but without any history of major depressive episode (no history of current or prior depressive episodes were excluded from analysis, N = 7). The sample used for analyses included 306 participants, with 95 males. Participant sex was not analyzed here as a predictor of the relationship between alpha asymmetry and depression status. A previous analysis of this dataset revealed no significant main effects or interaction effects between sex, frontal alpha asymmetry, and depression status when using the surface-laplacian transformation that is also used in this report (Stewart et al., 2010; also see meta-analyses by Thibodeau et al., 2006; and Wacker, Chavanon, & Stemmler, 2010). Participant selection is described in greater detail elsewhere (Stewart et al., 2010).
EEG data collection
Participants were scheduled to visit the laboratory for EEG recordings on four different days, scheduled at least 24hrs apart, within a two-week span. On each visit, two separate resting EEG recordings were obtained (eight EEG recordings for each participant in total over the four days). Data were averaged over the eight recording sessions for the analyses reported below, to examine trait-like indicators of function that have been related to risk for depression (c.f., Coan, Allen, & Nazarian, 2004).
Each recording session consisted of eight one-minute recordings. On each day’s visit, following the first resting EEG recording session and preceding the second, participants completed the directed facial action task (see Stewart, Coan, Towers, and Allen, 2011). Only the resting-state recordings are analyzed in this report. Each 1-minute session was either an eyes open or eyes closed session, and the order of eyes open/eyes closed alternated across sessions (OCCOCOOC or COOCOCCO). The current analysis includes four minutes of eye-open and four minutes of eyes-closed data resting-state data. EEG data were collected using a 64-channel electrode cap and NeuroScan Synamps2 amplifier (Charlotte,NC). Electrooculogram (EOG) sensors applied above and below the left eye recorded eye blinks; EOG sensors were also applied to the outer canthi of the left and right eyes to record rolling eye movements. All electrode impedances were less than or equal to 10KΩ for all recording sessions. Online-data were amplified with a gain of 2816, sampled at 1000Hz, and low-pass filtered at 200Hz. Signals were recorded using an online reference site immediately posterior to Cz. Four channels were not included in analyses: two mastoid channels, and two channels at the cranial base, lateral and ventral to the inion.
EEG preprocessing is described in greater detail in Stewart et al., 2010. Visual marking of raw data for non-biological artifact (e.g., amplifier clipping and cap shifts) was completed first. These segments were removed, and then an automatic artifact rejection algorithm rejected data segments where ocular activity exceeded ±75 microvolts in the vertical ocular channel, and rejected segments with large fast deviations in amplitude in any channel (e.g., direct current shifts and spikes) that may have eluded human inspection. Each minute of data was segmented into 2.048s epochs (Fast-Fourier Transform (FFT) necessitates the use of epochs with a length that is a power of 2). A Hamming window was used to attenuate artifacts at the edges of epochs resulting from the FFT. Epochs overlapped by 1.5s to mitigate the attenuation of spectral power at the edges of an epoch resulting from Hamming windowing. Bad channels were linearly interpolated. One copy of the online-referenced data was processed further with eLORETA (eLORETA transforms scalp voltages into a reference-free montage). Another copy of the data were transformed using the Current-Source Density (CSD; a Laplacian transformation of the surface data; see Kayser & Tenke, 2015), based on the spherical spline approach summarized by Perrin, Pernier, Bertrand, and Echallier (1989, 1990) and implemented using code from Cohen (2014; the laplacian_perrinX function with default settings).
Spectral power at the scalp was calculated using a Fast-Fourier Transformation (FFT) for the artifact-free epochs using the CSD-transformed data. Spectral power was averaged across all artifact-free epochs. Then, alpha band power (8-13 Hz) was extracted from the spectrum for all electrode sites, and log-transformed (to reduce skew). Log-transformations are a conventional approach for reducing skew in alpha asymmetry analyses (see Allen, Coan, & Nazarian, 2004). Alpha asymmetry scores were calculated by subtracting log-transformed activity at left electrodes from right electrodes (e.g., LN[F4]-LN[F3]).
Three-dimensional current-source density estimates for intracranial alpha-band power were computed using the eLORETA toolbox (exact low-resolution electrical tomography analysis; Pascual-Marqui, 2007) for each recording session using default settings. eLORETA estimates sources by constraining the solution to only gray-matter dense regions (6239 voxels at 5×5×5 mm) of the brain using an averaged model of the brain. Alpha-band power (8-13 Hz) for each voxel was normalized (total current density across all voxels = 1) and log-transformed. The eLORETA model brain includes 6239 total voxels, and 2981 left-sided voxels. The intracranial asymmetry metric was computed by finding all 2981 left-sided voxels and their 2981 right-sided counterpart voxels or the closest-neighbor (i.e., the eLORETA brain is asymmetrical); then, the log-transformed difference between the homologous voxels (e.g., LN[R-voxel] - LN[L-voxel]) was computed to create an intracranial asymmetry score.
The eLORETA brain can also estimate source activity for 84 Brodmann areas. To generalize significant voxel-wise outcomes (many other studies have examined only Brodmann areas), the results for Brodmann areas that included significant voxel-wise effects are also reported. For a Brodmann-level approach, the same technique used for voxel-wise analyses was used, but following subtraction of homologous voxels, data were averaged within an eLORETA-defined Brodmann area.
The relationship of MNI voxels to Brodmann areas is also defined by the eLORETA toolbox.
Statistical Analyses
Non-parametric statistics were used for all calculations unless otherwise noted. Medians were used for averaging over recording sessions. Median-based Z scores were calculated at each voxel using the ranksum function of the Matlab statistics toolbox (i.e., a Wilcoxon rank sum test used for statistical significance testing that also produces a robust Z score). Voxel-level thresholds were set at p < .05. A cluster-mass approach was used for multiple comparisons correction (Bullmore et al., 1999, Cohen, 2014). Contiguous voxels (6-way connectivity using the bwconncomp Matlab function) that were significant at p < .05 were treated as a cluster. The absolute values for test statistics within a cluster were then summed. Surrogate data were created by shuffling condition labels, then surrogate cluster-mass was computed, and the process was repeated 500 times to create a null distribution. Observed clusters with cluster-mass greater than the 95%ile of surrogate cluster mass were considered significant at p < .05. A similar approach was used for continuous measures (e.g., surface asymmetry correlations with intracranial asymmetry) that included Spearman correlation coefficients at the voxel level for cluster mass computation. Maximum statistics within a significant cluster are reported using Montreal Neurological Institute coordinates [X Y Z].
For comparisons involving MDD status, lifetime MDD status comparisons involved the 163 never-depressed participants compared to the 143 with a lifetime history of MDD (either current or past); comparisons for current MDD status compared the 163 never-depressed participants to the 62 participants with current MDD.
Results
Alpha power
Figure 1 displays intracranial alpha power estimates, and cooler colors indicate more alpha current density. Unsurprisingly, occipital and dorsal-parietal regions were characterized by the greatest amounts of alpha current density.
Figure 1. eLORETA estimated normalized alpha current density across the brain.
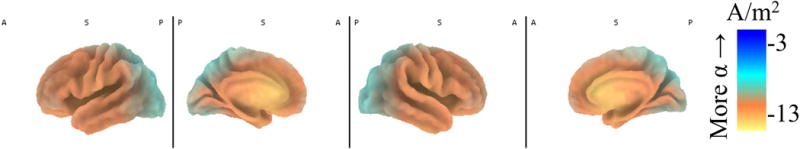
Intracranial eLORETA alpha (8Hz-13Hz) current density across the brain. Current density was normalized (current density for a voxel divided by the summed current density across all voxels) and then log-transformed. Cooler colors (i.e., blue) indicate more alpha power and less activity. Hotter colors (orange and yellow) indicate less alpha power and more activity. Parietal-occipital regions showed the greatest alpha power. Anterior medial regions were characterized by less alpha power and more activity at rest.
