TO THE EDITOR
Human skin undergoes structural and functional changes with aging. The epidermis becomes thinner, experiences a decline in barrier function, and has reduced replicative capacity (Zouboulis and Makrantonaki, 2011). In the dermis, the number of fibroblasts decreases, collagen and elastic fibers are lost, and the dermal-epidermal junction becomes flattened. Collectively, these changes make aged skin more susceptible to wounding, slower to heal from injury, and more prone to conditions such as allergic and irritant dermatitis (Gilchrest, 1989). Many factors contribute to aging-associated changes in the skin, and extrinsic causes such as UVR exposure are well known. However, the intrinsic factors that lead to age-associated skin changes are still incompletely understood.
Recently, the importance of ubiquitin-proteasome function in cellular, tissue, and organismal aging has become increasingly appreciated (Saez and Vilchez, 2014). The proteasome removes damaged or misfolded proteins from the cell. Impaired proteasome activity results in accumulation of aberrant proteins that disrupt cellular and tissue function. In general, proteasomal function declines with age; this decline has been noted in tissues including the liver, heart, kidney, brain, muscle, and skin (Saez and Vilchez, 2014). However, it is not established in the epidermis whether decline of proteasomal activity simply accompanies increasing age or whether it actively contributes to aging-related structural and functional changes. In this study, we evaluated whether depletion of proteasomal activity in youthful epidermal cells recapitulates biological hallmarks of epidermal aging.
The loss of proteasomal activity can be triggered by decreased expression of specific proteasomal subunits (Lee et al., 1999). We reanalyzed a comparative transcriptome study of youthful versus aged human skin (subjects <30 vs. >50 years, n = 5 each) (Chang et al., 2012). Consistent with conclusions of this prior study, we found that the expression of most proteasome subunits did not differ significantly between young and aged skin, with the notable exception of the regulatory subunit PSMD8 (Figure 1a, and see Supplementary Figure S1 and Supplementary Materials online), which showed an average decline of 26.8%. Because this study assessed mRNA transcript levels from biopsy samples that included all layers of the skin, we assessed whether PSMD8 protein levels were specifically reduced in aged epidermis. Samples were collected with written informed consent and approval by the University of CaliforniaeSan Diego Institutional Review Board. We isolated keratinocytes from neonatal (age < 1 year) and aged skin subjects (age > 65 years, n = 4 each) and performed a quantitative immunoblot analysis (Figure 1b and c). PSMD8 protein expression was reduced by approximately 30% in aged keratinocytes, concordant with the mRNA expression results of the previous study and supporting the finding that the PSMD8 declines with epidermal aging. Consistent with historical observations, aged keratinocyte progenitors replicated at a lower rate and displayed impaired capability for holoclone formation (Figure 1d–f, and see Supplementary Materials for methods).
Figure 1. Epidermal PSMD8 expression declines with aging and is associated with impaired proteasome function.
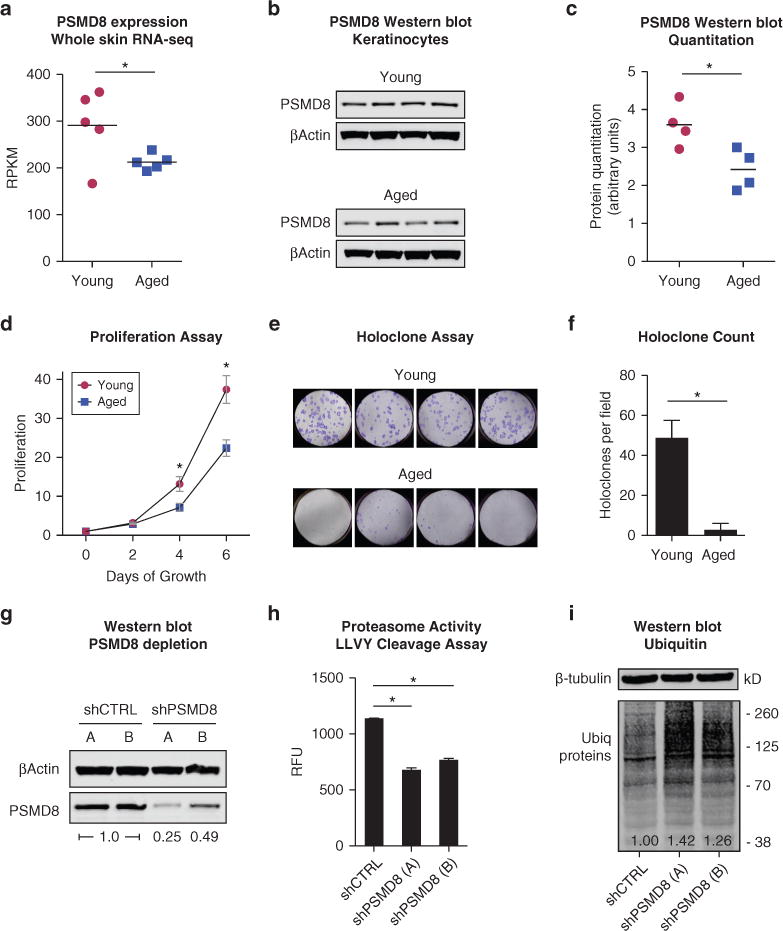
(a) Analysis of PSMD8 expression from a transcriptome study comparing mRNA expression in young (<30 years, n = 5, red circles) and aged (>50 years, n = 5, blue squares) skin samples (Chang et al., 2012). Expression compared by two-sided t test. *Q-value false discovery rate < 0.05. (b) Western blot of PSMD8 in young (<1 year) and aged (>65 years) keratinocytes, n = 4 each. (c) Quantification of protein expression using ImageStudioLite (Li-Cor, Lincoln, NE), corrected for total protein. Two-sided t test. *P < 0.05. (d) Proliferation assay of young versus aged keratinocyte progenitors, n = 4 each. Error bars indicate standard error