Abstract
Background
Accurate preoperative lymphoscintigraphy is vital to performing sentinel lymph node biopsy (SLNB) for cutaneous malignancies. Potential advantages of single-photon emission computed tomography with integrated computed tomography (SPECT/CT) include the ability to readily identify aberrant drainage patterns as well as provide the surgeon with three-dimensional anatomic landmarks not seen on conventional planar lymphoscintigraphy (PLS).
Methods
Patients with cutaneous malignancies who underwent SLNB with preoperative imaging using both SPECT/CT and PLS from 2011 to 2014 were identified.
Results
Both SPECT/CT and PLS were obtained in 351 patients (median age, 69 years; range, 5–94 years) with cutaneous malignancies (melanoma = 300, Merkel cell carcinoma = 33, squamous cell carcinoma = 8, other = 10) after intradermal injection of 99mtechnetium sulfur colloid (median dose 300 μCi). A mean of 4.3 hot spots were identified on SPECT/CT compared to 3.0 on PLS (p < 0.001). One hundred fifty-three patients (43.6 %) had identical findings between SPECT/CT and PLS, while 172 (49 %) had additional hot spots identified on SPECT/CT compared to only 24 (6.8 %) additional on PLS. SPECT/CT demonstrated additional nodal basins in 103 patients (29.4 %), compared to only 11 patients (3.1 %) with additional basins on PLS.
Conclusions
SPECT/CT is a useful adjunct that can help with sentinel node localization in challenging cases. It identified additional hot spots not seen on PLS in almost 50 % of patients. Because PLS identified hot spots not seen on SPECT/CT in 6.8 % of patients, we recommend using both modalities jointly. Long-term follow-up will be required to validate the clinical significance of the additional hot spots identified by SPECT/CT.
Most cutaneous malignancies, especially melanoma, have been shown to metastasize first to regional lymph nodes. Evaluation of the draining nodal basin with sentinel lymph node biopsy (SLNB) has become the standard of care for these patients because it offers significant prognostic value while limiting the morbidity of a complete lymph node dissection to only node-positive patients. 1–3 This procedure has been extensively evaluated in melanoma, where SLNB has been shown to lead to improved disease-free survival compared to nodal observation as demonstrated in the Multicenter Selective Lymphadenectomy Trial (MSLT-I). 4 SLNB has been routinely accomplished with the use of both radiocolloid and blue dye, which increases rates of detecting sentinel lymph nodes (SLNs) compared to blue dye alone. 5 For cutaneous malignancies, preoperative planar lymphoscintigraphy (PLS) is routinely utilized to visualize the number and location of SLNs, which can be particularly helpful in those sites with unpredictable drainage patterns.6, 7
Accurate preoperative PLS is vital to performing SLNB, though there are limitations to its use, especially in areas with complex anatomy, such as the head and neck, or a primary in close proximity to the draining nodal basin. A recently introduced modality to help facilitate better visualization, localization and removal of SLNs is single-photon emission computed tomography with integrated computed tomography (SPECT/CT). SPECT/CT has seen increased use in areas of anatomic constraints, such as the head and neck, for SLNs in the vicinity of the injection site or in patients with aberrant or unexpected drainage patterns. 8,9 Keidar et al. reported on this imaging technique and described SPECT/CT involving a low-dose CT system and a gamma camera. The CT and gamma camera capture images concurrently, which are then fused together to create a hybrid image. This shows the SLN in anatomically three-dimensional fashion. This is accomplished without changing the patient’s position. 9
Since its introduction, multiple studies have been conducted to evaluate potential advantages of SPECT/CT over conventional PLS alone.1, 9, 10 Stoffels et al. reported on the potential advantages of SPECT/CT in a retrospective study comparing 403 patients with clinically negative lymph nodes who had a SPECT/CT and PLS or PLS alone over an 8-year period. Both cohorts had similar tumor thickness, tumor ulceration, and age; however, the SPECT/CT cohort had significantly more tumors located on the head and neck. The authors showed SPECT/CT identified more SLNs compared to PLS, 2.40 versus 1.87, respectively (p < 0.001).1 This finding was consistent with previous, smaller studies.11, 12 The authors concluded the potential advantages of SPECT/CT encompassed additional three-dimensional anatomic detail, which could assist in more accurate placement of the surgical incision allowing the surgeon to identify the SLN with less dissection and overall less morbidity. The purpose of our study was to review our experience with the selective application of SPECT/CT to determine what additional information it provided while planning and performing a SLNB.