Reliability
Fig. 2 shows intraclass correlations (ICCs) for individual voxelwise power, power averaged across a Brodmann area, intracranial asymmetry using homologous voxels, and intracranial asymmetry scores using Brodmann areas. ICCs indicate the stability of intracranial source estimates across the eight EEG recordings collected in this study (four recording days, two recordings each day). These reflect the average measure ICC from a one-way random ANOVA model corresponding to Shrout and Fleiss (1979) ICC(1,k). Alpha-band eLORETA power was significantly more reliable when using Brodmann areas compared to voxels (Wilcoxon signed-rank test Z(6239) = −36.87, p < .01). Similarly, alpha-band eLORETA power was significantly more reliable when using homologous Brodmann areas for subtraction rather than homologous voxels for subtraction (Wilcoxon signed-rank test Z(6239) = −18.12, p < .01).
Figure 2. Intraclass correlation coefficients for four different metrics of intracranial activity.
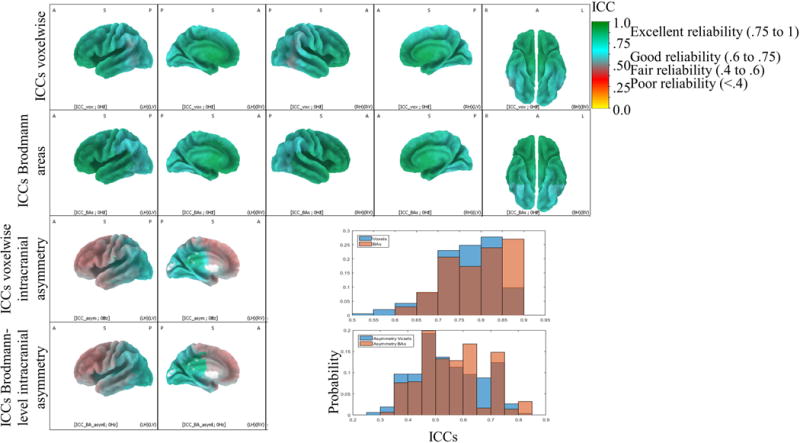
Intraclass correlation coefficients (ICCs) for various intracranial metrics. ICCs indicate the stability of intracranial source estimates across the eight EEG recordings collected in this study (four recording days, two recordings each day). Excellent reliability is colored green and includes coefficients greater than .75. Good reliability is colored blue and includes coefficients greater than .6 and less than .75. Fair reliability is colored pink or blue and includes coefficients greater than .4 and less than .6. Poor reliability is colored red and includes coefficients less than .4. Regions of dorsal-lateral, dorsal-medial, and polar PFC showed the lowest reliability, and these regions may be prone to state-like fluctuations in activity given their roles in on-demand executive functioning, working memory, and cortical organization. Top panel: ICCs for voxelwise eLORETA power estimates. Second from top panel: ICCs for eLORETA power estimates after averaging across 84 Brodmann areas. Second from bottom panel: ICCs for voxelwise intracranial asymmetry metric (LN[Right Voxel] – LN[Left Voxel]). White medial areas did not have asymmetry scores because of the asymmetrical eLORETA brain, nor was reliability computed. Bottom panel: ICCs for intracranial asymmetry metric for 84 Brodmann areas. Only the left hemisphere is displayed/colored for asymmetry scores (bottom two rows: left sagittal view and left medial view) because there is only one value for each pair of homologous left and right voxels. Bottom-right top panel: Histogram showing probability of ICC values for voxelwise eLORETA estimates and for eLORETA estimates after averaging within a Brodmann area. Bottom-right lower panel: Histogram showing probability of ICC values for voxelwise intracranial asymmetry scores and for average intracranial asymmetry scores within a Brodmann area.
Surface-source correlations
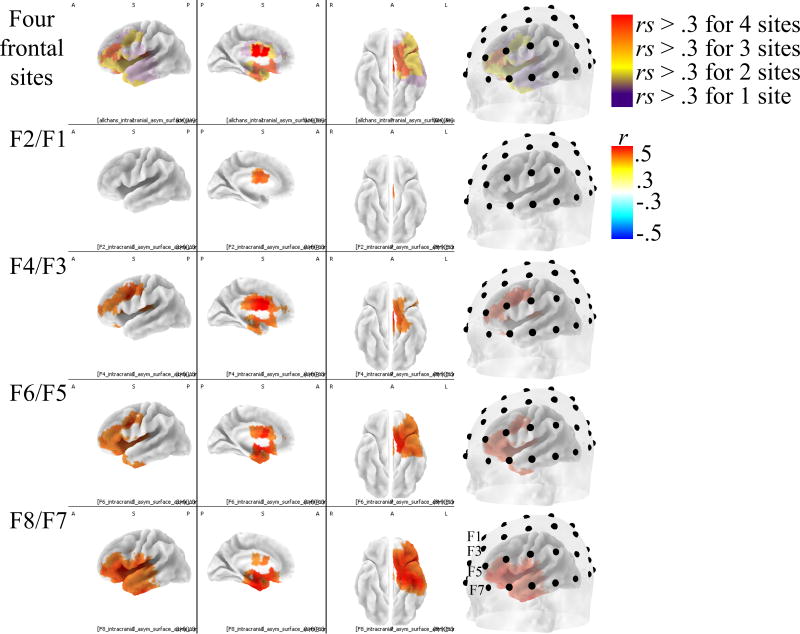
There were widespread correlations between intracranial asymmetry and surface asymmetry that were modest, but statistically reliable. For clarity, and to showcase the regions with the strongest relationships to scalp-level activity, scaling in Fig. 3 omitted correlations of smaller magnitude than ± .3. Surface-source correlations generally varied along a medial-lateral dimension along with the medial-lateral dimension of scalp electrode placement (max rs for F2/F1 r(306) = 0.39 at [−5 −5 30]; for F4/F3 r(306) = 0.53 at [−5 10 25]; for F6/F5 r(306) = 0.53 [−35 −5 20]; for F8/F7 r(306) = 0.52 at [−40 15 5]).
Figure 3. Correlations between alpha asymmetry recorded at the scalp and intracranial alpha asymmetry for four electrode locations.
Correlations between surface asymmetry scores and intracranial asymmetry scores. Positive correlations (red voxels) indicate regions where more relative left activity at the surface was associated with more relative left intracranial activity. Only the left hemisphere is displayed/colored because these were asymmetry scores; thus, there is only one value for each pair of homologous left and right voxels. First column: left sagittal view. Second column: left medial view. Third column: bilateral ventral view (right hemisphere is displayed, but only left hemisphere is displaying correlation coefficients). Fourth column: left sagittal view with translucent scalp overlaid to show electrode positions relative to sources. Only correlations (r) greater than ± .3 are displayed.
Depression and intracranial asymmetry
The relationship between depression and surface alpha asymmetry scores are displayed in Fig. 4. As reported elsewhere (Stewart et al., 2010), lifetime MDD participants were characterized by less left-than-right frontal activity compared to participants with no MDD history. Significant effects were pronounced at frontal (for F2/F1 Z = −1.85, p = .06; F4/F3 Z = −1.97, p = .05; F6/F5 Z = −2.07, p = .04; F8/F7 Z = −1.83, p = .07 and if averaging asymmetry scores over four frontal channels Z = −2.44, p = .01) and central-parietal (for CP4/CP3 Z = −2.29, p = .02; CP2/CP1 Z = −2.31, p = .02) electrode locations. Although largely consistent with the prior analyses of scalp reported data (Stewart et al., 2010), that report used repeated-measures analyses that accounted for within-person variation whereas this report averaged over recording sessions for simplicity (see Stewart et al., 2014 for scalp asymmetry averaged over sessions) and also used Wilcoxon nonparametric statistics to be comparable to the intracranial statistical analyses. Averaging over recording sessions instead of statistically modeling within-person variation also accounts for the effect sizes in Table 1 being smaller than in previous analyses of this dataset (cf. Stewart et al., 2010), and in a meta-analysis of the relationship between MDD and frontal alpha asymmetry (Thibodeau et al, 2006). Importantly, the focus of the present report was not on precise effect size estimation, but rather to localize generators of alpha asymmetry and MDD risk.