of the mean. (e) Holoclone assay of young versus aged keratinocytes. n = 4 each, three assays per replicate. One representative assay is shown. (f) Quantitation of holoclones from young and aged keratinocytes. *Two-sided t test, P < 0.05. (g) Western blot of keratinocytes infected with control (shCTRL) or PSMD8-targeted shRNA (shPSMD8). Relative PSMD8 expression compared with control is shown. (h) LLVY-peptide cleavage proteasome activity assay. n = 2 each, error bars indicate standard deviation. *Two-sided t test, P < 0.05. (i) Western blot of cell lysate for ubiquitylated proteins. Relative intensity of ubiquitylated proteins is shown. LLVY, Leu-Leu-Val-Tyr; RFU, relative fluorescent units; RNA-seq, RNA sequencing; RPKM, reads per kilobase per million; shRNA, short hairpin RNA.
Complete loss of proteasomal subunit proteins is lethal to cells. However, it is unclear whether partial subunit depletion, as seen in aging, is sufficient to impair keratinocyte function. To recapitulate partial PSMD8 depletion, we infected primary neonatal keratinocytes with short hairpin RNAs (shRNAs) against PSMD8. Two independent shRNAs (shA and shB) reduced protein expression by approximately 50% and approximately 75% (Figure 1g). A peptide cleavage assay showed that PSMD8 depletion reduced proteasomal activity (Figure 1h). PSMD8-depleted keratinocytes accumulated ubiquitylated proteins at 26-42% more than control keratinocytes (Figure 1i). Collectively, these data indicated that partial PSMD8 loss is sufficient to impair proteasomal function.
We next evaluated whether PSMD8 depletion in young keratinocytes would recapitulate biological features of aged keratinocytes. We performed a proliferation assay to assess cellular replicative capacity of PSMD8-depleted cells. Proliferation was markedly decreased in shPSMD8 cells compared with controls (Figure 2a). This could be due to decreased replication rate and/or increased cell death. To distinguish between these possibilities, we measured cell death in scramble versus PSMD8-depleted cells and found that PSMD8 knockdown resulted in an increased rate of cell death that accounts for part of the reduction in cell proliferation (Figure 2b). Viewed together, these data indicate that PSMD8 depletion results in both lower replication rate and increased cell death, leading to overall reduced replicative capacity. These findings are congruent with features described in aged human skin (Gilhar et al., 2004).
Figure 2. Depletion of PSMD8 in neonatal keratinocytes recapitulates hallmarks of epidermal aging.
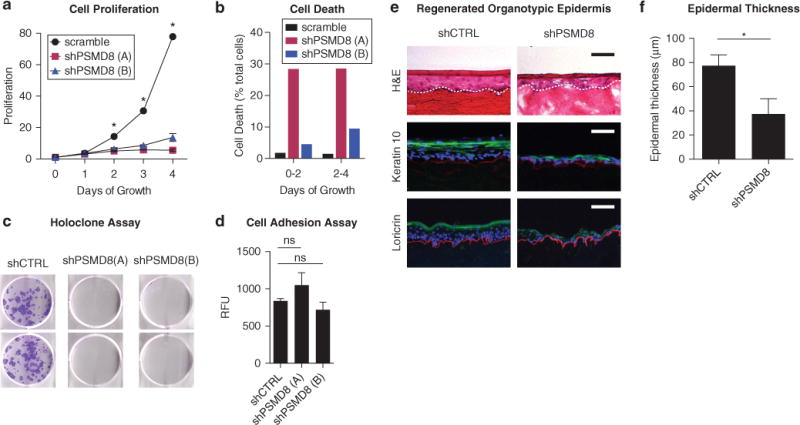
(a) Cell proliferation assay. Control and shPSMD8-depleted keratinocytes seeded in equal numbers and assayed over time course. n = 4. Error bars indicate standard error of the mean. *α = 0.05, t test with Holm-Sídák method. (b) Cell death assay. All adherent and floating cells were collected after 2 and 4 days of growth. Cells were labeled with propidium iodide, and live/dead cells were counted by flow cytometry. (c) Holoclone assay for shCTRL and shPSMD8 keratinocytes. (d) Cell adhesion assay. Calcein-labeled keratinocytes were seeded onto collagen matrix, nonbinding cells were washed away, and adherent cells were quantitated by fluorescence. n = 5. Error bars indicate standard deviation. (e) Organotypic epidermis. H&E staining (top row) and immunofluorescence for keratin 10 (green, middle row) and loricrin (green, bottom row) in epidermis regenerated from control and PSMD8-depleted neonatal keratinocytes. Collagen VII is stained in red to demarcate the basement membrane, with nuclei stained with Hoechst stain in blue. n = 3 per condition. Representative replicate is shown. Dashed white line indicates basement membrane. Scale bar = 50 μm. (f) Quantitation of epidermal thickness. n = 3. Error bars indicate standard error of the mean. *Two-sided t test, P < .05. CTRL, control; H&E, hematoxylin and eosin; ns, not significant to P < 0.05; RFU, relative fluorescent units; sh, short hairpin RNA.