Patients and Methods
After obtaining Institutional Review Board approval, a retrospective series of patients with cutaneous malignancies was identified from a single-institution database of all patients who had a SPECT/CT with PLS from 2011 to 2014. Cutaneous malignancies analyzed include melanoma, Merkel cell carcinoma, squamous cell carcinoma, and other cutaneous malignancies (Table 1). Demographic and clinicopathologic characteristics along with outcomes data were retrieved from the database. Patients were included even if they failed to map on PLS or SPECT/CT, or both.
TABLE 1.
Patient demographics and tumor characteristics
| Characteristics | All patients N=351 |
|---|---|
| Age at diagnosis | |
|
| |
| Median (range) | 69 (5–94) |
|
| |
| Gender (%) | |
|
| |
| Male | 250 (71.2) |
|
| |
| Cutaneous malignancies (%) | |
|
| |
| Melanoma | 300 (85.4) |
| Merkel cell carcinoma | 33 (9.4) |
| Squamous cell carcinoma | 8 (2.3) |
| Sarcomaa | 2 (0.6) |
| Otherb | 8 (2.3) |
|
| |
| Tumor location (%) | |
|
| |
| Head and neck | 228 (65) |
| Trunk | |
| Back | 38 (10.8) |
| Shoulder | 37 (10.5) |
| Chest/abdomen | 13(3.7) |
| Upper Extremity | 12 (3.5) |
| Lower extremity | 10 (2.8) |
| Genital | 8 (2.3) |
| Anorectal | 5 (1.4) |
Includes histological subtypes clear cell and synovial sarcoma
Other histologies include adnexal carcinoma, basal cell carcinoma and sebaceous carcinoma
Conventional PLS was performed using an intradermal injection of 99mtechnetium sulfur colloid divided into four equal aliquots of 0.4 mL, injected at the border of the lesion or the remaining scar in four quadrants. Anterior and posterior planar images, which include the injection site as well as expected regional lymphatic drainage sites, were obtained for 60 min. The lymphatic drainage sites (“hot spots”) were identified after imaging and marked with indelible ink by the nuclear medicine technologist on the patient’s skin. A SPECT/CT was then obtained and images of uptake of radiotracer in nodal basins or interval/in-transit nodes were recorded as hot spots and used in our data analysis.
Surgery was done the same or following day after the PLS and SPECT/CT. Lymphazurin blue dye was selectively used and 67.2 % of patients received it. A hand-held probe (Neoprobe, Neoprobe Corp.) was used to interrogate the area of the highest counts in the basin highlighted on the PLS and/or SPECT/CT. The sentinel nodes were removed, counted and sent to pathology for permanent section. The patients who failed to localize on imaging and had no nodes identified during the operation with the use of the probe or blue dye, were followed with serial ultrasounds every 4 months for years 1–3, every 6 months for years 4 and 5 and yearly thereafter.
Demographic data and clinical variables were collected and analyzed. χ2 tests were performed on categorical variables. Statistical significance was determined by a p ≤ 0.05. All analysis was done in R, version 3.1.0 (a statistical computing environment) (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 351 patients met the study criteria and were included for analysis. The median age of the cohort was 68.9 years (range, 5–94 years), and most patients (71.2 %) were male. The majority of primary cutaneous malignancies in this series were located on the head and neck (65.0 %). The next most common location was trunk (25.0 %), followed by the extremities (6.3 %) and genital/anorectal areas (3.7 %). The overwhelming majority of primary lesions were melanoma (85.4 %), while the second most common histology was Merkel cell carcinoma (9.4 %). Other types of cutaneous malignancy with a propensity to spread to the lymph nodes were included (Table 1 ). The median dose of 99mtechnetium sulfur colloid was 300 μCi.