Figure 4. Alpha asymmetry scores for MDD- and MDD+ participants at the surface.
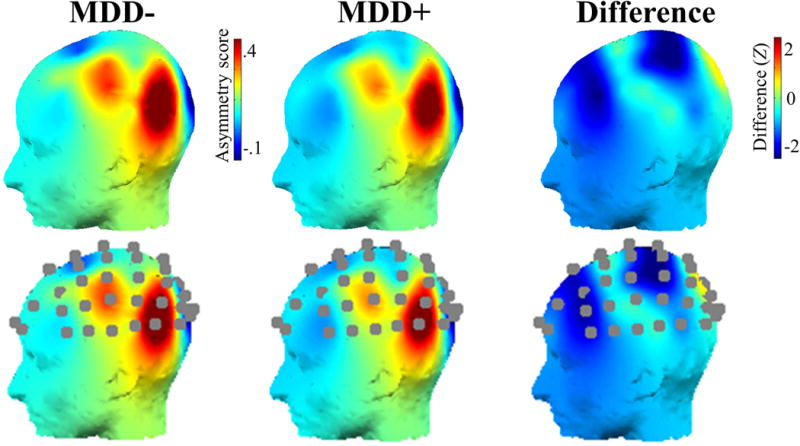
Top panel: Surface asymmetry scores for participants with no history of MDD and participants with a lifetime history of MDD, and the difference (Z scores) in surface asymmetry scores between MDD- and MDD+ participants. Bottom panel: same data as displayed in top panel, with channel locations additionally displayed. Only the left hemisphere is displayed because these were asymmetry scores, and thus there is only one value for each pair of homologous left and right electrodes.
Table 1.
Results from regression analyses of the incremental prediction of lifetime MDD status after accounting for anhedonia
| Life MDD | BDI anhedonia | SCID anhedonia | Life MDD & BDI anhedonia | Life MDD & SCID anhedonia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | t (p) | β | SE | t (p) | β | SE | t (p) | β | SE | t (p) | β | SE | t (p) | |
| (model | (model | (model | (model | (model | |||||||||||
| Fi (pi) | 1.36 (.26) | 2.41 (.09) | |||||||||||||
| (Constant) | .15 | .08 | 1.99 (.05) | −1.1e−16 | .06 | 1.9e−15(1) | .07 | .07 | 1.04 (.30) | .10 | .08 | 1.23 (.22) | .16 | .08 | 2.04 (.04) |
| Life MDD | −.33 | .11 | −2.92 (.004) | −.22 | .14 | −1.65 (.10) | −.28 | .13 | −2.18 (.03) | ||||||
| BDI anhedonia | −.16 | .06 | −2.81 (.005) | −.10 | .07 | −1.4 (.15) | |||||||||
| SCID anhedonia | −.28 | .13 | −2.07 (.04) | −.12 | .15 | −.83 (.41) | |||||||||
| R2 | .027 | .025 | .014 | .034 | .029 | ||||||||||
| F (p) | 8.51 (.003) | 7.87 (.005) | 4.27 (.04) | 5.32 (.005) | 4.54 (.01) | ||||||||||
Note. Results from regression models (Type III sums-of-squares) showing no significant change in incremental variance (Fi) for the prediction of intracranial alpha asymmetry scores in motor/premotor voxels after accounting for anhedonia. BDI-anhedonia was constructed using the average of four items (items 4, 12, 15, and 21) from the BDI that have been linked to anhedonia in previous reports. BDI was assessed on four occasions and averaged together. SCID anhedonia was a binary variable assessed once upon study intake. Results for tests of significant incremental variance (Fi, pi) were calculated according to: (Cohen & Cohen, 1983); where indicates the percent variance accounted for by the full model including Life MDD and anhedonia, and indicates the percent variance accounted for by lifetime MDD only. KA and kB indicate the number of predictors in the model. This notation is also reflected the table above.
Significant contrasts between depressed and healthy participants for intracranial asymmetry scores are displayed in Figure 5. Any lifetime history of MDD was related to less left lateralized precentral gyrus activity (Z(306) = −3.30, at [−35 −20 45], with reliability ICC = .54). The max voxel was close to dlPFC regions that were anticipated a priori based on previous reports (e.g., Lubar et al., 2003). This voxel was in Brodmann area 4, and the effect for activity averaged over the entirety of BA4 was suggestive of diminished left-lateralized activity for lifetime MDD participants (Z(306) = −1.59, p = .11, with reliability ICC = .50); a nearby region—BA2—did show a significant effect for depressions status on intracranial asymmetry (Z(306) = −2.30, p = .02, with reliability ICC = .53). The significant cluster also included more anterior voxels in the left dlPFC (most dorsal-anterior-lateral significant voxel: Z(306) = −2.22, at [−45 0 55], with reliability ICC = .46; also see lower panel of Fig. 5 showing trend-level contrasts between control and lifetime MDD participants uncorrected for multiple comparisons). This dorsal-anterior-lateral voxel was in BA6, but activity averaged over BA6 was unrelated to depression status (Z(306) = −.50, p = .62, with reliability ICC = .45). In short, lifetime MDD was related to less left-than-right activity in several motor and premotor brain regions.
Figure 5. Contrast between MDD participants and healthy controls for intracranial alpha asymmetry scores.
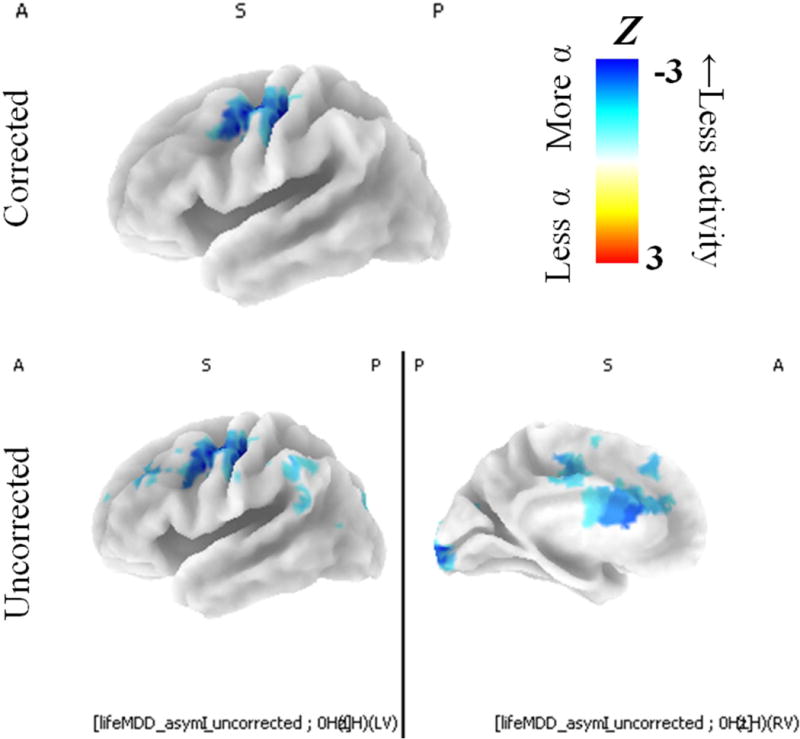
Intracranial asymmetry scores for MDD groups and healthy controls. Blue voxels indicate regions where MDD groups had smaller intracranial asymmetry scores (less relative left activity) than healthy controls. Participants with any lifetime MDD history (past or present) were characterized by less relative left mid-frontal activity compared to healthy controls. Top panel displays relationship between intracranial asymmetry after multiple comparisons correction, and bottom panel displays relationship between intracranial asymmetry with no multiple comparisons correction. Only the left hemisphere is displayed because these were asymmetry scores, and thus there is only one value for each pair of homologous left and right voxels. Top and left panels show left sagittal view. Bottom-right panel shows left medial view.
There were no statistically significant relationships between current MDD status and intracranial asymmetry scores when examining the entire left hemisphere after correcting for multiple comparisons (p > .05). There was also no significant relationship between current MDD and intracranial alpha asymmetry after averaging across the cluster of voxels identified as significant above (e.g., motor and premotor voxels; rank-order correlation r(306) = −.10, p = .07).