Next, we assessed whether clonogenic potential was affected by PSMD8 depletion. Control and shPSMD8 progenitors were seeded in a holoclone assay and cultured for 15 days. PSMD8-depleted cells were deficient in their ability to form holoclones (Figure 2c). To assess whether this could reflect a loss of adhesion, rather than effects on clonogenicity, we also performed a cell adhesion assay. No significant differences were observed in the ability of shPSMD8 cells to bind to the matrix substrate (Figure 2d), suggesting that reduced holoclone formation can be attributed to loss of clonogenic potential and not to failure of cell adhesion. Finally, we examined whether PSMD8 depletion affected epidermal tissue stratification. Organotypic epidermal tissue was regenerated with control and shPSMD8 progenitors. We noted two consistent observations: (i) PSMD8-depleted tissue formed thinner epidermis (Figure 2e and f), and (ii) immunofluorescence of early (keratin 10) and late (loricrin) structural/barrier proteins showed irregular expression of these key differentiation proteins (Figure 2e).
Taken together, these data identify a decline of PSMD8 expression in the epidermis with aging. Experimental depletion of PSMD8 in youthful epidermal progenitors recapitulated biological hallmarks of epidermal aging, including impairment of progenitor replicative and clonogenic capacity, thinning of epidermal tissue, and aberrant expression of structural and barrier proteins. In model organisms and some human cell types, restoring deficient proteasomal subunits is capable of improving function and promoting longevity (Chondrogianni et al., 2015; Tonoki et al., 2009). We overexpressed PSMD8 in aged keratinocytes but did not observe rescue of replicative capacity (see Supplementary Materials and Supplementary Figure S2 online). Repleting additional factors may be required to restore youthful cell function.
We hypothesize that proteasomal activity affects epidermal function in multiple ways. Globally, an impaired proteasome may inhibit clearance of aberrant proteins and disrupt cell cycle control. On a more specific level, proteasomal function may directly and indirectly affect activity of genetic regulators of epidermal renewal and homeostasis, leading to defects in barrier function. Our findings highlight the role of declining proteasomal function as an intrinsic contributor of epidermal aging and support future studies that better define the role of proteasomal regulation on epidermal homeostasis.
Supplementary Material
Acknowledgments
This work was supported by a grant from the La Roche-Posay Foundation and by an award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (AR067853) to BKS.
Abbreviations
- shRNA
short hairpin RNA
Footnotes
ORCID
Bryan K Sun: http://orcid.org/0000-0002-0740-0125
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2017.10.034.
References
- Chang ALS, Bitter PH, Qu K, Lin M, Rapicavoli NA, Chang HY. Rejuvenation of gene expression pattern of aged human skin by broadband light treatment: a pilot study. J Invest Dermatol. 2012;133:394–402. doi: 10.1038/jid.2012.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Voutetakis K, Kapetanou M, Delitsikou V, Papaevgeniou N, Sakellari M, et al. Proteasome activation: an innovative promising approach for delaying aging and retarding age-related diseases. Ageing Res Rev. 2015;23(Pt. A):37–55. doi: 10.1016/j.arr.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21(3 Pt. 2):610–3. doi: 10.1016/s0190-9622(89)70227-9. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Ullmann Y, Karry R, Shalaginov R, Assy B, Serafimovich S, et al. Ageing of human epidermis: the role of apoptosis, Fas and telomerase. Br J Dermatol. 2004;150:56–63. doi: 10.1111/j.1365-2133.2004.05715.x. [DOI] [PubMed] [Google Scholar]
- Lee C-K, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–3. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- Saez I, Vilchez D. The mechanistic links between proteasome activity, aging and age-related diseases. Curr Genomics. 2014;15:38–51. doi: 10.2174/138920291501140306113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, et al. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol. 2009;29:1095–106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis CC, Makrantokani E. Clinical aspects and molecular diagnostics of skin aging. Clin Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