Of the 351 patients, 333 patients (94.9 %) had at least one hot spot visualized on either PLS, SPECT/CT or both. The mean number of hot spots visualized on SPECT/CT was 4.3, which was significantly higher than the 3.0 visualized on PLS (p < 0.001). Of those 333, 314 had at least one node identified on final pathology as the sentinel node. PLS was unable to identify any hot spots in 29 patients (8.3 %); 20 of those had head and neck primaries. In 11 of these 29 patients, SPECT/CT was able to visualize at least one hot spot. Of these 11 patients, six had at least one node removed. Three of those six patients had a sentinel node positive for melanoma on final pathology. SPECT/CT was unable to identify a hot spot in 24 patients (6.8 %). Of those patients, PLS was able to identify at least one hot spot in six patients. Four of those six patients had a sentinel node removed and identified, with one patient having a node positive for melanoma.
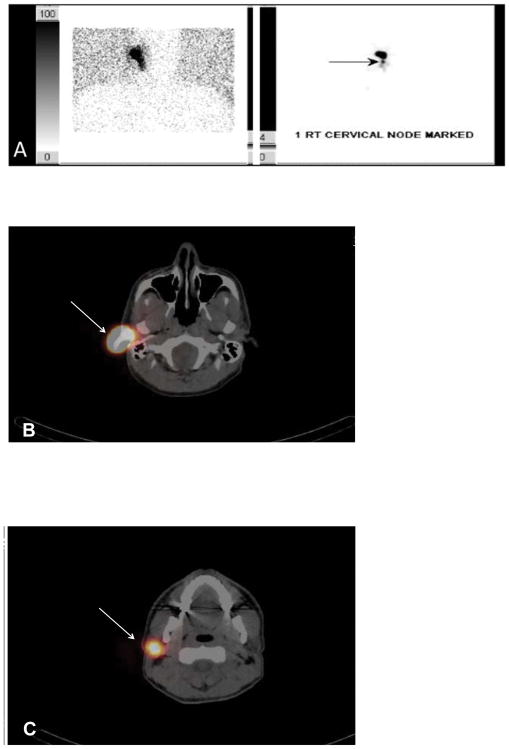
SPECT/CT and PLS produced identical results in 153 patients (43.6 %), while 172 patients (49.0 %) had more hot spots visualized on SPECT/CT compared to PLS. An example is depicted in Fig. 1. The patient was a 43-year-old man with a 19 mm melanoma of the right ear. The PLS showed only one right-sided lymph node near the primary site (Fig. 1a). SPECT/CT demonstrated one right parotid (Fig. 1c) and two right cervical nodes in levels IIB (Fig. 1d) and IV (Fig. 1e). One sentinel node was removed from the parotid and three were removed from the neck, which were all negative for disease.
FIG. 1.
(A) Planar lymphoscintigraphy revealed a right ear primary along with one right cervical sentinel lymph node. SPECT/CT demonstrated the primary site (B) and one parotid sentinel lymph node directly inferior to the primary (C) (white arrows). SPECT/CT also demonstrated one node in the right lateral neck level IIB (D) and one in level IV (E) (white arrows)
PLS and SPECT/CT were unable to identify a hot spot in 18 patients. Despite the lack of preoperative localization, eight of the 18 patients had at least one node identified by the surgeon intraoperatively with the gamma probe, which was confirmed on the final pathology. The remaining 10 patients (2.8 %) had no intraoperative identification of nodes with the gamma probe and no nodes reported on final pathology.