A continuous measures of depression severity—the BDI—was also examined in relation to intracranial asymmetry averaged across significant motor and premotor voxels. BDI scores were collected once at each recording session (four times). The BDI scores used for correlation with intracranial alpha asymmetry were the average across these four questionnaires. There was a significant relationship between BDI scores and intracranial alpha asymmetry in the motor/premotor cluster noted above (Spearman correlation r(306) = −.15, p = .01).
A 2×2 repeated-measures ANOVA was conducted using Group (MDD+ & MDD-) as a between-subjects factor and Hemisphere (Right & Left) as a within-subjects factor. MDD+ participants had a lifetime history of major depression, and MDD- participants had no reported history of major depression. Using significant motor and premotor voxels identified in the analysis above, we extracted homologous left and right voxels to test for the effect of an interaction between hemisphere and depression. Results revealed a significant Group x Hemisphere interaction (F(1, 304) = 7.84, p < .01; Greenhouse-Geisser corrected). To decompose the significant interaction and reveal hemisphere-specific contributions to intracranial asymmetry scores, pairwise analysis of group differences in hemispheric activity were examined. For example, an MDD- vs. MDD+ contrast of alpha power in left-hemisphere motor/premotor regions, and an MDD- vs. MDD+ contrast of alpha power for right-hemisphere motor/premotor regions. Pairwise tests revealed that MDD+ participants (M = .10, SD = 1.21) were characterized by a non-significant trend towards less left-hemisphere activity at rest (e.g., more left alpha power; t(304) = 1.65, p = .10, see Figure 6) than MDD- participants (M = −.09, SD = .77), there was no difference in right-hemisphere activity at rest between groups (p > .10; Figure 6).
Figure 6. Group*Hemisphere interaction for alpha power at motor and premotor voxels.
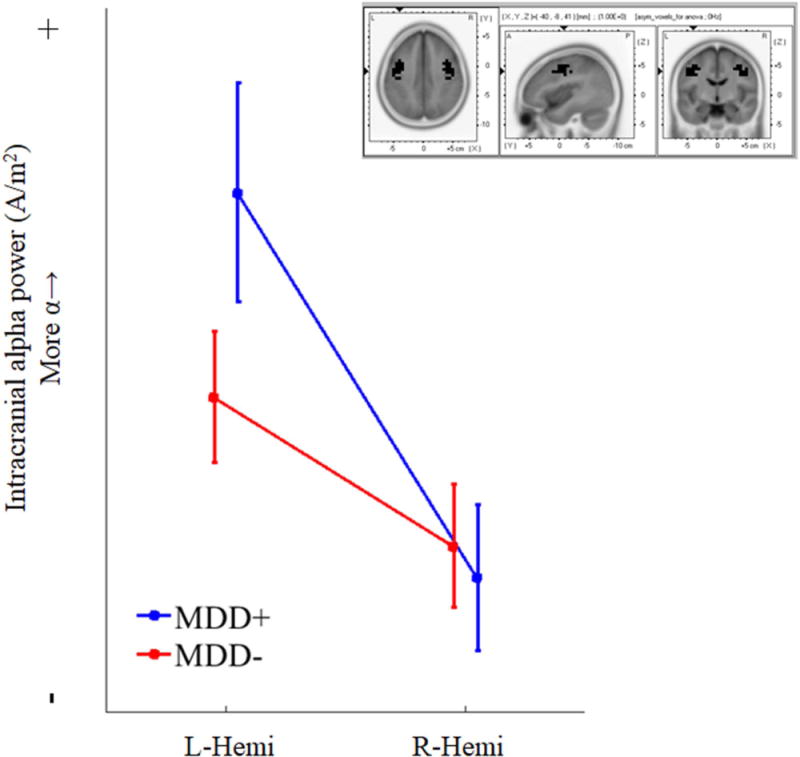
Note. Interaction between group (e.g., MDD history) and hemisphere for voxels identified as significant from intracranial asymmetry analyses (left and right hemisphere voxels used for interaction are shown in black in upper right panel). Participants with any history of depression (blue line) were characterized by more alpha power in left hemisphere motor and premotor voxels than control participants (red line). There was no difference in alpha power for right-hemisphere voxels. Upper right inset shows voxels used for analysis. Results indicate that MDD risk may be characterized by less left frontal activity. Bars indicate standard errors of the mean.
Significant effects were observed for intracranial asymmetry scores, and a Group*Hemisphere interaction showed less left frontal activity in MDD participants (at trend-level); however, single voxel-level alpha power effects were not apparent. These results showcase the importance of accounting for nuisance variables (e.g., skull thickness; total alpha) by using difference scores or ANOVA models (or residualization, see Allen, Coan, & Nazarian, 2004).
Asymmetry and depression overlap
The overlap of intra-cranial asymmetry voxels related to depression with surface asymmetry sources is displayed in Figure 7. The diminished activity observed for participants with a lifetime history of depression in the precentral gyrus cluster noted above overlapped with surface asymmetry generators for the channels F4/F3 and F6/F5. Most studies have used F4/F3 and F6/F5 in their analyses, and these results also support the use of those channels to detect frontal lobe activity associated with depression at the scalp. The most anterior voxels related to depression were the voxels also related to surface asymmetry scores at F4/F3 and F6/F5 (Fig. 7).
Figure 7. Overlap between voxels associated with scalp-level asymmetry scores and voxels associated with lifetime MDD.
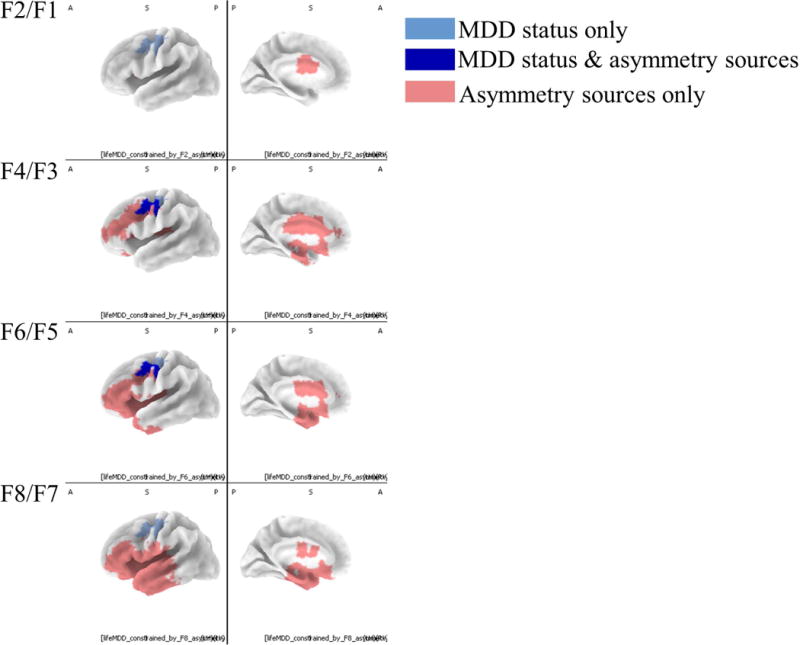
Intracranial asymmetry scores for MDD groups and healthy controls presented along with regions identified as predictive of surface asymmetry. Light blue voxels indicate regions where MDD groups had smaller intracranial asymmetry scores (less relative left activity) than healthy controls. Red voxels indicate regions that correlated (r greater than ±.3) with surface asymmetry scores. Dark blue voxels were related to both surface asymmetry and lifetime depression history. Only the left hemisphere is displayed because these were intracranial asymmetry scores, and thus there is only one value for each pair of homologous left and right voxels.
Post-hoc regression analyses for anhedonia
Because the results were suggestive of a precentral gyrus generator of frontal alpha asymmetry and depression risk, and because the precentral gyrus has been associated with intention to action (see Discussion), a post-hoc hypothesis was that anhedonia could account for the relationship between intracranial asymmetry in the precentral gyrus and lifetime MDD.
Anhedonia is conceptualized as a reduced sensitivity to behavioral reinforcers and reduced drive towards pleasurable stimuli, and has been noted previously as a core feature of MDD. The heterogeneity of DSM symptoms of MDD poses challenges for linking such a diverse phenotype to specific neural systems in the brain, but anhedonia is a more homogeneous and biologically plausible construct insofar as it represents neural sensitivity to certain classes of stimuli (Pizzagalli et al., 2005a). It may be the case that the relationship between precentral gyrus asymmetry and lifetime MDD is largely accounted for by a more homogeneous construct such as anhedonia.
Intracranial asymmetry in voxels that were related to lifetime MDD and frontal alpha asymmetry at F6/F5 (dark blue voxels in Fig. 7) were first averaged together to create the outcome variable. Anhedonia was operationalized as the average score across four days using the average of four BDI items related to anhedonia (Pizzagalli, Jahn, and O’Shea, 2005b): loss of pleasure (item 4), loss of interest (item 12), loss of energy (item 15), and loss of interest in sex (item 21). Scores could range from 0 to 3, with an obtained range of 0 to 3 (M = .47, MDN = .25; SD = .54). The correlation between BDI-anhedonia and lifetime MDD history was r(306) = .56 (Spearman rank-order correlation).
Table 1 shows the full regression results for the regression analyses of lifetime MDD and anhedonia. Regression models (Type III sums-of-squares) included either lifetime MDD as a predictor, anhedonia as a predictor, or lifetime MDD and anhedonia as a predictor (but not the MDD x anhedonia interaction term). After accounting for variance associated with anhedonia (i.e., the R2 from bivariate regression), lifetime MDD added no significant incremental variance (no significant change in R2) to the prediction of intracranial asymmetry scores (F(2, 302) = 1.36, p > .05), suggesting that anhedonia accounts for a substantial portion of the variance between lifetime MDD and intracranial asymmetry. Statistical significance of incremental variance (i.e., ΔR2) was calculated according to Cohen and Cohen (1983, p. 145).
The same analysis was done for the anhedonia item from the SCID that participants completed once upon study intake. Scores on the SCID anhedonia question were binary (e.g., participants could either endorse or not endorse anhedonia symptoms; M = .24, MDN = 0, SD = .43), and results were similar to the results reported above (F(2, 302) = 2.41, p > .05) for the (in)significance of incremental variance after adding lifetime MDD status as an additional predictor variable.
By comparison, lifetime MDD was incrementally predictive of intracranial alpha asymmetry scores after accounting for self-reported irritability (BDI item 11; F(2, 302) = 3.39, p < .05). Lifetime MDD was also incrementally predictive of intracranial alpha asymmetry after accounting for changes in appetite (BDI item 18; F(2, 302) = 4.25, p < .05). Overall, these two results suggest that the variance accounted for by anhedonia was somewhat specific to alpha asymmetry scores and not solely due to shared variance between BDI scores and lifetime MDD.
Altogether, the results indicate that anhedonia may be an important psychobiological dimension that substantially contributes to the relationship between alpha asymmetry at-rest and DSM-based diagnoses (e.g., multidimensional constructs).
Post-hoc regression analyses for anxiety
Depression and anxiety are frequently comorbid, and anxiety may moderate the relationship between mood and alpha asymmetry in some participants (Heller, Etienne, & Miller, 1995; Mathersul et al., 2008; Smith, Zambrano-Vazquez, & Allen, 2016).
Participants in the study completed the Penn-State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990) four times, once at each recording session. PSWQ scores were averaged across the four sessions (M = 49.43, SD = 13.81, range=16 – 78.33, rank-order correlation with lifetime MDD r(304) = .40). Participants in the study also completed the Spielberger State-Trait Anxiety Inventory Trait-scale (TAI; Spielberger, 1968) four times, and trait-anxiety scores (TAI) scores were averaged across sessions (M = 43.73, SD = 12.63, range=20 – 76.5, rank-order correlation with lifetime MDD r(305) = .55). Both PSWQ and TAI scores were averaged across sessions to create stable indices of trait-like anxiety.
There were no significant bivariate relationships between anxiety and intracranial alpha asymmetry scores after correcting for multiple comparisons in the analyses of the entire brain (p > .05). We next examined the effect of anxiety in a simultaneous regression with lifetime MDD using voxels identified as significant from the MDD analyses of the entire brain (e.g., voxels in Fig. 7). Table 2 shows the results of the simultaneous regressions for PSWQ and TAI. There were no significant main effects for either the PSWQ or TAI on alpha asymmetry scores, nor were there any significant anxiety X lifetime MDD interactions for alpha asymmetry scores in motor/premotor voxels.
Table 2.
Results from simultaneous regression of lifetime MDD, PSWQ, and TAI
| Life MDD & PSWQ | Life MDD & TAI | |||||
|---|---|---|---|---|---|---|
| β | SE | t (p) | β | SE | t (p) | |
|
|
||||||
| (Constant) | .15 | .08 | 1.82 (.07) | .16 | .09 | 1.76 (.08) |
| Life MDD | −.29 | .12 | −2.36 (.02) | −.27 | .14 | −2.02 (.04) |
| PSWQ | −.004 | .09 | −.05 (.96) | – | – | – |
| TAI | – | – | – | .01 | .10 | .14 (.88) |
| Life MDD x PSWQ | −.07 | .12 | −.54 (.59) | – | – | – |
| Life MDD x TAI | – | – | – | −.11 | .14 | −.80 (.42) |
| R2 | .028 | .029 | ||||
| F (p) | 2.9 (.04) | 3.02 (.03) | ||||
Note. Results of simultaneous regression for lifetime MDD and anxiety measures regressed on intracranial alpha asymmetry scores in motor-premotor voxels. There were no significant relationships between anxiety and intracranial alpha asymmetry, nor were there any significant lifetime MDD*anxiety interactions on alpha asymmetry scores.
Post-hoc tests for effects of Race, Ethnicity, and SES
To assess the generalizability of these results, the effects of Race, Ethnicity (Hispanic vs. Non-Hispanic), and SES were evaluated as potential main effects and moderators of the relationship between MDD and alpha asymmetry scores. Race and Ethnicity were assessed via self-report, and SES was assessed using the Hollingshead Four-Factor Index of Socioeconomic Status (Hollingshead, 1975). Race was operationalized as a dichotomous Caucasian vs. Non-Caucasian variable. Individual races were not examined because of small cell sizes and poor statistical power. There was no significant main effect of Race (β = .19, SE = .17, t = 1.12, p > .05) or significant MDD*Race interaction effect (β = −.08, SE = .26, t = −0.31, p > .05) on alpha asymmetry scores in a simultaneous regression model including the factors MDD, Race, and the MDD*Race interaction term. Using a similar regression model for Ethnicity (Hispanic vs. Non-Hispanic), there were also no significant main effects of Ethnicity (β = −.10, SE = .20, t = −0.50, p > .05), nor was there a statistically reliable Ethnicity*MDD interaction (β = .42, SE = .30, t = 1.38, p > .05). There were also no significant main effects (β = .03, SE = .08, t = 0.42, p > .05) or interactions (β = .04, SE = .12, t = 0.34, p > .05) for SES. Altogether, the results suggest that the relationship between lifetime MDD and alpha asymmetry is broadly generalizable across participants from different backgrounds.
Discussion
Alpha asymmetry and depression history were conjointly related to left dlPFC activity. The results are in line with previous results, and extend previous findings by linking intracranial sources of alpha and depression risk with scalp-level metrics that are frequently reported in the literature. Identified sources are supportive of an embodiment perspective of motivation.