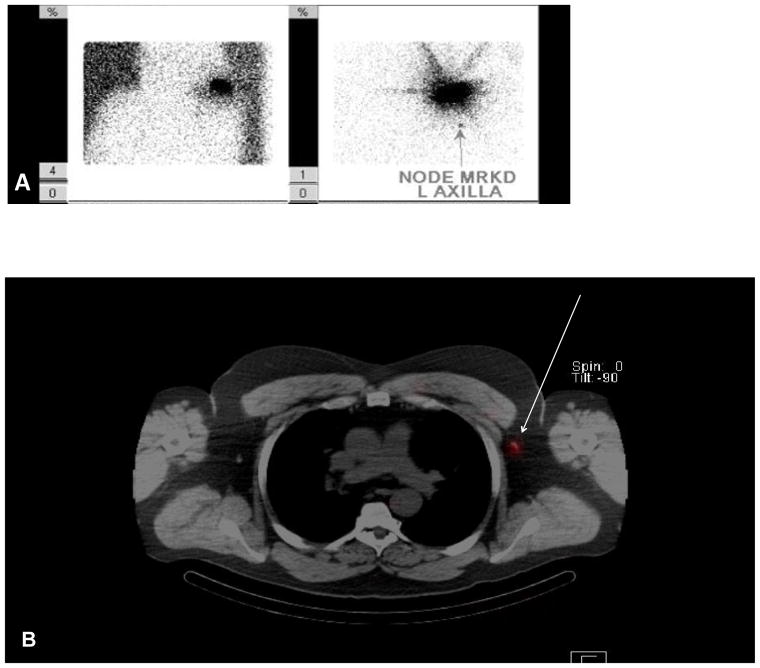
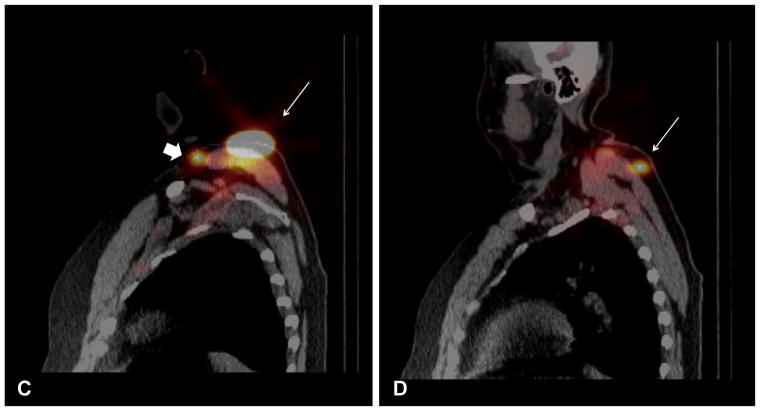
The mean number of hot spots seen on SPECT/CT was greater compared to PLS (Table 2), but SPECT/CT also demonstrated additional nodal basins not identified on PLS. An example of this is demonstrated in Fig. 2, which shows a 56-year-old male with a 1.0 mm melanoma of the left shoulder. The planar films show one node in the left axilla (Fig. 2a), which was seen on SPECT/CT (Fig. 2b). However, SPECT/CT also demonstrated additional sites not seen on PLS, with a node located in the left supraclavicular area (Fig. 2c) and an in-transit node overlying the left scapula (Fig. 2d). One node was removed from the left axilla and one from the supraclavicular area, both of which were negative for disease.
TABLE 2.
Comparison of nodes (“hot spots”) visualized to sentinel lymph nodes harvested
| Location | # of patients | Nodes visualized by SPECT/CT | Nodes visualized by PLS | Number of SLN’s harvested | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Mean | Range | Mean | Range | Mean | Range | ||
| Head and neck | 228 | 3.6 | 0–11 | 3.0 | 0–13 | 3.2 | 0–23 |
| Trunk | |||||||
| Back | 38 | 3.9 | 1–11 | 3.0 | 0–7 | 3.3 | 0–9 |
| Shoulder | 37 | 2.8 | 0–6 | 2.0 | 0–6 | 3.2 | 0–14 |
| Chest/abdomen | 13 | 3.6 | 0–11 | 2.0 | 0–9 | 2.8 | 0–7 |
| Upper extremity | 12 | 3.2 | 0–7 | 2.0 | 0–5 | 2.7 | 0–6 |
| Lower extremity | 10 | 8.4 | 1–13 | 6.0 | 1–10 | 2.5 | 1–6 |
| Genital | 8 | 4.1 | 1–6 | 3.0 | 1–6 | 3.3 | 1–7 |
| Anorectal | 5 | 5.0 | 3–8 | 3.0 | 2–4 | 3.0 | 1–4 |
|
| |||||||
| Total | 351 | 4.3 | 0–13 | 3.0 | 0–13 | 3.0 | 0–23 |
SPECT/CT single photon emission computed tomography with integrated CT, PLS planar lymphoscintigraphy, SLN’s sentinel lymph nodes
FIG. 2.
Planar lymphoscintigraphy revealed a left posterior shoulder primary with one node marked in the left axilla (A). SPECT/CT demonstrated one node located in the left axilla (white arrow) (B). SPECT/CT also demonstrated the left shoulder primary (white arrow) along with a left supraclavicular node (arrow head) (C) and a left posterior superficial subcutaneous node (white arrow) (D).