Present observations are consistent with several previous EEG source imaging reports, and are suggestive that left hemisphere differences in frontal intracranial alpha power (Fig. 6) may give rise to scalp-recorded asymmetry scores that have been linked to depression status. Lubar and others (2003) found less left-than-right activity in precentral and postcentral gyrus (similar to Fig. 1) and is the only resting-state study that examined the relationship between intracranial asymmetry scores and depression. Another report showed greater widespread right-frontal alpha power (i.e., decreases in right-frontal activity), but the authors did not specifically report an intracranial asymmetry score (Saletu, et al., 2010). Findings from a recent large-scale study showed a trend for less left frontal activity and more right frontal activity, but results did not meet statistical criteria after correcting for multiple comparisons, and researchers did not evaluate an intracranial asymmetry score (Arns et al., 2016). Another two reports reported null effects for depression and voxelwise alpha power (Coutin-Churchman and Moreno, 2008; Pizzagalli et al., 2002). Statistically null effects in some of these studies may have resulted from a multiple comparisons correction—single threshold Tmax—that can be especially conservative (Holmes, Blair, Watson, and Ford, 1996; Nichols and Holmes, 2002). Right-Left difference scores attenuate the effect of nuisance variables (e.g., skull thickness, overall alpha power) on alpha-based metrics, as do ANOVA models that include laterality as a factor, and the present study demonstrates the importance of this approach (also see Allen, Coan, & Nazarian, 2004): voxelwise alpha power was unrelated to MDD risk, but right-left difference scores and the Group*Hemisphere interaction revealed significant contributions of the left hemisphere in terms of MDD risk. The significant Group*Hemisphere interaction in Fig. 6 is in line with neurological/neuropsychological hypotheses that suggest left frontal damage is an antecedent to depression. More recently the left-dlPFC has been posited as a network hub that is important for cortical organization and is disrupted in MDD (Kaiser, Andrews-Hanna, Wager, and Pizzagalli 2015). In sum, the present findings converge with most previous reports, null findings from other studies may have been due to less-than-ideal multiple comparisons corrections or nuisance variables, and depression risk may be especially related to left hypoactivity.
Brain activity in regions conjointly related to surface asymmetry and depression, primarily in the precentral gyrus, may facilitate mobilization of the body for approach-motivated behaviors. There are important links between motor activity and frontal EEG alpha asymmetry: reports have suggested that pre-goal states (Harmon-Jones, Gable, and Price, 2011), approach-motivation body postures (Price and Harmon-Jones, 2010), approach-motivation facial expressions (Coan and Allen, 2003; Price, Hortensius, and Harmon-Jones, 2013; Stewart, Coan, Towers, & Allen, 2011), and contralateral hand contractions (Harmon-Jones, 2006) enhance left lateralized frontal brain function. Moreover, the EEG sources observed here are similar to sources noted in experimental studies (not resting-state studies) that have linked left dorsolateral activity with approach motivation (Berkman and Lieberman, 2010) and depression (Pizzagalli, et al., 2005; Spielberger et al., 2011), suggesting some degree of similarity between resting-state and experimentally-induced measures of emotion (see Allen and Cohen, 2014 for an example of how state-like fluctuations can contribute to a measure of a trait-like tendency). Price and others (2012) suggested that motivational approach states are embodied, that sensory-motor representations are coactivated with emotional states, and that emotional embodiment facilitates movement towards objects of desire and away from undesirable objects. Moreover, Harmon-Jones and coworkers have shown across several studies that motor actions cue approach motivation as indexed by more left-than-right frontal brain activity, increased self-reported approach-oriented affect, and increased approach-oriented behavioral responding (Allen & Harmon-Jones, 2001; Coan, Allen, & Harmon-Jones, 2001; Harmon-Jones, 2006; Harmon-Jones, Gable, & Price, 2011; Price & Harmon-Jones, 2011; although generators of these motor-approach relationships had not been examined). Hajcak and colleagues showed that peripheral motor responses induced via magnetic brain stimulation can be potentiated by motivationally-salient images (Hajcak et al., 2007). Event-related potentials (ERPs) that index intention to make motor responses also have generators in primary motor and premotor cortex (Cheyne, Bakhtazad, & Gaetz, 2006), and are modulated by depression (Novak, Novak, Lyman, & Foti, 2016).
Anhedonia has been noted as a core feature of depression and is characterized by a deficit in motivation to seek out rewards (Pizzagalli et al., 2005) and lack of interest in activities once considered pleasurable. Pizzagalli and colleagues (2005) reported that less left-than-right intracranial activity in the MFG and precentral gyrus predicted healthy participants’ behavioral sensitivity to reward, in line with the interpretation of effects here. In fact, a post-hoc analysis in this report demonstrated that lifetime MDD status did not add significant incremental variance to the prediction of precentral gyrus activity after accounting for anhedonia; thus, anhedonia symptoms may be characterized by a deficit in motor circuitry important for behaviorally mobilizing individuals to respond, perhaps especially to approach-relevant stimuli. Because many voxels were in motor regions, it is notable that the participants in this study were strongly right-handed, and some researchers have shown that handedness modulates the relationship between asymmetry scores and approach-related emotion self-reports (Brookshire & Casasanto, 2012; Harmon-Jones, 2006). The results here suggest that anhedonia and MDD are related to less left frontal activity, and initiation of motor movements often cues contralateral disinhibition concurrent with ipsilateral inhibition (Oberman, Pineda, and Ramachandran, 2007; Pfurtscheller, 2006; Pfurtscheller and Neuper, 1997). For example, less left and greater right activity in MDD participants may be related to diminished approach motivation pursuit (Spielberg, Heller, and Miller, 2013). These findings are consistent with a capability model of asymmetry (e.g., Stewart et al., 2014), especially in terms of a diminished capability to mount a behavioral response to motivationally salient cues (Allen, Harmon-Jones, and Cavender, 2001; Pizzagalli, Sherwood, Henriques, and Davidson, 2005). Altogether, the present results link results from resting-state EEG recordings with results from experimental studies that have emphasized the importance of embodied approach motivational states for depression risk.
Strengths of the present study were the large sample size and the use of a large recording montage with improved spatial sampling (nearly every report to date has used fewer than 30 channels), and the use of the current-source-density transform to enhance spatial specificity of surface recorded asymmetry. Statistical analyses were robust and balanced type I and type II error rates (Bullmore et al., 1999; Holmes and Nichols, 2002). The study is also directly comparable to previous reports from this well-characterized dataset (Allen and Cohen, 2014; Bismark et al., 2010; Stewart et al., 2010, 2011, 2014; Towers and Allen, 2009). The sample was limited to predominantly young individuals and early-onset depression, and thus is not likely to address factors that might precipitate late-onset depression (c.f. Kendler, Fiske, Gardner, & Gatz, 2009). The sample was medically healthy and results are probably not contaminated by medical illnesses which could confound results. The present sample is less representative of depression often observed in the clinic because participants with significant comorbidities were excluded (participants with alcoholism or significant anxiety). On the one hand, the sample probably accentuates neurophysiological features that may be specific to depression rather than psychological distress in general. On the other hand, the results may not generalize to depression typically observed in the clinic; for example, comorbidity in MDD can be more than 50% (Kessler et al., 2003). By comparison, the relationship between anhedonia and alpha asymmetry should be present irrespective of comorbidity. Reliability of voxel-based power was generally good or excellent, and reliability of intracranial asymmetry scores was generally fair and on par with reliability previously observed for surface asymmetry metrics (Allen et al., 2004; Hagemann, Naumann, Thayer, and Bartussek, 2002). Although internal consistency of alpha asymmetry scores is excellent (Towers & Allen, 2009), researchers interested in isolating trait-specific variance of frontal alpha asymmetry scores are encouraged to use repeated recordings in future intracranial asymmetry reports (Stewart et al., 2014; Hagemann et al., 2002). Finally, although the source estimation procedure used has demonstrated adequate performance empirically and the results are largely consistent with previous literature using other methods, eLORETA nonetheless represents only one approach for source estimation. Future studies will examine fluctuations in network connectivity as a function of resting-state alpha asymmetry.
Conclusion
The present findings recapitulate observations that less left frontal brain activity may be related to risk for Major Depressive Disorder, and extends previous findings to show that plausible sources of surface asymmetry that relate to depression history derive from intracranial asymmetry in dorsal-lateral frontal regions. Diminished motivational mobilization of motor scripts in premotor regions and precentral gyrus may be relevant for depression. Left frontal hypoactivity predicts lifetime history of depression. The results link resting-state recordings at the scalp to brain regions relevant to emotion and behavior, specifically anhedonia and neural systems that may facilitate mobilization of the body for approach-motivated behaviors. Previous reports of null effects may have been due to statistical corrections for multiple comparisons that were overly stringent, the impact of nuisance variables on uncorrected alpha power measures (e.g., asymmetry scores can mitigate nuisance effects of overall alpha power), or imperfect reliability of intracranial asymmetry measures. Results provide a basis for conceptualizing asymmetry in terms of preparation for and instantiation of action tendencies, and fit well with some existing models of asymmetry (Harmon-Jones, 2006; Price et al., 2012) and risk for MDD (Pizzagalli et al., 2005).
Acknowledgments
This research was supported in part by grants from the National Institute of Mental Health (R01–MH066902 and R21-MH101398) and from the National Alliance for Research on Schizophrenia and Depression (NARSAD). The authors wish to thank Jamie R. Velo, James A. Coan, David N. Towers, Jennifer L Stewart, Andrew W. Bismark, Craig Santerre, Eynav E. Accortt, Amanda Brody, Jay Hegde and myriad research assistants for their help on this project.
References
- Allen JJB, Cohen MX. Deconstructing the “resting” state: Exploring the temporal dynamics of frontal alpha asymmetry as an endophenotype for depression. Frontiers in Human Neuroscience. 2010;4:232. doi: 10.3389/fnhum.2010.00232. https://doi.org/10.3389/fnhum.2010.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38(04):685–693. http://dx.doi.org/10.1111/1469-8986.3840685. [PubMed] [Google Scholar]
- Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–280. doi: 10.1111/j.1469-8986.2003.00149.x. https://doi.org/10.3389/fnhum.2010.00232. [DOI] [PubMed] [Google Scholar]
- Arns M, et al. EEG alpha asymmetry as a gender-specific predictor of outcome to acute treatment with different antidepressant medications in the randomized iSPOT-D study. Clinical Neurophysiology. 2016;127(1):509–519. doi: 10.1016/j.clinph.2015.05.032. http://dx.doi.org/10.1016/j.clinph.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Clasen PC, Enock PM, Schnyer DM. Attention bias modification for major depressive disorder: Effects on attention bias, resting state connectivity, and symptom change. Journal of abnormal psychology. 2015;124(3):463. doi: 10.1037/abn0000049. http://dx.doi.org/10.1037/abn0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. Neuroscience. 2010;167(1):97–103. doi: 10.1016/j.neuroscience.2010.01.047. http://dx.doi.org/10.1016/j.neuroscience.2010.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman ET, Lieberman MD. Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience. 2010;22(9):1970–1979. doi: 10.1162/jocn.2009.21317. http://dx.doi.org/10.1162/jocn.2009.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismark AW, et al. Polymorphisms of the 5HT1A allele are linked to frontal brain electrical asymmetry. Biological Psychology. 2010;83:153–158. doi: 10.1016/j.biopsycho.2009.12.002. http://dx.doi.org/10.1016/j.biopsycho.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression?: A preliminary study. Biological psychology. 2006;72(1):46–50. doi: 10.1016/j.biopsycho.2005.06.010. http://dx.doi.org/10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Brookshire G, Casasanto D. Motivation and motor control: hemispheric specialization for approach motivation reverses with handedness. PLoS One. 2012;7(4):e36036. doi: 10.1371/journal.pone.0036036. http://dx.doi.org/10.1371/journal.pone.0036036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, et al. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE transactions on medical imaging. 1999;18(1):32–42. doi: 10.1109/42.750253. http://dx.doi.org/10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and cognition. 1987;6(2):175–183. doi: 10.1016/0278-2626(87)90118-7. http://dx.doi.org/10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event‐related beamforming approach. Human brain mapping. 2006;27(3):213–229. doi: 10.1002/hbm.20178. http://dx.doi.org/10.1002/hbm.20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Varieties of emotional experience during voluntary emotional facial expressions. Annals of the New York Academy of Sciences. 2003;1000(1):375–379. doi: 10.1196/annals.1280.034. http://dx.doi.org/10.1196/annals.1280.034. [DOI] [PubMed] [Google Scholar]
- Cohen MX. Analyzing neural time series data: theory and practice. MIT Press; 2014. [Google Scholar]
- Coutin-Churchman P, Moreno R. Intracranial current density (LORETA) differences in QEEG frequency bands between depressed and non-depressed alcoholic patients. Clinical Neurophysiology. 2008;119(4):948–958. doi: 10.1016/j.clinph.2007.12.013. http://dx.doi.org/10.1016/j.clinph.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta‐analytic study of changes in brain activation in depression. Human brain mapping. 2008;29(6):683–695. doi: 10.1002/hbm.20426. http://dx.doi.org/10.1080/09332480.2005.10722754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait? an application of latent state-trait theory. Journal of personality and social psychology. 2002;82(4):619. http://dx.doi.org/10.1037/0022-3514.82.4.619. [PubMed] [Google Scholar]
- Hajcak G, et al. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44(1):91–97. doi: 10.1111/j.1469-8986.2006.00487.x. http://dx.doi.org/10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, et al. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. The American journal of psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. http://dx.doi.org/10.1176/appi.ajp.2012.11071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon‐Jones E. Unilateral right‐hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology. 2006;43(6):598–603. doi: 10.1111/j.1469-8986.2006.00465.x. http://dx.doi.org/10.1111/j.1469-8986.2006.00465.x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Gable PA, Price TF. The influence of affective states varying in motivational intensity on cognitive scope. Frontiers in integrative neuroscience. 2011;6:73–73. doi: 10.3389/fnint.2012.00073. http://dx.doi.org/10.3389/fnint.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: Implications for neuropsychological models of emotion and psychopathology. Journal of abnormal psychology. 1995;104:327–327. doi: 10.1037//0021-843x.104.2.327. http://dx.doi.org/10.1037/0021-843X.104.2.327. [DOI] [PubMed] [Google Scholar]
- Hollingshead AA. Four-factor index of social status. Yale University; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Holmes AP, Blair RC, Watson JDG, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. Journal of Cerebral Blood Flow & Metabolism. 1996;16(1):7–22. doi: 10.1097/00004647-199601000-00002. http://dx.doi.org/10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Jackson DC, et al. Now you feel it, now you don't frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological science. 2003;14(6):612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. http://dx.doi.org/10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA psychiatry. 2015;72(6):603–611. doi: 10.1001/jamapsychiatry.2015.0071. http://dx.doi.org/10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: A tutorial review. International Journal of Psychophysiology. 2015;97(3):189–209. doi: 10.1016/j.ijpsycho.2015.04.012. http://dx.doi.org/10.1016/j.ijpsycho.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Fiske A, Gardner CO, Gatz M. Delineation of two genetic pathways to major depression. Biological psychiatry. 2009;65(9):808–811. doi: 10.1016/j.biopsych.2008.11.015. http://dx.doi.org/10.1016/j.biopsych.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. http://dx.doi.org/10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends in cognitive sciences. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. http://dx.doi.org/10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, et al. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003;19(4):1463–1476. doi: 10.1016/s1053-8119(03)00286-6. http://dx.doi.org/10.1016/S1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Laufs H, et al. Where the BOLD signal goes when alpha EEG leaves. Neuroimage. 2006;31(4):1408–1418. doi: 10.1016/j.neuroimage.2006.02.002. http://dx.doi.org/10.1016/j.neuroimage.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Lubar JF, Congedo M, Askew JH. Low-resolution electromagnetic tomography (LORETA) of cerebral activity in chronic depressive disorder. International Journal of Psychophysiology. 