SPECT/CT also demonstrated additional nodal basins in 103 patients (29.4 %). Of those 103 patients, 94 had at least one node identified in the SPECT/CT localized basin confirmed on final pathology. Fourteen of those 94 patients had at least one SLN positive for metastatic disease, and of those 14, the positive SLN was identified in a basin that was not visualized on the PLS in five patients. PLS showed drainage to nodal basins not visualized on SPECT/CT in only 11 patients (3.1 %), while nine of the 11 had at least one node identified in those basins on final pathology. Only one patient of those nine had a node positive for disease in a basin not visualized on the SPECT/CT.
Discussion
SLNB is an important prognostic and staging tool used in cutaneous malignancies with the propensity to spread to regional lymph nodes. This has been shown to be important in cutaneous melanoma and other cutaneous malignancies, where if occult nodal metastases are found, a completion lymph node dissection of that nodal basin is indicated. 13 SLNB has been shown to be the only reliable method of identifying occult metastatic disease in clinically negative regional nodes and is considered the standard of care.13, 14 Accurate preoperative lymphoscintigraphy is vital in helping accurately identify SLNs in cutaneous malignancies. SPECT/CT is a newer imaging modality that has seen increasing use and has been compared to PLS in a number of studies. The potential benefit of improved localization of SLNs, especially in the head and neck region, has been demonstrated in previous studies. 10–12, 15,16 The purpose of our study was to determine what additional information was elucidated by the addition of SPECT/CT to PLS in patients with cutaneous malignancies.
Conventional two-dimensional PLS can be a useful tool, but PLS has been shown to accurately identify the number of SLNs in only 81 % of nodal basins by Jansen et al. The authors report that PLS showed drainage to 393 SLNs in 255 lymphatic fields in 199 patients. In 48 lymphatic fields (19 %) in 46 patients, the number of SLNs was different from what was visualized on SPECT.17 Potential explanations for this low identification rate have been suggested in the literature. Jansen et al. stated that nodes could have been superimposed on each other or shine-through from the primary site might have made visualization of nodes difficult. The authors also stated that the planar imaging was not able to identify some nodes or lymphatic channels because of the low resolution of the planar imaging itself. 17 We reported SPECT/CT was able to visualize more nodes in 172 patients. This was clearly seen in one of our patients diagnosed with a right ear melanoma (Fig. 1). The PLS showed only one right cervical node, but the SPECT/CT was able to identify a node in the right parotid gland. This node was not seen on PLS either because of the shine-through effect of the primary lesion or the lack of three-dimensional anatomic definition.
Drainage to minor basins, such as the popliteal or epitrochlear, and/or interval nodes (i.e., lattisumus, 12th rib, trapezius) has been reported in select patients. 18 Another advantage of SPECT/CT is the ability to identify and localize unusual lymphatic drainage to interval and minor nodal basin nodes. This was clearly depicted in one of our patients with a left shoulder melanoma primary (Fig. 2). The PLS showed drainage to only one left axilla node, while the SPECT/CT was also able to identify a left scapular in-transit node. In our study we reported multiple or different nodal basins in 30 % of patients, with the majority of those being head and neck. This is similar to what Even-Sapir et al. reported in 2003. They showed multiple basins in 33 % of patients with a head and neck primary and 50 % with a trunk melanoma 19. Our lower rate of identification of hot spots on PLS may be related to the higher preponderance of head and neck melanomas. It is important to identify not only multiple nodes, but nodes in different basins, as these nodes may be positive for micrometastatic disease. In our study, five patients (four head and neck and one trunk) had at least one SLN positive for micrometastatic disease identified in a basin not seen on PLS.
SPECT/CT has been shown to add value when drainage to the pelvis is seen on PLS.20 SPECT/CT can identify additional pelvic nodes and allows for identification of presacral, aortocaval, paraaortic and abdominal wall nodes that may not be visualized by routine PLS.21 These can be followed with cross-sectional imaging if the superficial SLNs are negative. Alternatively, if the superficial or deep pelvic nodes are positive, they could be dissected with an open or robotic pelvic node dissection or followed with imaging. 22
The recognized limitations of our study are derived from the inherent flaws of the retrospective design and missing data points for some patients. The major limitation of the study is not being able to know if all the additional nodes identify on SPECT/CT were SLNs that potentially contained metastatic melanoma or not. Another limitation of this study is not including a complete cost analysis. At present, additional reimbursement is not provided for SPECT/CT at our institution because there are no available billing codes. The patient is charged the cost of PLS with colloid ($2,000 at our institution) whether or not a SPECT/CT is obtained. Stoffels et al. analyzed the cost-effectiveness of SPECT/CT and SLNB or SLNB alone in patients with melanoma. The authors reported a cost savings of 35 % when SPECT/CT was utilized to aid SLNB, which was explained by a significant decrease in operating time (p = 0.002) and hospital stay (p < 0.001). 23
This study has provided the opportunity to compare patients with cutaneous malignancies who had both a SPECT/CT and PLS. A future direction is to organize a multi-institutional study to prospectively compare the two imaging modalities to each other, analyzing such variables such as time needed to identify SLNs, placement of incisions or costs.
Conclusions
Even though the advantages are difficult to quantify, SPECT/CT is a useful adjunct and can help with localization of SLNs in selected cases. While SPECT/CT identified almost 50 % more nodes, PLS was still able to identify nodes not seen in 6.8 % of patients on SPECT/CT. We recommend both modalities be employed in selective and specific situations, such as head and neck cutaneous malignancies, when SLNB is performed. This may help increase the identification of hot spots over using one modality alone. We recommend SPECT/CT whenever detailed anatomic localization might facilitate more accurate placement of SLNB incisions and when shine-through on PLS interferes with localization.
Footnotes
Disclosure Jonathan S. Zager, MD: consulting fees from Amgen, Castle Biosciences, Provectus; research support from Amgen, Castle Biosciences, Provectus, and Delcath; advisory board for Delcath. Maki Yamamoto, MD: consulting fees from Castle Biosciences. Vernon K. Sondak, MD: consulting fees from Amgen, Bristol-Myers Squibb, Genentech, Merck, Novartis, and Provectus. The other authors declare no conflict of interest.
References
- 1.Stoffels I, Boy C, Poppel T, Kuhn J, et al. Association between sentinel lymph node excision with or without preoperative SPECT/CT and metastatic node detection and disease-free survival in melanoma. JAMA. 2012;308:1007–14. doi: 10.1001/2012.jama.11030. [DOI] [PubMed] [Google Scholar]
- 2.Schadendorf D, Fisher DE, Garbe C, et al. Melanoma. Nat Rev Dis Primers. 2015;1:15003. doi: 10.1038/nrdp.2015.3. [DOI] [PubMed] [Google Scholar]
- 3.Livingstone E, Windemuth-Kieselbach C, Eigentler TK, et al. A first prospective population-based analysis investigating the actual practice of melanoma diagnosis, treatment and follow-up. Eur J Cancer. 2011;47:1977–89. doi: 10.1016/j.ejca.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapteijn BA, Nieweg OE, Liem I, et al. Localizing the sentinel node in cutaneous melanoma: gamma probe detection versus blue dye. Ann Surg Oncol. 1997;4:156–60. doi: 10.1007/BF02303799. [DOI] [PubMed] [Google Scholar]
- 6.Statius Muller MG, Hennipman FA, Van Leeuwen PA, et al. Unpredictability of lymphatic drainage patterns in melanoma patients. Eur J Nucl Med Mol Imaging. 2002;29:255–61. doi: 10.1007/s00259-001-0670-8. [DOI] [PubMed] [Google Scholar]