2003;49(3):175–185. doi: 10.1016/s0167-8760(03)00115-6. http://dx.doi.org/10.1016/S0167-8760(03)00115-6. [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. http://dx.doi.org/10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers C. The global burden of disease: 2004 update. World Health Organization; 2008. http://dx.doi.org/10.1016/B978-0-12-803678-5.00175-2. [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8(4):560. doi: 10.1037/a0012811. http://dx.doi.org/10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- Mathewson KE, et al. Pulsed Out of Awareness: EEG Alpha Oscillations Represent a Pulsed-Inhibition of Ongoing Cortical Processing. 2011;i(2):99. doi: 10.3389/fpsyg.2011.00099. http://dx.doi.org/10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, et al. Validation of ICA-based myogenic artifact correction for scalp and source-localized EEG. Neuroimage. 2010;49(3):2416–2432. doi: 10.1016/j.neuroimage.2009.10.010. http://dx.doi.org/10.1016/j.neuroimage.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Greischar LL, Davidson RJ. Validation of regression‐based myogenic correction techniques for scalp and source‐localized EEG. Psychophysiology. 2009;46(3):578–592. doi: 10.1111/j.1469-8986.2009.00787.x. http://dx.doi.org/10.1111/j.1469-8986.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the penn state worry questionnaire. Behaviour research and therapy. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. http://dx.doi.org/10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra-and inter-hemispheric lesion location using meta-analysis. The Journal of neuropsychiatry and clinical neurosciences. 2003;15(4):422–430. doi: 10.1176/jnp.15.4.422. http://dx.doi.org/10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human brain mapping. 2002;15(1):1–25. doi: 10.1002/hbm.1058. http://dx.doi.org/10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK, Hwang I, Sampson NA, Kessler RC. Mental disorders, comorbidity and suicidal behavior: results from the National Comorbidity Survey Replication. Molecular psychiatry. 2010;15(8):868–876. doi: 10.1038/mp.2009.29. http://dx.doi.org/10.1038/mp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak KD, Foti D. Teasing apart the anticipatory and consummatory processing of monetary incentives: An event‐related potential study of reward dynamics. Psychophysiology. 2015;52(11):1470–1482. doi: 10.1111/psyp.12504. http://dx.doi.org/10.1111/psyp.12504. [DOI] [PubMed] [Google Scholar]
- Novak BK, Novak KD, Lynam DR, Foti D. Individual differences in the time course of reward processing: Stage-specific links with depression and impulsivity. Biological Psychology. 2016;119:79–90. doi: 10.1016/j.biopsycho.2016.07.008. http://dx.doi.org/10.1016/j.biopsycho.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press; USA: 2006. [Google Scholar]
- Nusslock R, et al. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. Journal of Abnormal Psychology. 2011;120(2):497. doi: 10.1037/a0022940. http://dx.doi.org/10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes TR, et al. Functional coupling of simultaneous electrical and metabolic activity in the human brain. Human brain mapping. 2004;21(4):257–270. doi: 10.1002/hbm.20004. http://dx.doi.org/10.1002/hbm.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: a link between action observation and social skills. Social cognitive and affective neuroscience. 2007;2(1):62–66. doi: 10.1093/scan/nsl022. http://dx.doi.org/10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21(1):99–111. doi: 10.1016/j.neuroimage.2003.08.026. http://dx.doi.org/10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, MacNamara A, Goldstein RZ, Hajcak G. Event-related induced frontal alpha as a marker of lateral prefrontal cortex activation during cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2012;12(4):730–740. doi: 10.3758/s13415-012-0107-9. http://dx.doi.org/10.3758/s13415-012-0107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv preprint arXiv. 2007;0710:3341. [Google Scholar]
- Pfurtscheller G. The cortical activation model (CAM) Progress in brain research. 2006;159:19–27. doi: 10.1016/S0079-6123(06)59002-8. http://dx.doi.org/10.1016/S0079-6123(06)59002-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neuroscience letters. 1997;239(2):65–68. doi: 10.1016/s0304-3940(97)00889-6. http://dx.doi.org/10.1016/S0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, et al. Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biological psychiatry. 2002;52(2):73–85. doi: 10.1016/s0006-3223(02)01313-6. http://dx.doi.org/10.1016/S0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness a source-localization study. Psychological Science. 2005a;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. http://dx.doi.org/10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological psychiatry. 2005b;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. http://dx.doi.org/10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TF, Harmon‐Jones E. Approach motivational body postures lean toward left frontal brain activity. Psychophysiology. 2011;48(5):718–722. doi: 10.1111/j.1469-8986.2010.01127.x. http://dx.doi.org/10.1111/j.1469-8986.2010.01127.x. [DOI] [PubMed] [Google Scholar]
- Price TF, Peterson CK, Harmon-Jones E. The emotive neuroscience of embodiment. Motivation and Emotion. 2012;36(1):27–37. http://dx.doi.org/10.1007/s11031-011-9258-1. [Google Scholar]
- Price TF, Hortensius R, Harmon-Jones E. Neural and behavioral associations of manipulated determination facial expressions. Biological psychology. 2013;94(1):221–227. doi: 10.1016/j.biopsycho.2013.06.001. http://dx.doi.org/10.1016/j.biopsycho.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clinical EEG and neuroscience. 2010;41(4):203–210. doi: 10.1177/155005941004100407. http://dx.doi.org/10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- Scheeringa R, Petersson KM, Kleinschmidt A, Jensen O, Bastiaansen MC. EEG alpha power modulation of FMRI resting-state connectivity. Brain connectivity. 2012;2(5):254–264. doi: 10.1089/brain.2012.0088. http://dx.doi.org/10.1089/brain.2012.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychological bulletin. 1979;86(2):420. doi: 10.1037//0033-2909.86.2.420. http://dx.doi.org/10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, et al. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. Neuroimage. 2011;54(1):661–670. doi: 10.1016/j.neuroimage.2010.08.037. http://dx.doi.org/10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Heller W, Miller GA. Hierarchical brain networks active in approach and avoidance goal pursuit. Frontiers in human neuroscience. 2013;7:284–284. doi: 10.3389/fnhum.2013.00284. http://dx.doi.org/10.3389/fnhum.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. The State-trait Anxiety Inventory (STAI): Test Manual for Form X. Consulting Psychologists Press; 1968. [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJ. Resting frontal EEG asymmetry as an endophenotype for depression risk: sex-specific patterns of frontal brain asymmetry. Journal of abnormal psychology. 2010;119(3):502. doi: 10.1037/a0019196. http://dx.doi.org/10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJB. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. Journal of Affective Disorders. 2011;129:167–174. doi: 10.1016/j.jad.2010.08.029. http://dx.doi.org/10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48:82–95. doi: 10.1111/j.1469-8986.2010.01035.x. http://dx.doi.org/10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJB. Resting and Task-Elicited Prefrontal Brain Asymmetry in Depression: Support for the Capability Model. Psychophysiology. 2014;51:446–455. doi: 10.1111/psyp.12191. http://dx.doi.org/10.1111/psyp.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT. Functions of the frontal lobes: relation to executive functions. Journal of the international neuropsychological Society. 2011;17(05):759–765. doi: 10.1017/S1355617711000695. http://dx.doi.org/10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Szucs D, Ioannidis JP. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. bioRxiv. 2016;071530 doi: 10.1371/journal.pbio.2000797. http://dx.doi.org/10.1371/journal.pbio.2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. Journal of abnormal psychology. 2006;115(4):715. doi: 10.1037/0021-843X.115.4.715. http://dx.doi.org/10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Towers DN, Allen JJB. A better estimate of the internal consistency reliability of frontal EEG asymmetry scores. Psychophysiology. 2009;46:132–142. doi: 10.1111/j.1469-8986.2008.00759.x. http://dx.doi.org/10.1111/j.1469-8986.2008.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Chavanon ML, Stemmler G. Resting EEG signatures of agentic extraversion: New results and meta-analytic integration. Journal of Research in Personality. 2010;44(2):167–179. http://dx.doi.org/10.1016/j.jrp.2009.12.004. [Google Scholar]
- Wager TD, Kang J, Johnson TD, Nichols TE, Satpute AB, Barrett LF. A Bayesian model of category-specific emotional brain responses. PLoS Comput Biol. 2015;11(4):e1004066. doi: 10.1371/journal.pcbi.1004066. http://dx.doi.org/10.1371/journal.pcbi.1004066. [DOI] [PMC free article] [PubMed] [Google Scholar]