- 7.Thompson JF, Uren RF, Shaw HM, et al. Location of sentinel lymph nodes in patients with cutaneous melanoma: new insights into lymphatic anatomy. J Am Coll Surg. 1999;189:195–204. doi: 10.1016/s1072-7515(99)00108-8. [DOI] [PubMed] [Google Scholar]
- 8.Summer WE, Ross MI, Mansfield, et al. Implications of lymphatic drainage to unusual sentinel node sites in patients with primary cutaneous melanoma. Cancer. 2002;95:354–60. doi: 10.1002/cncr.10664. [DOI] [PubMed] [Google Scholar]
- 9.Keidar Z, Israel O, Krausz Y. SPECT/CT in tumor imaging: technical aspects and clinical applications. Semin Nucl Med. 2003;33:205–18. doi: 10.1053/snuc.2003.127310. [DOI] [PubMed] [Google Scholar]
- 10.Valdes-Olmos RA, Rietbergen DD, Vidal-Sicart S. SPECT/CT and sentinel node lymphoscintigraphy. Clin Transl Imaging. 2014;2:491–504. [Google Scholar]
- 11.Klode J, Poeppel T, Boy C, et al. Advantages of preoperative hybrid SPECT/CT in detection of sentinel lymph nodes in cutaneous head and neck malignancies. J Eur Acad Dermatol Venereol. 2011;25:1213–21. doi: 10.1111/j.1468-3083.2010.03954.x. [DOI] [PubMed] [Google Scholar]
- 12.Vermeeren L, Valdés Olmos RA, Klop WM, et al. SPECT/CT for sentinel lymph node mapping in head and neck melanoma. Head Neck. 2011;33:1–6. doi: 10.1002/hed.21392. [DOI] [PubMed] [Google Scholar]
- 13.Ambe CM, Sondak VK. Sentinel lymph node biopsy in melanoma and other cutaneous malignancies. Am J Hematol Oncol. 2014;10:3–10. [Google Scholar]
- 14.Mariani G, Erba P, Manca G, et al. Radioguided sentinel lymph node biopsy in patients with malignant cutaneous melanoma: the nuclear medicine contribution. J Surg Oncol. 2004;85:141–51. doi: 10.1002/jso.20027. [DOI] [PubMed] [Google Scholar]
- 15.Haerle SK, Stoeckli SJ. SPECT/CT for lymphatic mapping of sentinel nodes in early squamous cell carcinoma of the oral cavity and oropharynx. Int J Mol Imaging. 2011;2011:1060–8. doi: 10.1155/2011/106068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez R, Payoux P, Gantet P, Esquerré JP, Boutault F, Paoli JR. Multimodal image registration for localization of sentinel nodes in head and neck squamous cell carcinoma. J Oral Maxillofac Surg. 2004;62:1497–504. doi: 10.1016/j.joms.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Jansen L, Nieweg OE, Kapteijn AE, et al. Reliability of lymphoscintigraphy in indicating the number of sentinel nodes in melanoma patients. Ann Surg Oncol. 2000;7:624–30. doi: 10.1007/BF02725343. [DOI] [PubMed] [Google Scholar]
- 18.Zager JS, Puleo CA, Sondak VK. What is the significance of the in transit or interval sentinel node in melanoma? Ann Surg Oncol. 2011;18:3232–4. doi: 10.1245/s10434-011-1996-5. [DOI] [PubMed] [Google Scholar]
- 19.Even-Sapir E, Lerman H, Lievshitz G, et al. Lymphoscintigraphy for sentinel node mapping using a hybrid SPECT/CT system. J Nucl Med. 2003;44:1413–20. [PubMed] [Google Scholar]
- 20.Kretschmer L, Altenvoerde G, Meller J, et al. Dynamic lymphoscintigraphy and image fusion of SPECT and pelvic CT-scans allow mapping of aberrant pelvic sentinel lymph nodes in malignant melanoma. Eur J Cancer. 2003;39:175–83. doi: 10.1016/s0959-8049(02)00534-8. [DOI] [PubMed] [Google Scholar]
- 21.Heffernan-Jimenez A, Ellmann A, Sado H, et al. Results of a prospective multicenter international atomic energy agency sentinel node trial on the value of SPECT/CT over planar imaging in various malignancies. J Nucl Med. 2015;56:1338–44. doi: 10.2967/jnumed.114.153643. [DOI] [PubMed] [Google Scholar]
- 22.Dossett LA, Castner NB, Pow-Sang JM, et al. Robotic-assisted transperitoneal pelvic lymphadenectomy for metastatic melanoma: early outcomes compared with open pelvic lymphadenectomy. J Am Coll Surg. 2016;222:702–9. doi: 10.1016/j.jamcollsurg.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffels I, Muller M, Geisel MH, et al. Cost-effectiveness of preoperative SPECT/CT combined with lymphoscintigraphy vs. lymphoscintigraphy for sentinel lymph node excision in patients with cutaneous malignant melanoma. Eur J Nucl Med Mol Imaging. 2014;41:1723–31. doi: 10.1007/s00259-014-2771-1. [DOI] [PubMed] [Google Scholar]